The Quality Characteristics Formation and Control of Salted Eggs: A Review
Abstract
1. Introduction
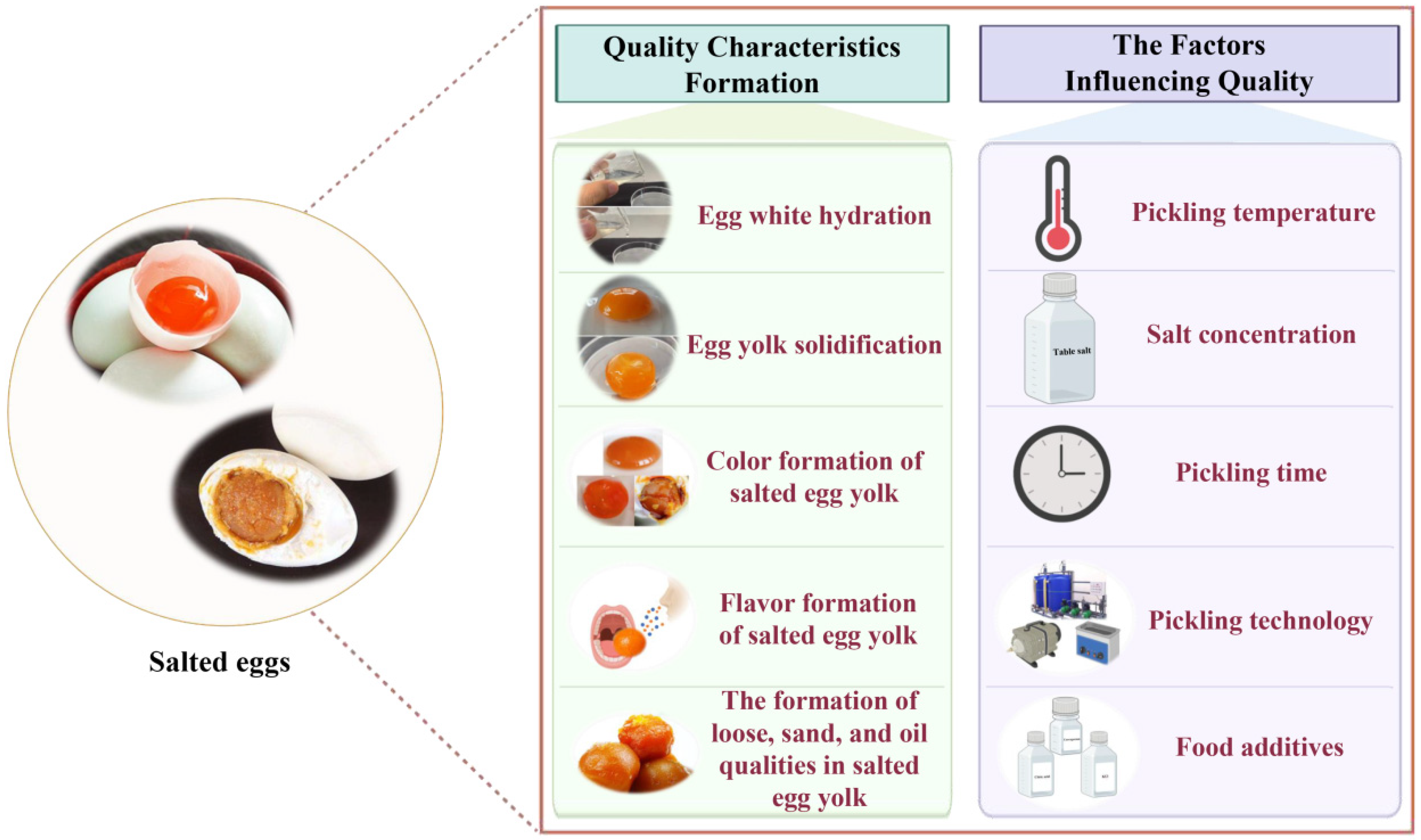
2. Quality Formation Mechanism of Salted Eggs
2.1. Egg White Hydration
2.2. Gelling of Salted Egg Yolk
2.3. Color Formation of Salted Egg Yolk
2.4. Flavor Formation of Salted Egg Yolk
2.5. The Formation of Loose, Sand, and Oil Qualities in Salted Egg Yolk
3. Factors Affecting the Quality of Salted Eggs
3.1. Effect of Pickling Temperature on the Quality of Salted Eggs
3.2. Effect of Salt Concentration on the Quality of Salted Eggs
3.3. Effect of Pickling Time on the Quality of Salted Eggs
3.4. Effect of Pickling Technology on the Quality of Salted Eggs
3.5. The Influence of Food Additives on the Quality of Salted Eggs
4. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Yao, X.; Xu, J.; Adhikari, B.; Lv, W.; Chen, H. Mooncake production waste: Nutritional value and comprehensive utilization of salted duck egg white. J. Food Process. Preserv. 2022, 46, e16772. [Google Scholar] [CrossRef]
- Sun, X.; He, L.; Yang, H.; Wu, W.; Yue, L.; Peng, W.; Jin, G.; Jin, Y. Intermittent ultrasound assisted in speeding up the pickling speed of salted eggs. Sci. Technol. Food Ind. 2018, 39, 204–211. (In Chinese) [Google Scholar] [CrossRef]
- Zou, L.; Zhao, Y.; Qiu, J.; Weng, L.; Liu, J.; Jiang, H. Optimization of process parameters in two-stage brining of salted eggs with low NaCl content. Int. J. Food Eng. 2018, 4, 200–205. [Google Scholar] [CrossRef]
- Chen, Y.C.; Liaw, R.B.; Liao, Y.S.; Wanangkarn, A.; Chen, W.S.; Tan, F.J. Molecular identification and relative abundance of microorganisms in douchi koji and salted egg white sufu during processing. Anim. Sci. J. 2021, 92, e13567. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhao, J.; Liang, H.; Deng, Q.; Wan, C.; Li, B.; Zhou, B. Desalination of salted duck egg white assisted by gelatin: Foaming and interface properties of the mixed system. Food Hydrocoll. 2022, 124, 107260. [Google Scholar] [CrossRef]
- Zhou, N.; Yao, Y.; Wu, N.; Du, H.; Xu, M.; Zhao, Y.; Tu, Y. Front Cover: VF-4 and DR-8 derived from salted egg white inhibit inflammatory activity via NF-κB/PI3K-Akt/MAPK signal transduction pathways in HT-29 cells induced by TNF-α. Mol. Nutr. Food Res. 2022, 66, 2270008. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Watanuki, C.; Ariizumi, M.; Shigematsu, Y.; Kobayashi, H.; Hasegawa, M.; Watanabe, K. Super chilling enhances preservation of the freshness of salted egg yolk during long-term storage. J. Food Sci. 2009, 74, E62–E69. [Google Scholar] [CrossRef]
- Novia, D.; Vebriyanti, E.; Hakim, H.F. Research Article Evaluation of Heating the Gambier Liquid Waste on the Quality of Raw Salted Eggs. Int. J. Poult. Sci. 2017, 16, 369–373. [Google Scholar] [CrossRef][Green Version]
- Lai, K.; Ko, W.; Lai, T. Effect of NaCl penetration rate on the granulation and oil-off of the yolk of salted duck egg. Food Sci. Technol. Int. 1997, 3, 269–273. [Google Scholar] [CrossRef][Green Version]
- Kaewmanee, T.; Benjakul, S.; Visessanguan, W. Effect of acetic acid and commercial protease pretreatment on salting and characteristics of salted duck egg. Food Bioprocess Technol. 2012, 5, 1502–1510. [Google Scholar] [CrossRef]
- Sun, J.; Du, J.; Xiang, J.; Zeng, X.; Li, K. Effect of washing and disinfection before pickling on free amino acids in salted eggs. J. Food Saf. Qual. 2021, 12, 6160–6168. (In Chinese) [Google Scholar] [CrossRef]
- Omana, D.; Liang, Y.; Kav, N.; Wu, J. Proteomic analysis of egg white proteins during storage. Proteomics 2011, 11, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, P.; Liu, H.; Zhang, X.; Gao, Z. Changes in physiochemical properties of egg white during vacuum curing of salted eggs. Food Res. Dev. 2022, 43, 6–13. (In Chinese) [Google Scholar]
- Kaewmanee, T.; Benjakul, S.; Visessanguan, W. Changes in chemical composition, physical properties and microstructure of duck egg as influenced by salting. Food Chem. 2009, 112, 560–569. [Google Scholar] [CrossRef]
- Zuo, S. The Gel Property of Ovomucin and Its Effect on Egg White Gel. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2013. (In Chinese). [Google Scholar]
- Sun, X.; Huang, J.; Zeng, H.; Wu, J. Protein-resistant property of egg white ovomucin under different pHs and ionic strengths. J. Agric. Food Chem. 2018, 66, 11034–11042. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Tsai, J.S.; Pan, B.S. Pickling time and electrodialysis affects functional properties of salted duck egg white. J. Food Biochem. 1999, 23, 607–618. [Google Scholar] [CrossRef]
- Oliveira Dos Santos, D.; Selia Dos Reis Coimbra, J.; De Sousa, R.D.C.S.; Da Silva, C.A.S.; Barreto, S.L.D.T.; Minim, L.A. Solubility of quail (Coturnix coturnix japonica) egg white protein. J. Food Process Eng. 2008, 31, 684–693. [Google Scholar] [CrossRef]
- Croguennec, T.; Nau, F.; Brule, G. Influence of pH and salts on egg white gelation. J. Food Sci. 2002, 67, 608–614. [Google Scholar] [CrossRef]
- Kaewmanee, T.; Benjakul, S.; Visessanguan, W. Effect of NaCl on thermal aggregation of egg white proteins from duck egg. Food Chem. 2011, 125, 706–712. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Wang, C.; Zhang, M.; Xu, Y.; Zhou, B.; Su, Y.; Yang, Y. Characteristics of gelling and water holding properties of hen egg white/yolk gel with NaCl addition. Food Hydrocoll. 2018, 77, 887–893. [Google Scholar] [CrossRef]
- Nasabi, M.; Labbafi, M.; Mousavi, M.E.; Madadlou, A. Effect of salts and nonionic surfactants on thermal characteristics of egg white proteins. Int. J. Biol. Macromol. 2017, 102, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, Y.; Xu, M.; Yao, Y.; Nie, X.; Du, H.; Tu, Y. Changes in aggregation behavior of raw and cooked salted egg yolks during pickling. Food Hydrocoll. 2018, 80, 68–77. [Google Scholar] [CrossRef]
- Ibarz, A. Rheology of salted egg yolk. J. Texture Stud. 1993, 24, 63–71. [Google Scholar] [CrossRef]
- Ai, M.-M.; Guo, S.-G.; Zhou, Q.; Wu, W.-L.; Jiang, A.-M. The investigation of the changes in physicochemical, texture and rheological characteristics of salted duck egg yolk during salting. LWT-Food Sci. Technol. 2018, 88, 119–125. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, F.; Yang, Y.; Xiong, C.; Xu, M.; Tu, Y. Gelation behavior of egg yolk under physical and chemical induction: A review. Food Chem. 2021, 355, 129569. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lv, Y.; Mo, X.; Duan, S.; Tong, Q. Effects of freezing and thawing treatment on the rheological and textural characteristics and micro-structure of heat-induced egg yolk gels. J. Food Eng. 2018, 216, 144–150. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Y.; Xu, M.; Yao, Y.; Nie, X.; Du, H.; Tu, Y. Effects of salting treatment on the physicochemical properties, textural properties, and microstructures of duck eggs. PLoS ONE 2017, 12, e0182912. [Google Scholar] [CrossRef]
- Kiosseoglou, V. Egg yolk protein gels and emulsions. Curr. Opin. Colloid Interface Sci. 2003, 8, 365–370. [Google Scholar] [CrossRef]
- Anton, M.; Le Denmat, M.; Beaumal, V.; Pilet, P. Filler effects of oil droplets on the rheology of heat-set emulsion gels prepared with egg yolk and egg yolk fractions. Colloids Surf. B Biointerfaces 2001, 21, 137–147. [Google Scholar] [CrossRef]
- Kaewmanee, T.; Benjakul, S.; Visessanguan, W.; Gamonpilas, C. Effect of sodium chloride and osmotic dehydration on viscoelastic properties and thermal-induced transitions of duck egg yolk. Food Bioprocess Technol. 2013, 6, 367–376. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Y.; Xu, M.; Yao, Y.; Wu, N.; Du, H.; Tu, Y. Changes in physico-chemical properties, microstructure, protein structures and intermolecular force of egg yolk, plasma and granule gels during salting. Food Chem. 2019, 275, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, A.; Kiosseoglou, V.; Alevisopoulos, S.; Kasapis, S. Small deformation measurements of single and mixed gels of low cholesterol yolk and egg white. J. Texture Stud. 2000, 31, 225–244. [Google Scholar] [CrossRef]
- Omana, D.A.; Wang, J.; Wu, J. Ovomucin—A glycoprotein with promising potential. Trends Food Sci. Technol. 2010, 21, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Anton, M.; Beaumal, V.; Gandemer, G. Adsorption at the oil–water interface and emulsifying properties of native granules from egg yolk: Effect of aggregated state. Food Hydrocoll. 2000, 14, 327–335. [Google Scholar] [CrossRef]
- Strixner, T.; Sterr, J.; Kulozik, U.; Gebhardt, R. Structural study on hen-egg yolk high density lipoprotein (HDL) granules. Food Biophys. 2014, 9, 314–321. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Xu, M.; Yao, Y.; Wu, N.; Du, H.; Tu, Y. Effects of strong alkali treatment on the physicochemical properties, microstructure, protein structures, and intermolecular forces in egg yolks, plasma, and granules. Food Chem. 2020, 311, 125998. [Google Scholar] [CrossRef]
- Geng, S.; Wang, H.; Wang, X.; Ma, X.; Xiao, S.; Wang, J.; Tan, M. A non-invasive NMR and MRI method to analyze the rehydration of dried sea cucumber. Anal. Methods-UK 2015, 7, 2413–2419. [Google Scholar] [CrossRef]
- Lin, S.; Yang, S.; Li, X.; Chen, F.; Zhang, M. Dynamics of water mobility and distribution in soybean antioxidant peptide powders monitored by LF-NMR. Food Chem. 2016, 199, 280–286. [Google Scholar] [CrossRef]
- Bao, Z.; Tian, Y.; Gao, J.; Da, K.; Lin, S. Effect of partial substitution of sodium salt on the quality of salted quail eggs. J. Food Biochem. 2021, 45, e13941. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Z.; Xiao, H.; Wang, Y.; Bai, J. Enhanced mass transfer of osmotic dehydration and changes in microstructure of pickled salted egg under pulsed pressure. J. Food Eng. 2013, 117, 141–150. [Google Scholar] [CrossRef]
- Wibawanti, J.; Meihu, M.; Hintono, A.; Pramono, Y.B. The characteristics of salted egg in the presence of liquid smoke. J. Apl. Teknol. Pangan 2013, 1, 68–70. [Google Scholar] [CrossRef]
- Wen, C.; Su, Y.; Tao, Z.; Cheng, Z.; Zhou, D.; Wang, T.; Zhou, Y. Dietary supplementation with microencapsulated lutein improves yolk color and lutein content in fresh and cooked eggs of laying hens. J. Poult. Sci. 2020, 58, 97–102. [Google Scholar] [CrossRef]
- Frigg, M.; Whitehead, C.; Weber, S. Absence of effects of dietary α-tocopherol on egg yolk pigmentation. Br. Poult. Sci. 1992, 33, 347–353. [Google Scholar] [CrossRef]
- Panaite, T.D.; Nour, V.; Saracila, M.; Turcu, R.P.; Untea, A.E.; Vlaicu, P.A. Effects of linseed meal and carotenoids from different sources on egg characteristics, yolk fatty acid and carotenoid profile and lipid peroxidation. Foods 2021, 10, 1246. [Google Scholar] [CrossRef] [PubMed]
- Fan, J. Study on Key Technology and Formation Mechanism of Salted Egg Yolk Using Fresh Egg Yolk. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2011. (In Chinese). [Google Scholar]
- Zamora, R.; Nogales, F.; Hidalgo, F.J. Phospholipid oxidation and nonenzymatic browning development in phosphatidylethanolamine/ribose/lysine model systems. Eur. Food Res. Technol. 2005, 220, 459–465. [Google Scholar] [CrossRef]
- Tan, J.; Yao, Y.; Wu, N.; Du, H.; Xu, M.; Liao, M.; Zhao, Y.; Tu, Y. Color, physicochemical characteristics and antioxidant activities of preserved egg white pickled at different temperatures. LWT-Food Sci. Technol. 2022, 164, 113685. [Google Scholar] [CrossRef]
- Dang, K.L.M.; Le, T.Q.; Songsermpong, S. Effect of ultrasound treatment in the mass transfer and physical properties of salted duck eggs. Agric. Nat. Resour. 2014, 48, 942–953. [Google Scholar]
- Yang, H.; Jin, Y.; Sun, X.; Jin, G.; Ma, M. Effects of different NaCl addition levels on the gel properties of egg yolk plasma. Mod. Food Sci. Technol. 2018, 34, 165–171. (In Chinese) [Google Scholar] [CrossRef]
- Li, M.; Ling, X.; Li, X.; Xia, N.; Teng, J.; Wei, B.; Huang, L.; Li, W. Analysis of volatile flavor compounds in sea duck egg white during salting based on Gas Chromatography Ion Mobility Spectrometry. Food Sci. 2022, 1–13. (In Chinese) [Google Scholar]
- Singh, A.; Ramaswamy, H. Effect of high pressure processing on color and textural properties of eggs. J. Food Res. 2013, 2, 11–24. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Zheng, Y.; Sun, Q.; Wang, J.; Zheng, B.; Guo, Z. Structural characteristics and emulsifying properties of myofibrillar protein-dextran conjugates induced by ultrasound Maillard reaction. Ultrason. Sonochem. 2021, 72, 105458. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, Y.; Zhou, B.; Xu, W.; Xiang, X.; Huang, Q.; Li, S. Improvement of quality and flavor of salted egg yolks by ultrasonic assisted cooking. Ultrason. Sonochem. 2021, 75, 105579. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.C.; Darfler, J.; Lifshitz, A. Factors affecting the discoloration of hard-cooked egg yolks. Poult. Sci. 1967, 46, 664–672. [Google Scholar] [CrossRef]
- Jiang, B.; Mine, Y. Phosphopeptides derived from hen egg yolk phosvitin: Effect of molecular size on the calcium-binding properties. Biosci. Biotechnol. Biochem. 2001, 65, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, H.; Li, Y.; Dai, Y.; Li, H.; Liu, Y. Analysis of the causes of the “black circles” in the vacuum cooked salted duck eggs. Mod. Food Sci. Technol. 2021, 37, 234–241. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, S.; La, P.; Ding, R.; Li, Y.; Pan, C. Improvement of sensory quality of salted egg yolk. China Food Ind. 2008, 12, 41–43. (In Chinese) [Google Scholar]
- Yu, P. Changes of Flavor Compounds in Salted Egg Yolk Processing and Preliminary Study on the Causes. Master’s Thesis, Tianjin University of Science and Technology, Tianjin, China, 2017. (In Chinese). [Google Scholar]
- Zhou, Q. Studies on Texture and Flavor of Salty Yolk. Master’s Thesis, Tianjin University of Science and Technology, Tianjin, China, 2012. (In Chinese). [Google Scholar]
- Paraskevopoulou, A.; Amvrosiadou, S.; Biliaderis, C.G.; Kiosseoglou, V. Mixed whey protein isolate-egg yolk or yolk plasma heat-set gels: Rheological and volatile compounds characterisation. Food Res. Int. 2014, 62, 492–499. [Google Scholar] [CrossRef]
- Yang, P.; Zheng, Y.; You, M.; Song, H.; Zou, T. Characterization of key aroma-active compounds in four commercial egg flavor Sachimas with differing egg content. J. Food Biochem. 2019, 43, e13040. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.; Zhang, N.; Chen, H.; Sun, B.; Zhang, Y. Analysis of volatile compounds of fried eggs by solid phase microextraction coupled with gas chromatography-mass spectroscopy. Fine Chem. 2014, 31, 218–224. [Google Scholar] [CrossRef]
- Ren, L.; Ma, J.; Xu, W.; Lv, Y.; Tong, Q. Stability of low density lipoprotein particles affect the formation of off-flavor in thermal egg yolk. Food Res. Int. 2022, 154, 111029. [Google Scholar] [CrossRef]
- Chen, Z. Characterization of Egg Yolk Flavoring Fast Prepared by Microwave Irradiation. Master’s Thesis, Jiangnan University, Wuxi, China, 2020. (In Chinese). [Google Scholar]
- Su, Y.; Chen, Z.; Li, J.; Chang, C.; Gu, L.; Yang, Y. Characterization of salted egg yolk flavoring prepared by enzyme hydrolysis and microwave irradiation. Food Chem. 2021, 338, 127913. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhang, T.; Wang, X.; Song, Y.; Wan, H.; Wang, H.; Yang, P.; Tan, M. Influence of salting processes on water and lipid dynamics, physicochemical and microstructure of duck egg. LWT-Food Sci. Technol. 2018, 95, 143–149. [Google Scholar] [CrossRef]
- Wang, C. The physico-chemical properties of low density lipoprotein during granulation of salted chicken egg. J. Chin. Agri. Chem. Goc. 1991, 29, 406–414. [Google Scholar]
- Anton, M.; Denmat, M.L.; Gandemer, G. Thermostability of hen egg yolk granules: Contribution of native structure of granules. J. Food Sci. 2000, 65, 581–584. [Google Scholar] [CrossRef]
- Laca, A.; Paredes, B.; Rendueles, M.; Díaz, M. Egg yolk granules: Separation, characteristics and applications in food industry. LWT-Food Sci. Technol. 2014, 59, 1–5. [Google Scholar] [CrossRef]
- Samli, H.E.; Agma, A.; Senkoylu, N. Effects of storage time and temperature on egg quality in old laying hens. J. Appl. Poult. Res. 2005, 14, 548–553. [Google Scholar] [CrossRef]
- Bao, Z.; Kang, D.; Li, C.; Zhang, F.; Lin, S. Effect of salting on the water migration, physicochemical and textural characteristics, and microstructure of quail eggs. LWT-Food Sci. Technol. 2020, 132, 109847. [Google Scholar] [CrossRef]
- Cao, D.; Feng, F.; Xiong, C.; Li, J.; Xue, H.; Zhao, Y.; Wang, Y.; Tu, Y.; Zhao, Y. Changes in lipid properties of duck egg yolks under extreme processing conditions. Poult. Sci. 2021, 100, 101140. [Google Scholar] [CrossRef]
- Ganesan, P.; Kaewmanee, T.; Benjakul, S.; Baharin, B. Comparative study on the nutritional value of pidan and salted duck egg. Korean J. Food Sci. Anim. Res. 2014, 34, 1–6. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Yang, M.-H.; Lin, J.-H.; Lee, M.-H. Lipid profile and oxidative stability of commercial egg products. J. Food Drug Anal. 2005, 13, 78–83. [Google Scholar] [CrossRef]
- Sharif, M.K.; Saleem, M.; Javed, K. Food materials science in egg powder industry. In Role of Materials Science in Food Bioengineering; Academic Press: Cambridge, MA, USA, 2018; pp. 505–537. [Google Scholar] [CrossRef]
- Milinsk, M.; Murakami, A.; Gomes, S.; Matsushita, M.; De Souza, N. Fatty acid profile of egg yolk lipids from hens fed diets rich in n-3 fatty acids. Food Chem. 2003, 83, 287–292. [Google Scholar] [CrossRef]
- Fatwa, S.; Suhaidi, I.; Ginting, S. The quality characteristics of frozen salted egg yolk salting using various media. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 454, p. 012115. [Google Scholar] [CrossRef]
- Yang, N.; Jin, Y.; Xu, Y.; Bin, Y.; Xu, X. Effect of pressure cooking on physicochemical properties of salted eggs. RSC Adv. 2016, 6, 97089–97095. [Google Scholar] [CrossRef]
- Long, M.; Song, Y.; Du, Q.; Zhou, D.; Cai, H.; Zhan, G. Effect of pickling temperature and concentration of salt solution on lipid of duck egg yolk. Trans. Chin. Soc. Agric. Eng. 2015, 31, 281–288. [Google Scholar] [CrossRef]
- Xu, F. The Study of Fast Pickled Egg Based on Alternating Ice and Hot Therapy and Key Technologies for Intelligent Equipment. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2017. (In Chinese). [Google Scholar]
- Lai, K.; Chung, W.; Jao, C.; Hsu, K. Oil exudation and histological structures of duck egg yolks during brining. Poult. Sci. 2010, 89, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, M. Study on New Pickling Technique of High Quality Salted Egg Yolk and Its Quality Analysis. Master’s Thesis, Nanchang University, Nanchang, China, 2015. (In Chinese). [Google Scholar]
- Xu, J.; Liu, H.; Yang, X.; Zhang, C.; Wang, Y.; Zhao, F.; Liu, X. Speed up the process of salted eggs by vacuum technique and the changes of fatty acid in yolk. Food Sci. Technol. 2015, 102–107. (In Chinese) [Google Scholar] [CrossRef]
- Pu, Y.; Du, J.; Liang, Z.; Pi, J.; Pan, A.; Wu, Y.; Shen, J.; Li, Q.; Xiang, J.; Xu, X. Study on processing of salted egg with circulating water. China Poult. 2010, 32, 25–27. (In Chinese) [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, J.; Wu, J.; Gao, Z.; Xie, X.; Wang, Z.; Wang, X. The effect on quality of pickled salted duck eggs using the novel method of pulsed pressure osmotic dehydration. J. Food Process. Preserv. 2018, 42, e13581. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S.; Zhang, Y.; Zhang, R. Parameter optimization for quickly salted egg by using ultrasonic-pulsed pressure technology. Trans. Chin. Soc. Agric. Eng. 2013, 29, 286–292. [Google Scholar]
- Huang, J.Y.; Fang, T.J. Use of recycled mustard pickle brine in the production of salted duck eggs. Int. J. Food Eng. 2018, 14, 27–30. [Google Scholar] [CrossRef]
- Suretno, N.; Novitasari, E.; Rivaie, A. The fat content and the preferences of salted duck egg enriched with black and white pepper. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 653, p. 012086. [Google Scholar] [CrossRef]
- Yuan, R.; Gao, L.; Bai, H.; Ding, D.; Shen, F.; Shao, P.; Zhang, Z. Research on the using of carrageenan in the curing of eggs. J. Agric. Sci. 2017, 38, 86–89. (In Chinese) [Google Scholar] [CrossRef]
- Sun, J.; Du, J.; Yang, H.; Xiang, J.; Hu, T. Adding antioxidants during pickling to improve the interior quality of salted egg yolk. Mod. Food Sci. Technol. 2021, 37, 182–191. (In Chinese) [Google Scholar] [CrossRef]
- Sun, J.; Du, J.; Yang, H.; Xiang, J.; Hu, T. Effect of acid curing agent on salted egg. Food Sci. Technol. 2021, 46, 45–50. (In Chinese) [Google Scholar] [CrossRef]
- Harlina, P.W.; Shahzad, R.; Ma, M.; Wang, N.; Qiu, N. Effects of galangal extract on lipid oxidation, antioxidant activity and fatty acid profiles of salted duck eggs. J. Food Meas. Charact. 2019, 13, 1820–1830. [Google Scholar] [CrossRef]
- Thohari, I.; Rosyidi, D. Pengaruh kosentrasi sari kunyit putih (Curcuma zediaria) terhadap kualitas telur asin ditinjau dari aktivitas antioksidan, total fenol, kadar protein dan kadar garam. J. Ilmu Teknol. Has. Ternak 2017, 10, 46–53. [Google Scholar] [CrossRef]
- Novia, D.; Juliyarsi, I. Quality characteristics of salted egg soaked with Aloe vera solution. Int. J. Adv. Sci. Eng. Inf. Technol. 2019, 9, 434–440. [Google Scholar] [CrossRef]
- Harlina, P.W.; Meihu, M.; Legowo, A.M.; Pramono, Y.B. The effect of supplementation garlic oil as an antibacterial activity and salting time on the characteristics of salted egg. J. Apl. Teknol. Pangan. 2012, 1, 121–128. [Google Scholar]
- Aliño, M.; Grau, R.; Fuentes, A.; Barat, J.M. Influence of low-sodium mixtures of salts on the post-salting stage of dry-cured ham process. J. Food Eng. 2010, 99, 198–205. [Google Scholar] [CrossRef]
- Ariviani, S.; Fitriasih, N.H.; Ishartini, D. Development of low sodium salted eggs and its antioxidant potential. J. Gizi Diet. Indones. 2018, 5, 51–59. [Google Scholar] [CrossRef]
- Tian, Y.; Jin, H.; Guo, S.; Lin, S.; Bao, Z. Effects of different metal ions on the physicochemical properties and microstructure of egg white gel. J. Sci. Food Agric. 2021, 102, 3308–3315. [Google Scholar] [CrossRef]
- Liu, H.; Feng, F.; Xue, H.; Gao, B.; Han, T.; Li, R.; Hu, X.; Tu, Y.; Zhao, Y. Effects of partial replacement of NaCl by KCl and CaCl2 on physicochemical properties, microstructure, and textural properties of salted eggs. J. Food Sci. 2022, 8, 795–807. [Google Scholar] [CrossRef] [PubMed]





| Quality Characteristics Index of Salted Eggs | The Main Reason for Quality Characteristics Formation | Induction/Acceleration of Quality Characteristics Formation Methods | References |
|---|---|---|---|
| Egg white hydration | A high concentration of salt can dissociate the protein structure and affect the charge of the protein. | Acid treatment of eggshells, ultrasonic treatment | [10,15] |
| Gelling of salted egg yolk | Salt promotes the molecular interaction of egg yolks and the formation of disulfide bonds, gradually forming the gel network structure. | Pulsating pressure, liquid smoke treatment | [28,41,42] |
| Color formation of salted egg yolk | Yolk lipids exude, the number of lipid-soluble carotenoids increases, and oxidation of lipids | Ultrasonic treatment, high-pressure vacuum cooking | [47,52,54] |
| Flavor formation of salted egg yolk | Lipids oxidation and Strecker degradation | Enzymatic hydrolysis and microwave co-processing, high-temperature treatment | [59,60,65] |
| The formation of loose, sand, and oil qualities in salted egg yolk | Salt breaks down the lipoprotein, causing the yolk particles to become more numerous and the arrangement becomes more and more compact to form irregular polyhedras, and the oil oozes into the particle gaps. | Pressure cooking, ultrasonic-assisted cooking | [26,52,79] |
| Factors Affecting the Quality Characteristics of Salted Eggs | Main Affected Parts | Influence Reasons | References |
|---|---|---|---|
| Pickling temperature | Egg white, egg yolk | Increasing temperature can accelerate the penetration rate of salt. | [46,80] |
| Salt concentration | Egg white, egg yolk | The higher the salt concentration, the stronger the chemical bond of the egg yolk protein, which is conducive to the coagulation of the egg yolk. | [20,23] |
| Pickling time | Egg white, egg yolk | The longer the pickling time, the greater the difference in salt concentration between the inside and outside of the egg yolk, causing the egg yolk to gel. | [14,24,83] |
| Pickling technology | Egg white, egg yolk | Reduce salt penetration resistance and increase mass transfer rate. | [85,87] |
| Food additives | Egg shell, egg white, egg yolk | Adjust the permeability of eggshell and penetration rate of salt, improve the antioxidant activity and flavor of salted eggs. | [91,93,96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chen, S.; Yao, Y.; Wu, N.; Xu, M.; Zhao, Y.; Tu, Y. The Quality Characteristics Formation and Control of Salted Eggs: A Review. Foods 2022, 11, 2949. https://doi.org/10.3390/foods11192949
Li X, Chen S, Yao Y, Wu N, Xu M, Zhao Y, Tu Y. The Quality Characteristics Formation and Control of Salted Eggs: A Review. Foods. 2022; 11(19):2949. https://doi.org/10.3390/foods11192949
Chicago/Turabian StyleLi, Xiaoya, Shuping Chen, Yao Yao, Na Wu, Mingsheng Xu, Yan Zhao, and Yonggang Tu. 2022. "The Quality Characteristics Formation and Control of Salted Eggs: A Review" Foods 11, no. 19: 2949. https://doi.org/10.3390/foods11192949
APA StyleLi, X., Chen, S., Yao, Y., Wu, N., Xu, M., Zhao, Y., & Tu, Y. (2022). The Quality Characteristics Formation and Control of Salted Eggs: A Review. Foods, 11(19), 2949. https://doi.org/10.3390/foods11192949






