Effect of Hydrothermal Treatment on the Structure and Functional Properties of Quinoa Protein Isolate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of QPI
2.3. Hydrothermal Treatment of QPI
2.4. Structural Properties of QPI
2.4.1. Particle Size
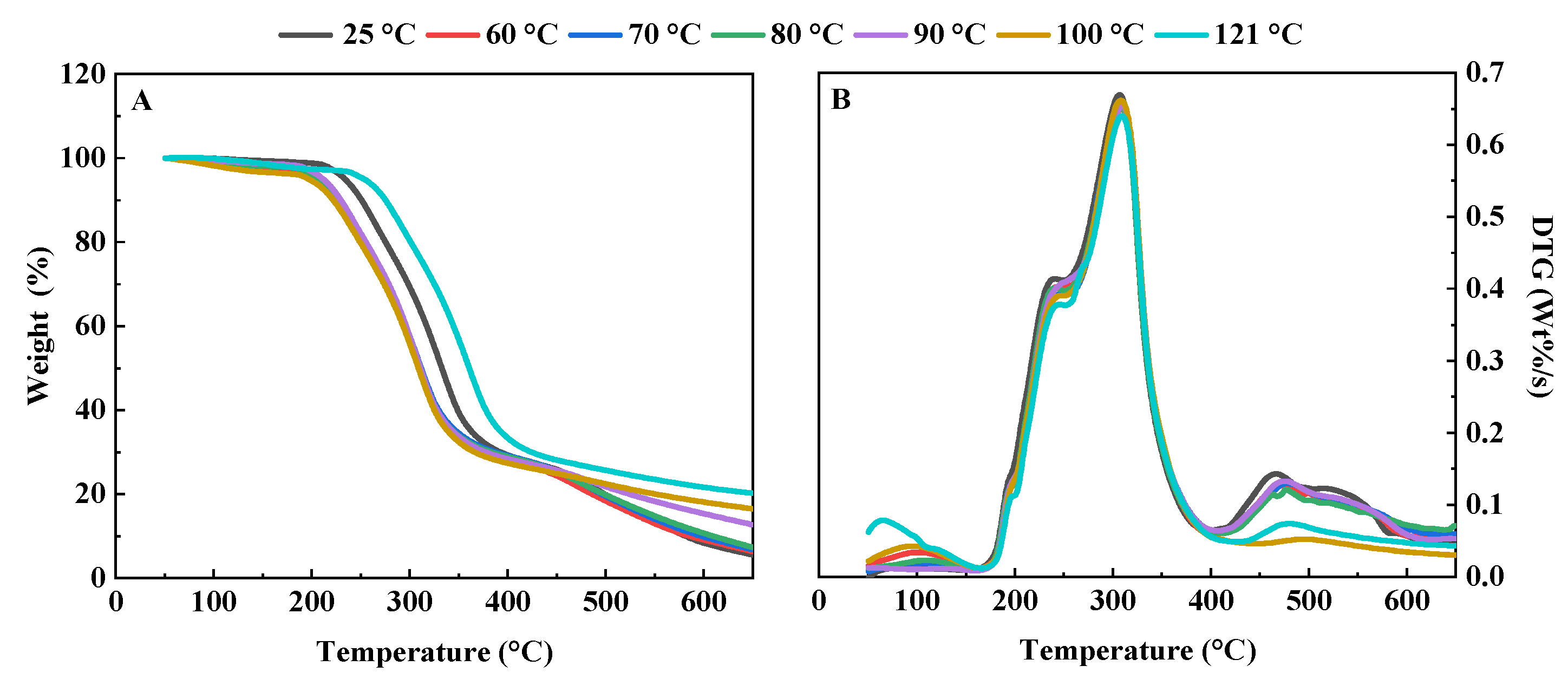
2.4.2. Thermogravimetric Characteristics
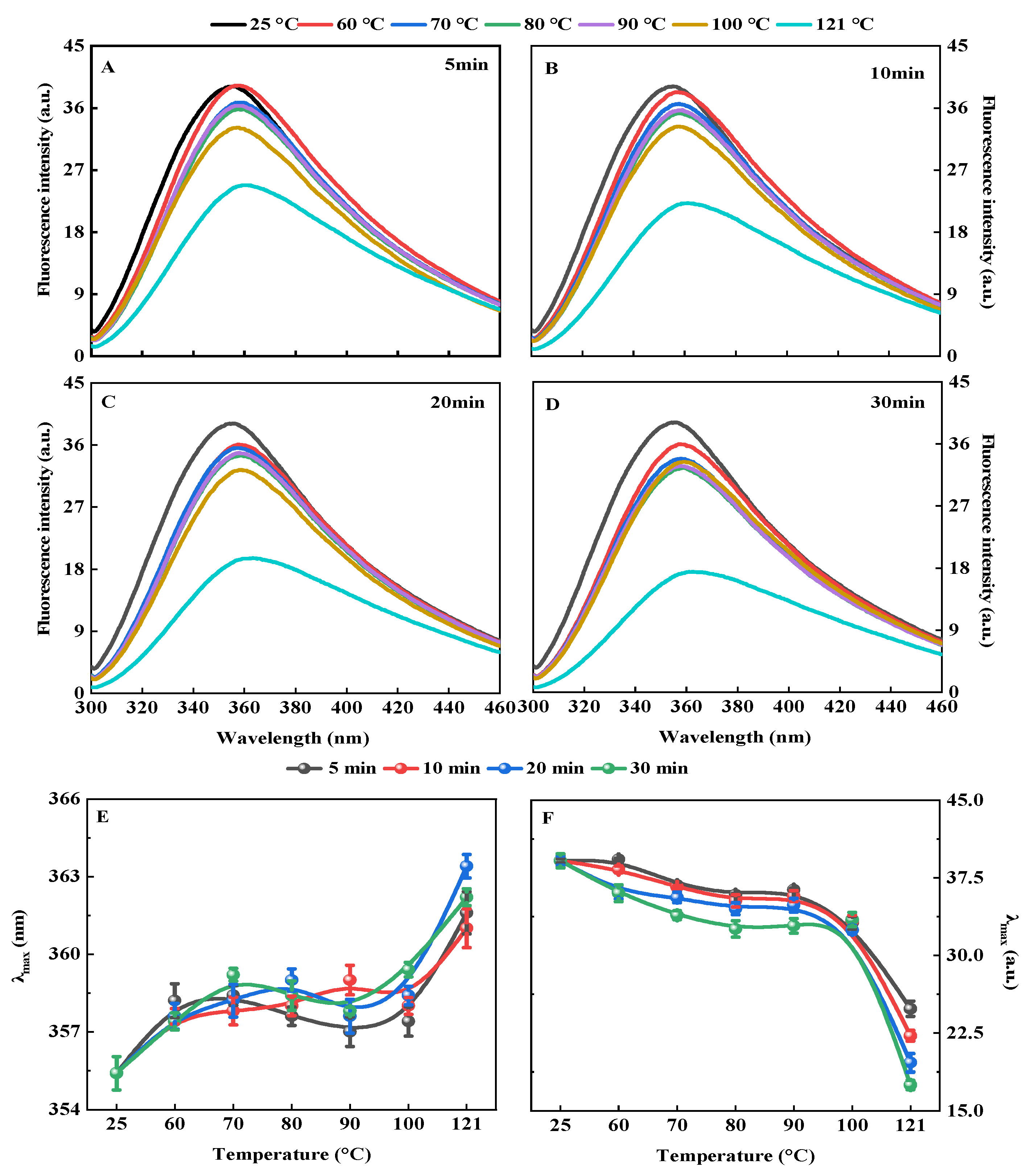
2.4.3. Intrinsic Fluorescence Spectrum
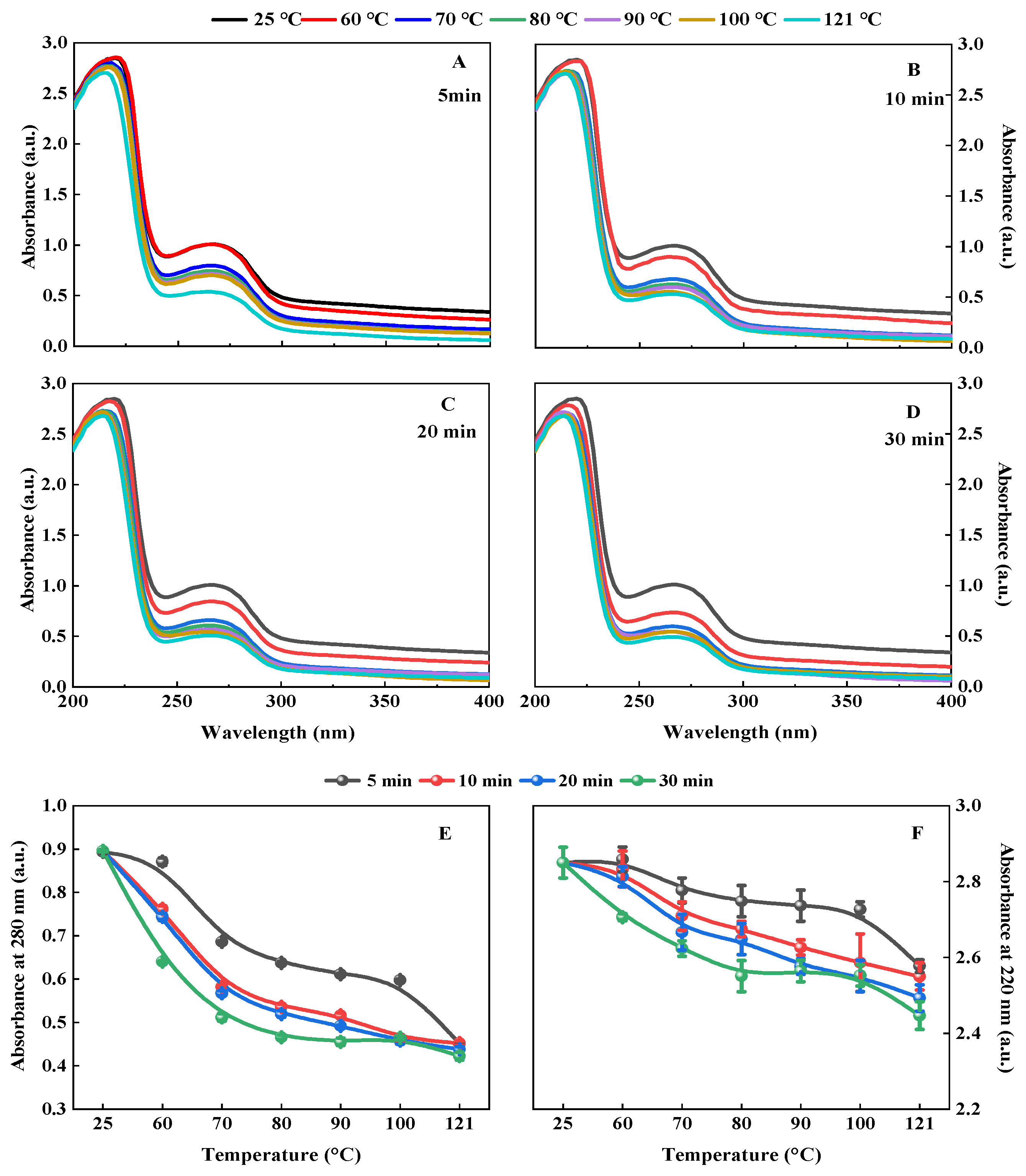
2.4.4. Ultra-Violet (UV) Spectrum
2.4.5. FTIR
2.5. Determination of Functional Properties of QPI
2.5.1. Solubility
2.5.2. Water-Holding Capacity (WHC) and Oil-Holding Capacity (OHC)
2.5.3. Emulsifying Activity (EA) and Emulsion Stability (ES)
2.6. Data Analysis
3. Results and Discussion
3.1. Structural Properties of QPI
3.1.1. Particle Size
3.1.2. Thermogravimetric Characteristics
3.1.3. Intrinsic Fluorescence Spectrum
3.1.4. Ultra-Violet (UV) Spectrum
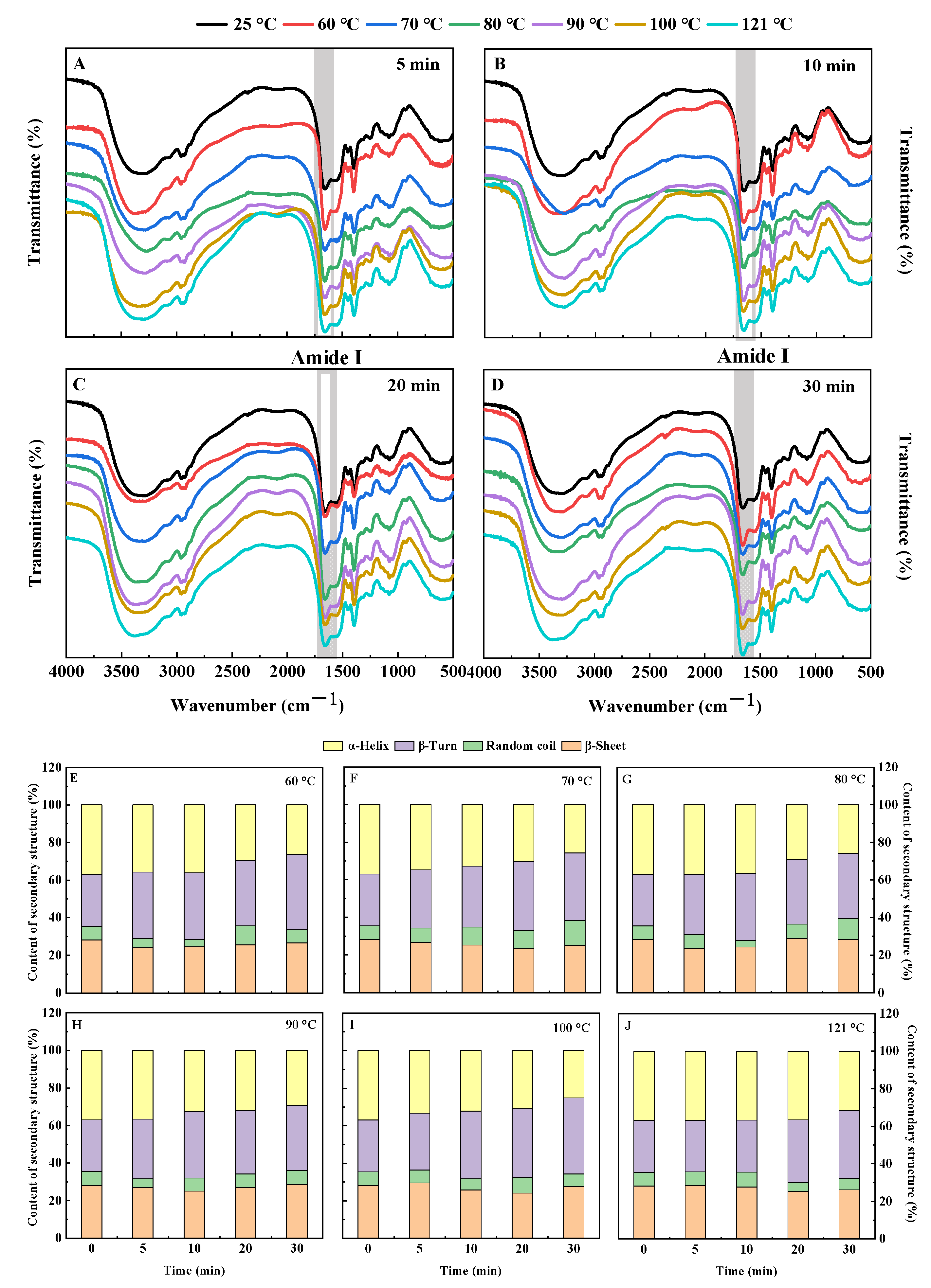
3.1.5. Fourier Transform Infrared Spectrum (FTIR)
3.2. Determination of Functional Properties of QPI
3.2.1. Solubility
3.2.2. Water-Holding Capacity (WHC) and Oil-Holding Capacity (OHC)
3.2.3. Emulsifying Activity (EA) and Emulsion Stability (ES)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz, G.A.; Xiao, W.; Boekel, M.V.; Minor, M.; Stieger, M. Effect of extraction pH on heat-induced aggregation, gelation and microstructure of protein isolate from quinoa (Chenopodium quinoa Willd). Food Chem. 2016, 209, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Santos, M.; Mauro, R.; Samman, N.; Matos, A.S.; Torres, D. Protein content and amino acids profile of pseudocereals. Food Chem. 2016, 193, 55. [Google Scholar] [CrossRef] [PubMed]
- Dakhili, S.; Abdolalizadeh, L.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Quinoa protein: Composition, structure and functional properties. Food Chem. 2019, 299, 125161.1–125161.10. [Google Scholar] [CrossRef] [PubMed]
- Brinegar, C.; Goundan, S. Isolation and characterization of chenopodin, the 11S seed storage protein of quinoa (Chenopodium quinoa). J. Agric. Food Chem. 1993, 41, 182–185. [Google Scholar] [CrossRef]
- Brinegar, C.; Sine, B.; Nwokocha, L. High-cysteine 2S seed storage proteins from quinoa (Chenopodium quinoa). J. Agric. Food Chem. 1996, 44, 1621–1623. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Effect of pH and holding time on the characteristics of protein isolates from Chenopodium seeds and study of their amino acid profile and scoring. Food Chem. 2019, 272, 165–173. [Google Scholar] [CrossRef]
- Elsohaimy, S.; Refaay, T.; Zaytoun, M. Physicochemical and functional properties of quinoa protein isolate. Ann. Agric. Sci.-Cairo 2015, 60, 297–305. [Google Scholar] [CrossRef]
- Chauhan, G.; Cui, W.; Eskin, N. Effect of saponin on the surface properties of quinoa proteins. Int. J. Food Prop. 1999, 2, 13–22. [Google Scholar] [CrossRef]
- Ma, C.Y.; Harwalkar, V.R. Chemical characterization and functionality assessment of oat protein fractions. J. Agric. Food Chem. 1984, 32, 144–149. [Google Scholar] [CrossRef]
- Ashraf, S.; Saeed, S.M.G.; Sayeed, S.A.; Ali, R. Impact of microwave treatment on the functionality of cereals and legumes. Int. J. Agric. Biol. 2012, 14, 356–370. [Google Scholar]
- Li, F.; Kong, X.; Zhang, C.; Hua, Y. Effect of heat treatment on the properties of soy protein-stabilised emulsions. Int. J. Food Sci. Technol. 2011, 46, 1554–1560. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Cadesky, L.; Walkling-Ribeiro, M.; Kriner, K.T.; Karwe, M.V.; Moraru, C.I. Structural changes induced by high-pressure processing in micellar casein and milk protein concentrates. Int. J. Dairy Technol. 2017, 100, 7055–7070. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Chen, J.; Xiong, Y. Structural and emulsifying properties of soy protein isolate subjected to acid and alkaline pH-shifting processes. J. Agric. Food Chem. 2009, 57, 7576–7583. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mu, T.; Miao, Z.; Goffin, D. Effect of heat treatments on the structure and emulsifying properties of protein isolates from cumin seeds (Cuminum cyminum). Food Sci. Technol. Int. 2018, 24, 673–687. [Google Scholar] [CrossRef]
- Zhao, M.; Xiong, W.; Chen, B.; Zhu, J.; Wang, L. Enhancing the solubility and foam ability of rice glutelin by heat treatment at pH 12: Insight into protein structure. Food Hydrocoll. 2020, 103, 105626. [Google Scholar] [CrossRef]
- Zhao, C.; Chu, Z.; Miao, Z.; Liu, J.; Liu, J.; Xu, X.; Wu, Y.; Qi, B.; Yan, J. Ultrasound heat treatment effects on structure and acid-induced cold set gel properties of soybean protein isolate. Food Biosci. 2020, 39, 100827. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, M.; Zhang, X.; Li, X.; Wang, C. The effect of heat treatment on the microstructure and functional properties of whey protein from goat milk. Int. J. Dairy Technol. 2019, 103, 1289–1302. [Google Scholar] [CrossRef]
- Espinosa, M.; Guevara-Oquendo, V.H.; Newkirk, R.W.; Yu, P. Effect of heat processing methods on the protein molecular structure, physicochemical, and nutritional characteristics of faba bean (low and normal tannin) grown in western Canada. Anim. Feed Sci. Technol. 2020, 269, 114681. [Google Scholar] [CrossRef]
- Yu, N.; Jiang, C.; Ning, F.; Hu, Z.; Shao, S.; Zou, X.; Meng, X.; Xiong, H. Protein isolate from Stauntonia brachyanthera seed: Chemical characterization, functional properties, and emulsifying performance after heat treatment. Food Chem. 2020, 345, 128542. [Google Scholar] [CrossRef]
- Malik, M.A.; Saini, C.S. Improvement of functional properties of sunflower protein isolates near isoelectric point: Application of heat treatment. LWT-Food Sci. Technol. 2018, 98, 411–417. [Google Scholar] [CrossRef]
- Wang, L.; Dong, J.; Zhu, Y.; Shen, R.; Wu, L.; Zhang, K. Effects of microwave heating, steaming, boiling and baking on the structure and functional properties of quinoa (Chenopodium quinoa Willd.) protein isolates. Int. J. Food Sci. Technol. 2020, 56, 14706. [Google Scholar] [CrossRef]
- Vondel, J.; Lambrecht, M.A.; Housmans, J.; Rousseau, F.; Delcour, J.A. Impact of hydrothermal treatment on denaturation and aggregation of water-extractable quinoa (Chenopodium quinoa Willd.) protein. Food Hydrocoll. 2021, 115, 106611. [Google Scholar] [CrossRef]
- Cerdán-Leal, M.A.; López-Alarcón, C.A.; Ortiz-Basurto, R.I.; Luna-Solano, G.; Jiménez-Fernández, M. Influence of heat denaturation and freezing-lyophilization on physicochemical and functional properties of quinoa protein isolate. Cereal Chem. 2019, 97, 373–381. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Improvement in the functional properties of quinoa (Chenopodium quinoa) protein isolates after the application of controlled heat-treatment: Effect on structural properties. Food Struct. 2021, 28, 100189. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Structural modification of quinoa seed protein isolates (QPIs) by variable time sonification for improving its physicochemical and functional characteristics. Ultrason. Sonochem. 2019, 58, 104700. [Google Scholar] [CrossRef]
- Hall, A.E.; Moraru, C.I. Structure and function of pea, lentil and faba bean proteins treated by high pressure processing and heat treatment. LWT-Food Sci. Technol. 2021, 152, 112349. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, Y.; Li, S.; Wang, W. Fabrication and characterization of acid soluble collagen stabilized Pickering emulsions. Food Hydrocoll. 2020, 106, 105875. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymanska-Chargot, M.; Mis, A.; Wilczewska, A.Z.; Markiewicz, K.H. Effect of dietary fibre polysaccharides on structure and thermal properties of gluten proteins—A study on gluten dough with application of FT-Raman spectroscopy, TGA and DSC. Food Hydrocoll. 2017, 69, 410–426. [Google Scholar] [CrossRef]
- Guo, L.; Fang, Y.; Shao, Z.P.; Fang, S.; Li, Y.; Chen, J.; Meng, Y. pH-induced structural transition during complexation and precipitation of sodium caseinate and ε -poly-L-lysine. Int. J. Biol. Macromol. 2020, 154, 644–653. [Google Scholar] [CrossRef]
- Vera, A.; Valenzuela, M.A.; Yazdani-Pedram, M.; Tapia, C.; Abugoch, L. Conformational and physicochemical properties of quinoa proteins affected by different conditions of high-intensity ultrasound treatments. Ultrason. Sonochem. 2019, 51, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, R.; Yuan, W. Composition and secondary structure of proteins isolated from six different quinoa varieties from china. J. Cereal Sci. 2020, 95, 103036. [Google Scholar] [CrossRef]
- Wang, S.; Wang, T.; Sun, Y.; Cui, Y.; Yu, G.; Jiang, L. Effects of high hydrostatic pressure pretreatment on the functional and structural properties of rice bran protein hydrolysates. Foods 2022, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Li, S. Physicochemical and functional properties of Chinese quince seed protein isolate. Food Chem. 2019, 283, 539–548. [Google Scholar] [CrossRef]
- Lawal, O.S.; Adebowale, K.O.; Adebowale, Y.A. Functional properties of native and chemically modified protein concentrates from bambarra groundnut. Food Res. Int. 2007, 40, 1003–1011. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, W.; Yi, J.; Liu, N.; Cao, Y.; Lu, J.; Decker, E.A.; McClements, D.J. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018, 106, 853–861. [Google Scholar] [CrossRef]
- Jia, X.; Wang, J.; Lu, X.; Zheng, B.; Guo, Z. Structure and dilatational rheological behavior of heat-treated lotus (Nelumbo nucifera Gaertn.) seed protein. LWT-Food Sci. Technol. 2019, 116, 108579. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Fan, J.; Zhang, B.; Chen, H. The effects of phosphorylation modification on the structure, interactions and rheological properties of rice glutelin during heat treatment. Food Chem. 2019, 297, 124978. [Google Scholar] [CrossRef]
- Zhao, X.; Hou, C.; Tian, M.; Zhou, Y.; Yang, R.; Wang, X.; Gu, Z.; Wang, P. Effect of water-extractable arabinoxylan with different molecular weight on the heat-induced aggregation behavior of gluten. Food Hydrocoll. 2020, 99, 105318. [Google Scholar] [CrossRef]
- Ogungbenle, H.N. Nutritional evaluation and functional properties of quinoa (Chenopodium quinoa) flour. Int. J. Food Sci. Nutr. 2009, 54, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Eugenia, S.M.; Paula, V.; Eduardo, M.S.R.; Ritva, R.C.; Alberto, L.E.; Gabriela, P.T. Physico-chemical and functional characterization of protein isolated from different quinoa varieties (Chenopodium quinoa willd). Cereal Chem. J. 2015, 93, 275–281. [Google Scholar]
- Lqari, H.; Vioque, J.; Pedroche, J.; Millán, F. Lupinus angustifolius protein isolates: Chemical composition, functional properties and protein characterization. Food Chem. 2002, 76, 349–356. [Google Scholar] [CrossRef]
- Yu, L.; Yang, W.; Sun, J.; Zhang, C.; Bi, J.; Yang, Q. Preparation, characterisation and physicochemical properties of the phosphate modified peanut protein obtained from Arachin Conarachin L. Food Chem. 2015, 170, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Ganesan, K.; Selvaraj, K.; Rao, P. Studies on the functional properties of protein concentrate of kappaphycus alvarezii (Doty) Doty–An edible seaweed. Food Chem. 2014, 153, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Saini, C.S. Heat treatment of sunflower protein isolates near isoelectric point: Effect on rheological and structural properties. Food Chem. 2019, 276, 554–561. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, L.; Zheng, X.; Ai, B.; Yang, Y.; Pan, Y.; Sheng, Z. Effect of steam explosion treatments on the functional properties and structure of camellia (Camellia oleifera Abel.) seed cake protein. Food Hydrocoll. 2019, 93, 189–197. [Google Scholar] [CrossRef]
- Chao, D.; Aluko, R.E. Modification of the structural, emulsifying, and foaming properties of an isolated pea protein by thermal pretreatment. CyTA-J. Food 2018, 16, 357–366. [Google Scholar] [CrossRef]
- Zhong, L.; Ma, N.; Wu, Y.; Zhao, L.; Ma, G.; Pei, F.; Hu, Q. Characterization and functional evaluation of oat protein isolate-Pleurotus ostreatus β-glucan conjugates formed via Maillard reaction. Food Hydrocoll. 2019, 87, 459–469. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, S.; Feng, X.; Wang, R.; Zeng, M.; Zhao, Y. Effect of heat treatment on physicochemical state and in vitro digestion of salt-soluble protein from pacific oyster (Crassostrea gigas). LWT-Food Sci. Technol. 2020, 134, 110126. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Yong, L.; Guo, W.; Xu, Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, F.; Xue, W.; Lee, L. FTIR spectra studies on the secondary structures of 7s and 11s globulins from soybean proteins using AOT reverse micellar extraction. Food Hydrocoll. 2008, 22, 568–575. [Google Scholar] [CrossRef]
- He, R.; He, H.Y.; Chao, D.; Ju, X.; Aluko, R. Effects of high pressure and heat treatments on physicochemical and gelation properties of rapeseed protein isolate. Food Bioprocess Technol. 2014, 7, 1344–1353. [Google Scholar] [CrossRef]
- Keerati-U-Rai, M.; Miriani, M.; Iametti, S.; Bonomi, F.; Corredig, M. Structural changes of soy proteins at the oil–water interface studied by fluorescence spectroscopy. Colloids Surf. B 2012, 93, 41–48. [Google Scholar] [CrossRef]
- Peng, Q.; Khan, N.A.; Wang, Z.; Yu, P. Moist and dry heating-induced changes in protein molecular structure, protein subfractions, and nutrient profiles in camelina seeds. J. Dairy Sci. 2013, 97, 446–457. [Google Scholar] [CrossRef]
- Meziani, S.; Jasniewski, J.; Ribotta, P.; Arab-Tehrany, E.; Muller, J.M.; Ghoul, M.; Desobry, S. Influence of yeast and frozen storage on rheological, structural and microbial quality of frozen sweet dough. J. Food Eng. 2012, 109, 538–544. [Google Scholar] [CrossRef]
- Shilpashree, B.G.; Arora, S.; Chawla, P.; Tomar, S.K. Effect of succinylation on physicochemical and functional properties of milk protein concentrate. Food Res. Int. 2015, 72, 223–230. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Shi, K.; Wang, S.; Chen, J.; Liu, Y.; Huang, Q. Composition, secondary structure, and self-assembly of oat protein isolate. J. Agric. Food Chem. 2009, 57, 4552–4558. [Google Scholar] [CrossRef]
- Shima, M.; Maryam, S.; Farhad, A.; Zahra, E.D.; Elnaz, H.; Nader, S.; Ali, A.M.M. Effect of dry heating on physico-chemical, functional properties and digestibility of camel whey protein. Int. Dairy J. 2018, 86, 9–20. [Google Scholar]
- Shao, Y.; Lin, K.; Kao, Y. Modification of foaming properties of commercial soy protein isolates and concentrates by heat treatments. J. Food Qual. 2016, 39, 695–706. [Google Scholar] [CrossRef]
- Beck, S.M.; Knoerzer, K.; Sellahewa, J.; Emin, M.A.; Arcot, J. Effect of different heat-treatment times and applied shear on secondary structure, molecular weight distribution, solubility and rheological properties of pea protein isolate as investigated by capillary rheometry. J. Food Eng. 2017, 208, 66–76. [Google Scholar] [CrossRef]
- Lv, S.; Sun, L.; Zhao, S.; Bao, Y. Effect of dry heat stabilisation on the functional properties of rice bran proteins. Int. J. Food Sci. Technol. 2017, 52, 1836–1843. [Google Scholar] [CrossRef]
- Yu, P.; Kyriakopoulou, K.; Rahmani, A.; Venema, P.; Goot, A. Isochoric moisture heating as a tool to control the functionality of soy protein. LWT-Food Sci. Technol. 2021, 150, 111979. [Google Scholar]
- Khan, S.H.; Butt, M.S.; Sharif, M.K.; Sameen, A.; Mumtaz, S.; Sultan, M.T. Functional properties of protein isolates extracted from stabilized rice bran by microwave, dry heat, and parboiling. J. Agric. Food Chem. 2011, 59, 2416–2420. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wen, J.; Ma, X.; Lin, F.; Zhuo, J.; Bing, D. Structural, functional properties and immunomodulatory activity of isolated Inca peanut (Plukenetia volubilis L.) seed albumin fraction. Int. J. Biol. Macromol. 2018, 118, 1931–1941. [Google Scholar] [CrossRef]
- Hu, H.; Li-Chan, E.C.Y.; Wan, L.; Tian, M.; Pan, S. The effect of high intensity ultrasonic pre-treatment on the properties of soybean protein isolate gel induced by calcium sulfate. Food Hydrocoll. 2013, 32, 303–311. [Google Scholar] [CrossRef]
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O.E.; Pilosof, A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Du, Y.; Jiang, Y.; Zhu, X.; Xiong, H.; Shi, S.; Hu, J.; Peng, H.; Zhou, Q.; Sun, W. Physicochemical and functional properties of the protein isolate and major fractions prepared from Akebia trifoliata var. australis seed. Food Chem. 2012, 133, 923–929. [Google Scholar] [CrossRef]
- Nidhina, N.; Muthukumar, S.P. Antinutritional factors and functionality of protein-rich fractions of industrial guar meal as affected by heat processing. Food Chem. 2015, 173, 920–926. [Google Scholar] [CrossRef]
- Tang, C.; Ma, C. Heat-induced modifications in the functional and structural properties of vicilin-rich protein isolate from kidney (Phaseolus vulgaris L.) bean. Food Chem. 2009, 115, 859–866. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Structural modification in album (Chenopodium album) protein isolates due to controlled thermal modification and its relationship with protein digestibility and functionality. Food Hydrocoll. 2020, 103, 105708. [Google Scholar] [CrossRef]


| Size (nm) | Temperature (°C) | Time (min) | |||
|---|---|---|---|---|---|
| 5 | 10 | 20 | 30 | ||
| 25 | 11.54 ± 1.10 Ea | 11.54 ± 1.10 Fa | 11.54 ± 1.10 Ea | 11.54 ± 1.10 Fa | |
| 60 | 13.57 ± 0.53 Db | 14.23 ± 0.99 Eab | 14.35 ± 0.59 Dab | 15.67 ± 1.09 Ea | |
| 70 | 14.78 ± 0.65 Db | 16.13 ± 0.53 Db | 16.44 ± 1.10 Cb | 18.61 ± 0.96 Da | |
| D[4,3] | 80 | 16.76 ± 0.64 Cc | 18.84 ± 0.98 Cb | 21.09 ± 0.79 Ba | 22.01 ± 0.77 Ca |
| 90 | 18.11 ± 0.45 Cb | 20.59 ± 1.11 BCb | 21.24 ± 0.61 Bb | 21.64 ± 1.30 Ca | |
| 100 | 20.20 ± 0.31 Bc | 21.87 ± 0.48 Bb | 20.52 ± 0.46 Bc | 24.47 ± 0.74 Ba | |
| 121 | 34.49 ± 0.41 Ac | 143.02 ± 0.49 Ab | 49.58 ± 0.41 Aa | 49.69 ± 0.87 Aa | |
| 25 | 7.37 ± 0.52 Da | 7.37 ± 0.52 Da | 7.37 ± 0.52 Da | 7.37 ± 0.52 Ea | |
| 60 | 10.90 ± 0.40 Cb | 9.62 ± 0.54 Cc | 10.04 ± 0.43 Cbc | 12.94 ± 0.62 Da | |
| 70 | 9.69 ± 0.44 Cc | 10.37 ± 0.45 Cbc | 10.92 ± 0.34 Cb | 11.95 ± 0.62 Da | |
| D[5,0] | 80 | 9.85 ± 0.36 Cb | 10.36 ± 0.46 Cb | 14.60 ± 0.30 Ba | 15.49 ± 0.35 Ca |
| 90 | 9.84 ± 0.13 BCd | 13.05 ± 0.44 Bc | 14.87 ± 0.37 Bb | 15.72 ± 0.35 Ca | |
| 100 | 12.33 ± 0.28 Bd | 13.94 ± 0.36 Bc | 15.03 ± 0.18 Bb | 17.87 ± 0.61 Ba | |
| 121 | 24.51 ± 0.42 Ac | 30.55 ± 0.39 Ab | 36.16 ± 0.16 Aa | 36.36 ± 0.65 Aa | |
| Capacity (%) | Temperature (°C) | Time (min) | |||
|---|---|---|---|---|---|
| 5 | 10 | 20 | 30 | ||
| 25 | 143.53 ± 2.30 Da | 143.53 ± 2.30 Aa | 143.53 ± 2.30 Ea | 143.53 ± 2.30 Ea | |
| 60 | 144.80 ± 0.95 Da | 145.33 ± 3.97 Aa | 145.87 ± 1.52 Da | 146.07 ± 1.08 Da | |
| 70 | 147.40 ± 0.31 Cb | 152.47 ± 2.36 Aa | 152.07 ± 0.72 Ca | 152.80 ± 0.95 Ca | |
| water | 80 | 153.73 ± 1.54 Ba | 153.93 ± 3.62 Aa | 154.27 ± 0.74 Ba | 157.20 ± 2.60 Ba |
| 90 | 153.00 ± 3.85 Bb | 158.13 ± 1.83 Aa | 161.67 ± 0.85 Aa | 161.47 ± 1.15 Aa | |
| 100 | 161.47 ± 3.62 Aa | 152.13 ± 0.99 Ab | 147.60 ± 1.00 Dc | 145.73 ± 1.14 Dc | |
| 121 | 152.33 ± 5.28 Ba | 151.20 ± 0.47 Aab | 146.53 ± 0.92 Dab | 145.00 ± 3.59 DEc | |
| 25 | 151.80 ± 0.64 Ea | 151.80 ± 0.64 Ea | 151.80 ± 0.64 Ea | 151.80 ± 0.64 Ea | |
| 60 | 152.87 ± 2.16 DEa | 154.33 ± 1.90 Da | 155.40 ± 3.60 Da | 156.00 ± 1.40 Da | |
| 70 | 154.00 ± 1.87 Db | 154.53 ± 0.63 Db | 155.80 ± 2.17 Dab | 159.87 ± 3.67 Ca | |
| oil | 80 | 159.27 ± 2.16 Cb | 166.73 ± 3.38 Aa | 167.40 ± 2.06 Aa | 168.00 ± 1.56 Ba |
| 90 | 163.40 ± 0.36 Bc | 166.20 ± 1.17 Abc | 167.67 ± 2.33 Ab | 173.33 ± 2.42 Aa | |
| 100 | 171.33 ± 2.73 Aa | 162.20 ± 5.46 Bb | 162.13 ± 4.30 Bb | 157.07 ± 0.90 Db | |
| 121 | 160.60 ± 2.54 Ca | 159.07 ± 4.03 Ca | 159.67 ± 5.49 Ca | 156.60 ± 1.56 Da | |
| Temperature (°C) | Time (min) | |||
|---|---|---|---|---|
| 5 | 10 | 20 | 30 | |
| 25 | 6.55 ± 0.38 Da | 6.55 ± 0.38 Da | 6.55 ± 0.38 Ca | 6.55 ± 0.38 Da |
| 60 | 7.65 ± 0.25 CDb | 7.72 ± 0.39 CDb | 8.06 ± 0.73 BCb | 9.17 ± 0.30 BCa |
| 70 | 7.95 ± 0.28 BCDc | 8.73 ± 0.46 BCbc | 9.46 ± 0.52 Bb | 10.57 ± 0.64 ABa |
| 80 | 9.56 ± 0.68 ABb | 10.19 ± 0.40 ABb | 11.57 ± 0.71 Aa | 11.68 ± 0.66 Aa |
| 90 | 11.05 ± 0.38 Aa | 11.76 ± 0.67 Aa | 11.82 ± 0.58 Aa | 12.05 ± 0.38 Aa |
| 100 | 10.31 ± 0.59 Aa | 9.84 ± 0.80 Bab | 8.68 ± 0.36 Bb | 8.64 ± 0.59 Cb |
| 121 | 9.28 ± 0.29 ABCa | 8.46 ± 0.37 BCab | 7.72 ± 0.39 BCb | 6.16 ± 0.56 Dc |
| Temperature (°C) | Time (min) | |||
|---|---|---|---|---|
| 5 | 10 | 20 | 30 | |
| 25 | 56.08 ± 0.59 Da | 56.08 ± 0.59 Da | 56.08 ± 0.59 Ca | 56.08 ± 0.59 Fa |
| 60 | 57.13 ± 0.88 CDb | 64.37 ± 2.70 Ca | 65.87 ± 0.68 Ba | 66.10 ± 2.14 Ca |
| 70 | 58.13 ± 1.73 Cb | 65.34 ± 2.30 BCa | 68.51 ± 0.86 Aa | 68.00 ± 0.41 Ba |
| 80 | 63.57 ± 0.66 Bb | 68.62 ± 1.42 Aa | 68.86 ± 1.06 Aa | 64.21 ± 0.50 Db |
| 90 | 64.15 ± 1.10 Bb | 69.63 ± 0.39 Aab | 68.62 ± 1.42 Aab | 71.25 ± 1.60 Aa |
| 100 | 70.49 ± 1.74 Aa | 69.27 ± 1.45 Aa | 67.37 ± 0.74 ABab | 64.48 ± 1.79 CDb |
| 121 | 70.55 ± 0.45 Aa | 66.31 ± 2.20 Bb | 65.80 ± 2.09 Bb | 61.76 ± 1.27 Ec |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Wang, B.; Zhao, B.; Meng, Y.; Chen, J.; Yang, F. Effect of Hydrothermal Treatment on the Structure and Functional Properties of Quinoa Protein Isolate. Foods 2022, 11, 2954. https://doi.org/10.3390/foods11192954
He X, Wang B, Zhao B, Meng Y, Chen J, Yang F. Effect of Hydrothermal Treatment on the Structure and Functional Properties of Quinoa Protein Isolate. Foods. 2022; 11(19):2954. https://doi.org/10.3390/foods11192954
Chicago/Turabian StyleHe, Xingfen, Bin Wang, Baotang Zhao, Yuecheng Meng, Jie Chen, and Fumin Yang. 2022. "Effect of Hydrothermal Treatment on the Structure and Functional Properties of Quinoa Protein Isolate" Foods 11, no. 19: 2954. https://doi.org/10.3390/foods11192954
APA StyleHe, X., Wang, B., Zhao, B., Meng, Y., Chen, J., & Yang, F. (2022). Effect of Hydrothermal Treatment on the Structure and Functional Properties of Quinoa Protein Isolate. Foods, 11(19), 2954. https://doi.org/10.3390/foods11192954