Octenyl Succinic Anhydride-Modified Starch Attenuates Body Weight Gain and Changes Intestinal Environment of High-Fat Diet-Fed Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation and Characterization of OS Starch
2.2.2. Animal Experiment
2.2.3. Body Composition, Lee Index, and Food Intake of Mice Fed Different Diets
2.2.4. Blood Samples Preparation
2.2.5. Intraperitoneal Glucose Tolerance Tests (IPGTT)
2.2.6. Insulin Tolerance Test (ITT)
2.2.7. Histopathological Examination
2.2.8. Collection of Mice Feces
2.2.9. Determination of Fecal Fat Content
2.2.10. Determination of the Short-Chain Fatty Acid (SCFA) Concentrations in Mice Feces
2.2.11. Fecal Sample Preparation and 16S rDNA Gene Sequencing
2.2.12. Statistical Analysis
3. Results and Discussion
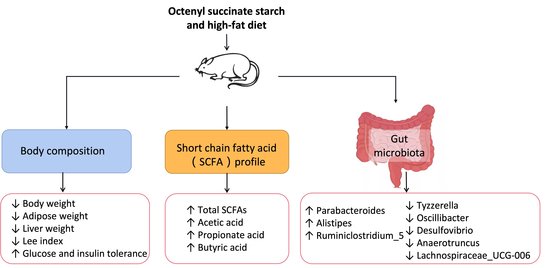
3.1. Effects of OS Starch on Body Composition and Food and Energy Intake of Mice Fed an HF Diet
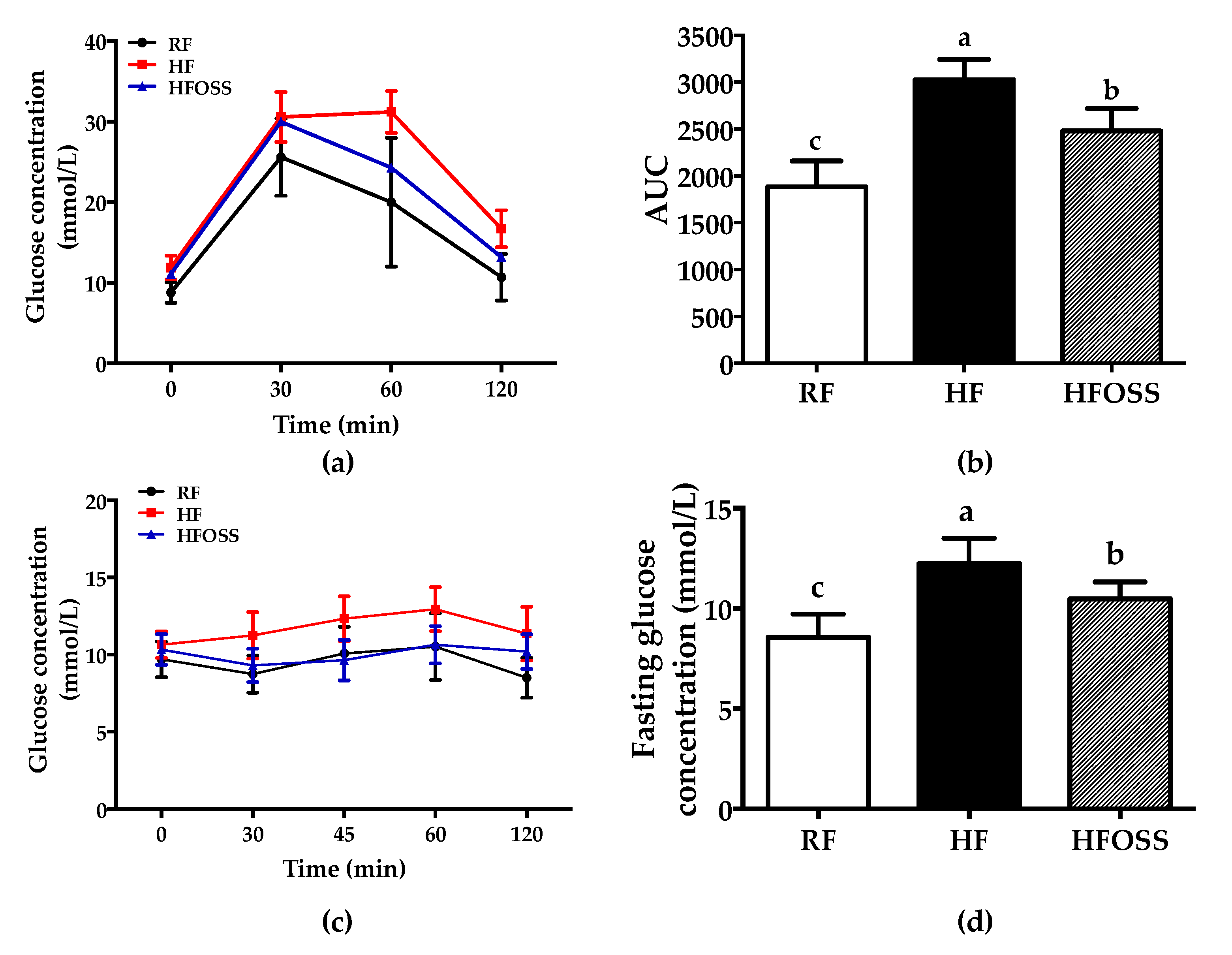
3.2. Effects of OS Starch on Glucose and Insulin Tolerance in Mice Fed a High-Fat Diet
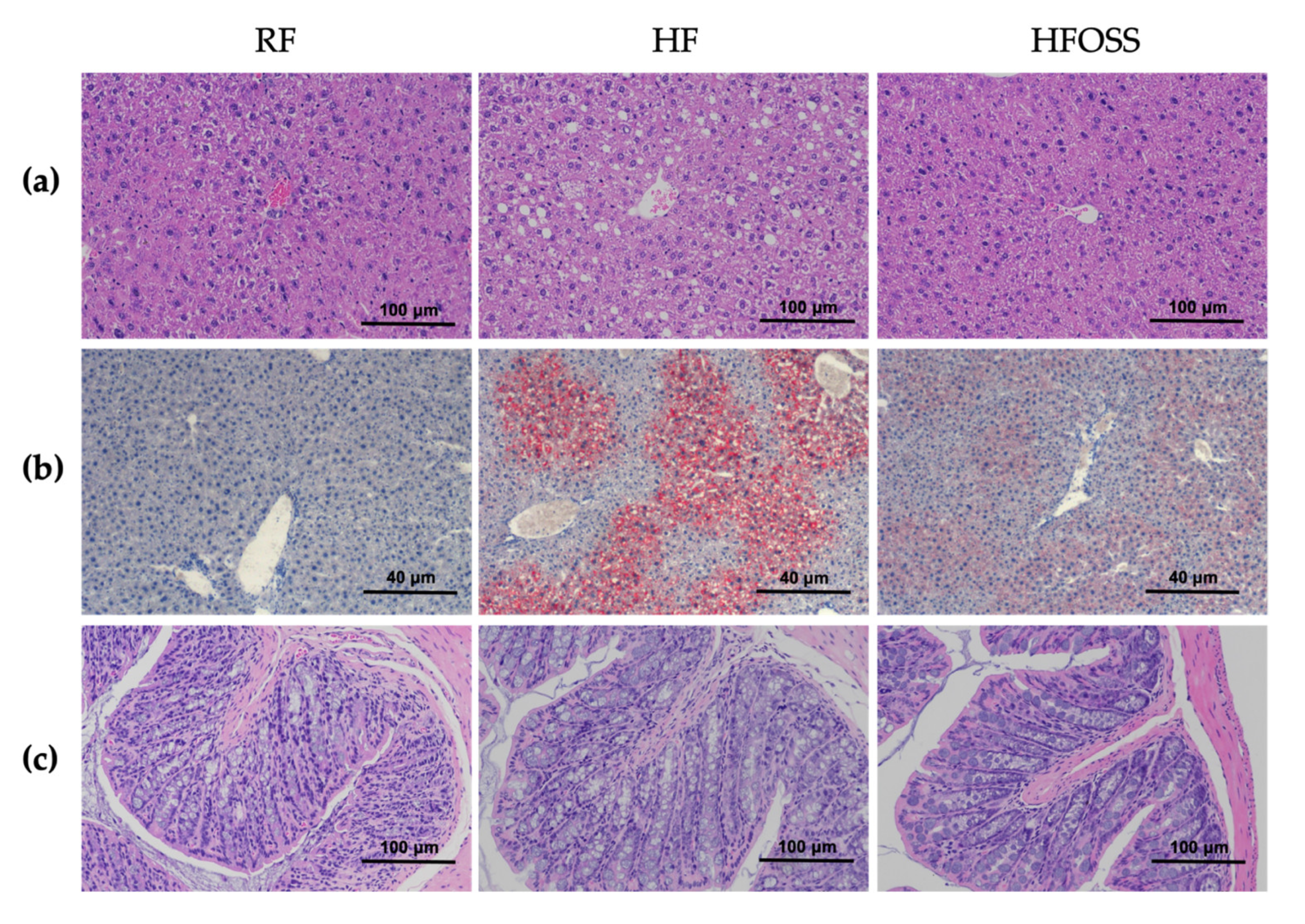
3.3. Effects of OS Starch on the Histology of Liver and Colon Tissues
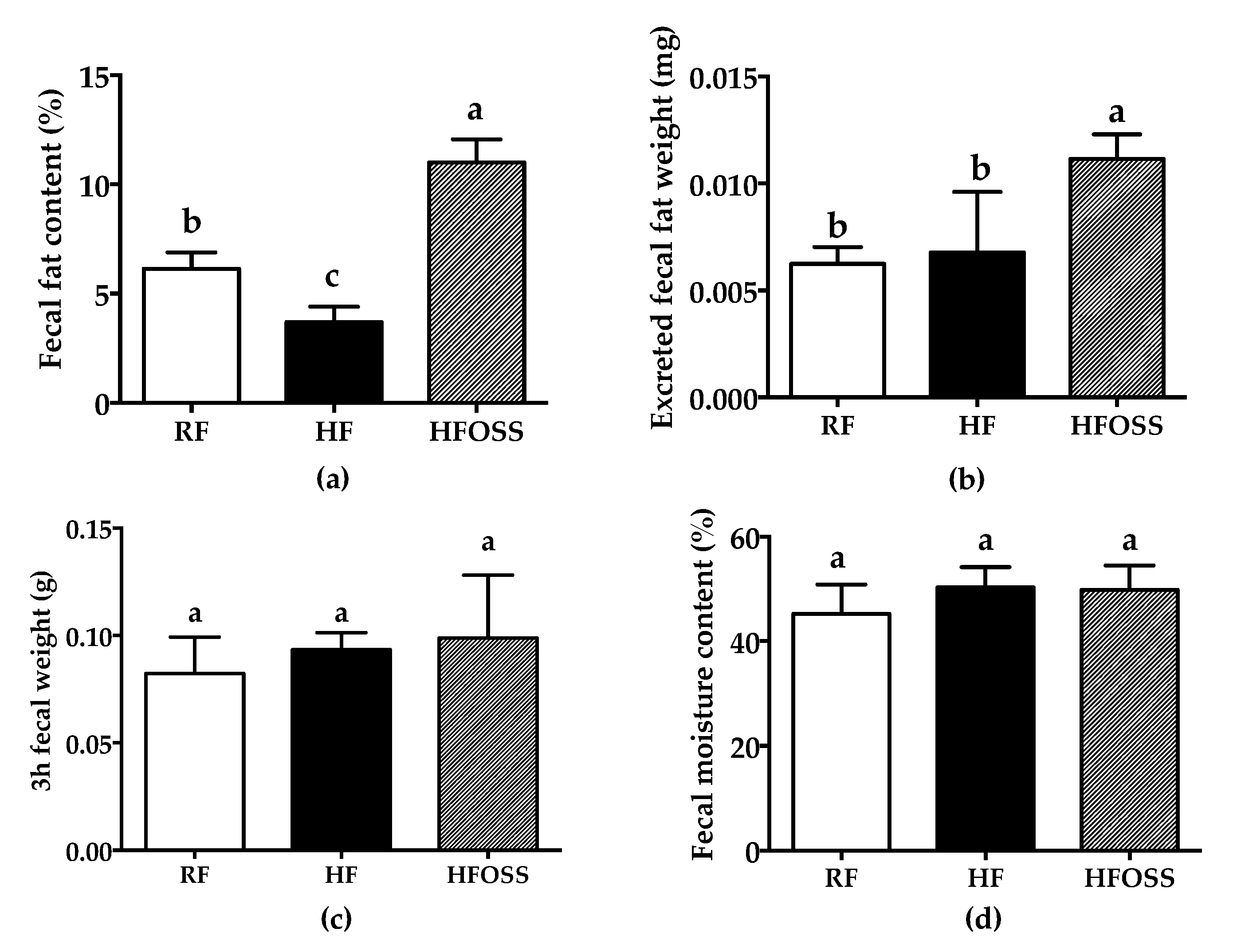
3.4. Effects of OS Starch on the Fecal Fat Content of Mice Fed a High-Fat Diet
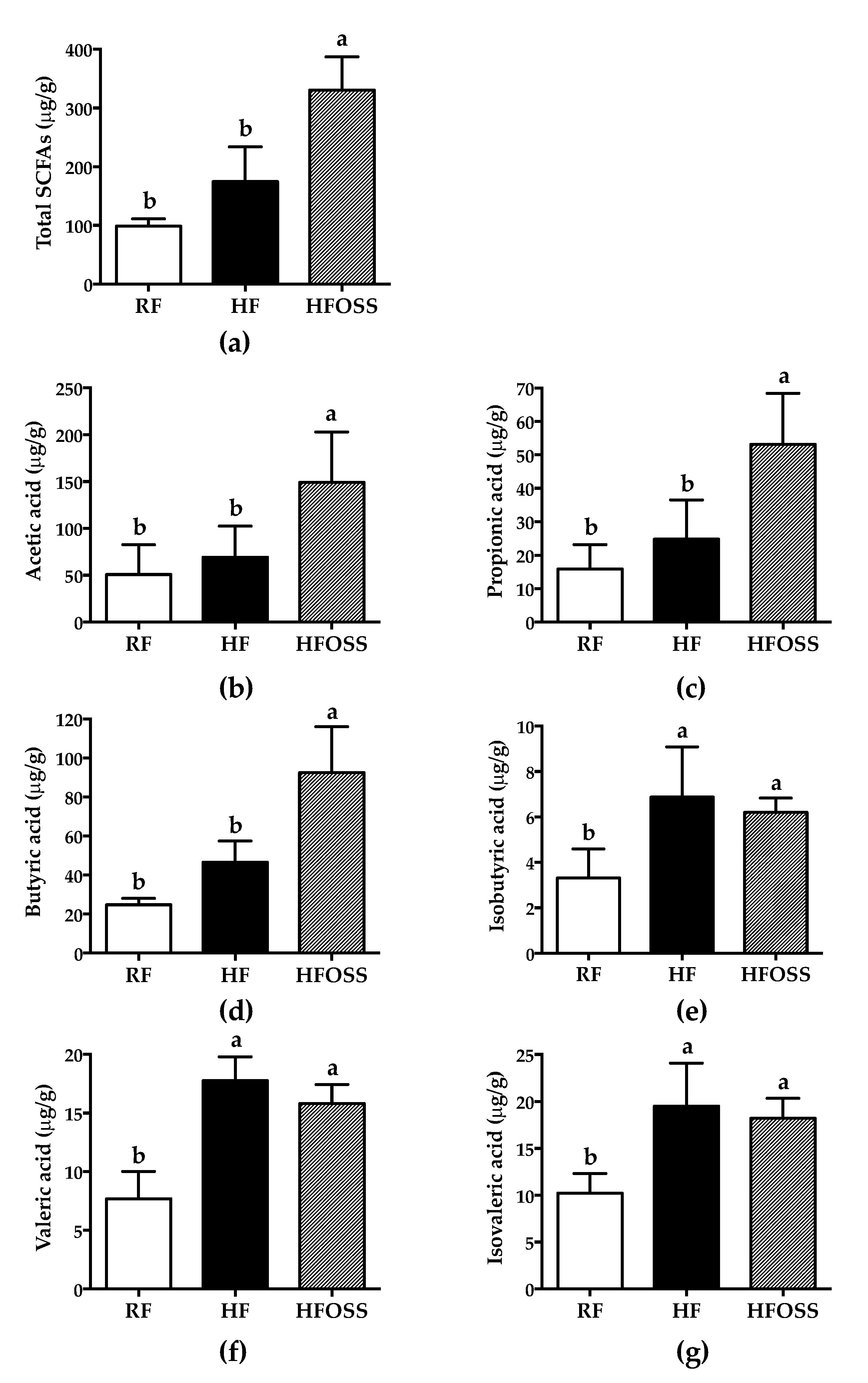
3.5. Effects of OS Starch on the Fecal SCFA Composition of Mice Fed a High-Fat Diet
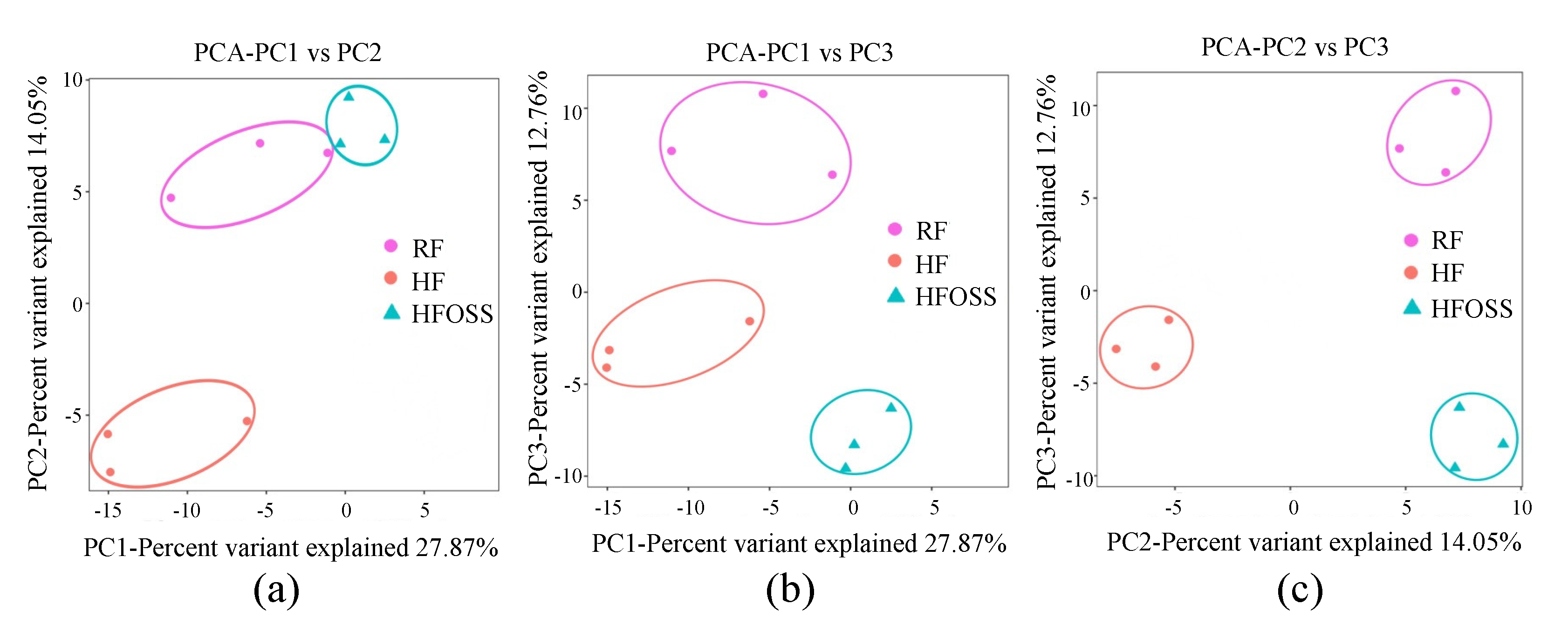
3.6. Effects of OS Starch on Fecal Microbiota Structure
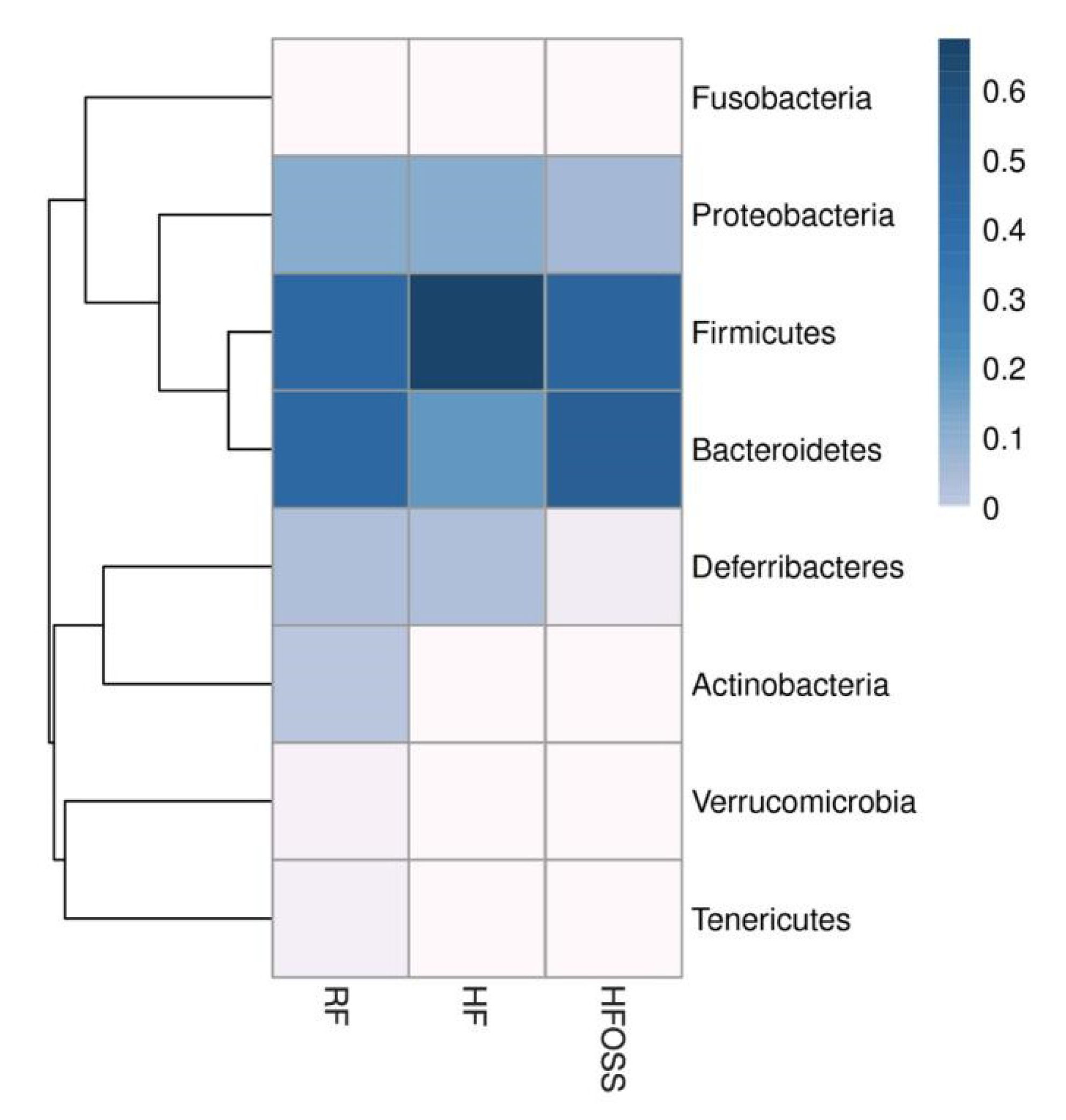
3.6.1. Gut Microbiota Composition at the Phylum Level
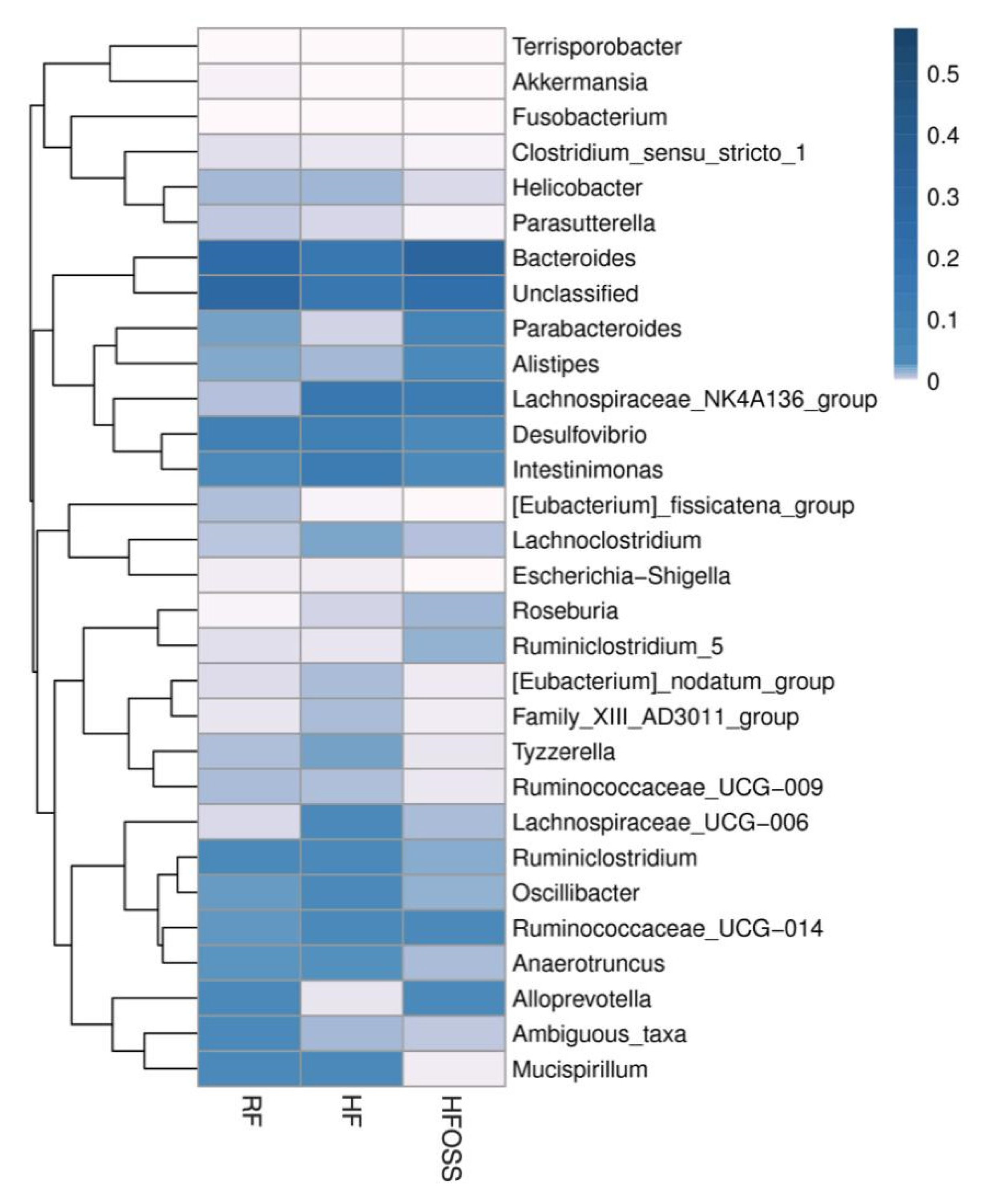
3.6.2. Gut Microbiota Composition at the Genus Level
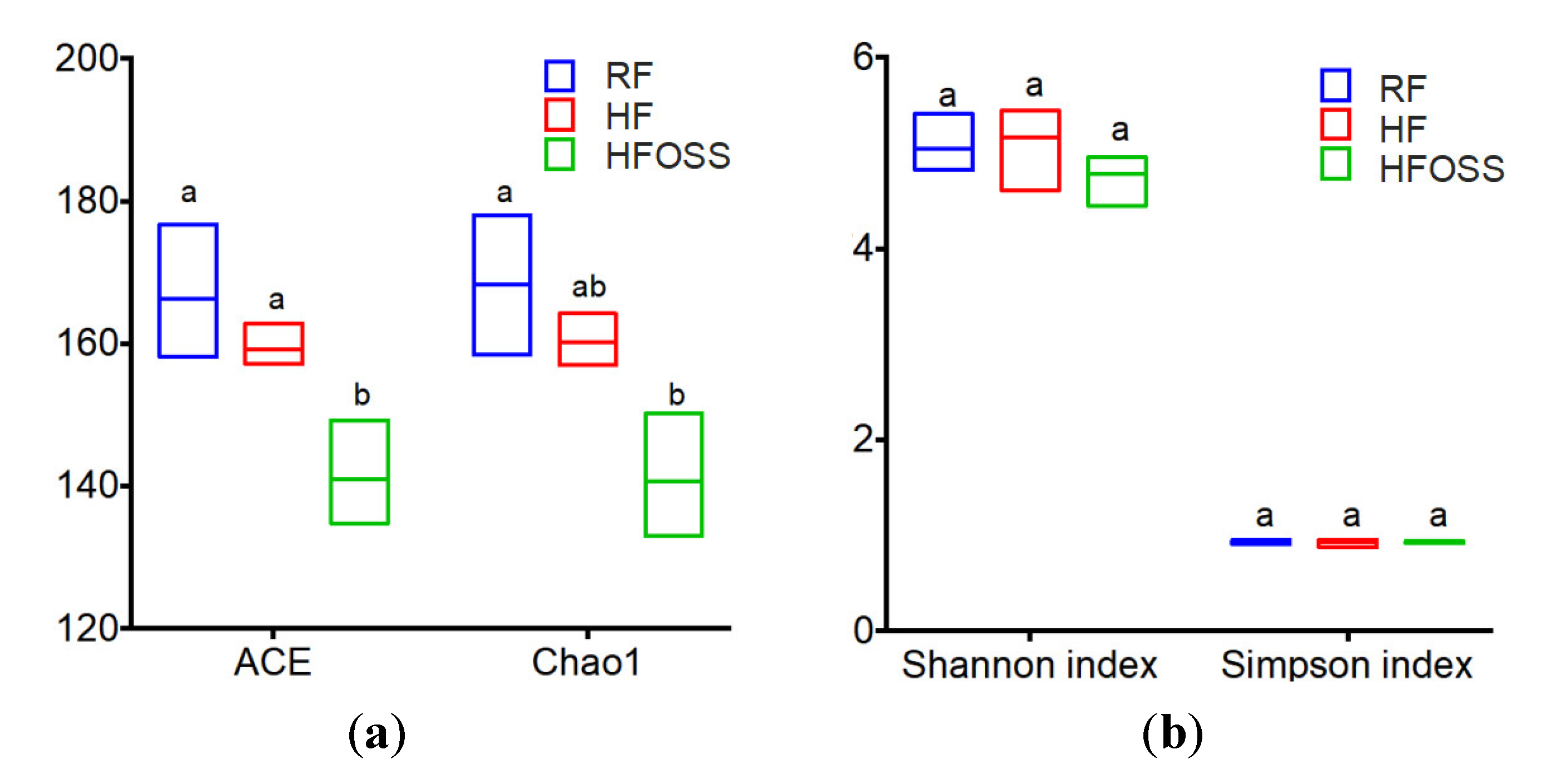
3.6.3. Community Richness and Diversity of Fecal Microbiota
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bai, Y.; Shi, Y.-C. Structure and preparation of octenyl succinic esters of granular starch, microporous starch and soluble maltodextrin. Carbohydr. Polym. 2011, 83, 520–527. [Google Scholar] [CrossRef]
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, C.G.; Wurzburg, O.B. Polysaccharide Derivatives of Substituted Dicarboxylic Acids. U.S. Patent 2661349, 1 December 1953. [Google Scholar]
- Tesch, S.; Gerhards, C.; Schubert, H. Stabilization of emulsions by OSA starches. J. Food Eng. 2002, 54, 167–174. [Google Scholar] [CrossRef]
- Altuna, L.; Herrera, M.L.; Foresti, M.L. Synthesis and characterization of octenyl succinic anhydride modified starches for food applications. A review of recent literature. Food Hydrocoll. 2018, 80, 97–110. [Google Scholar] [CrossRef]
- Hategekimana, J.; Masamba, K.G.; Ma, J.; Zhong, F. Encapsulation of vitamin E: Effect of physicochemical properties of wall material on retention and stability. Carbohydr. Polym. 2015, 124, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Wolf, B.W.; Wolever, T.M.S.; Bolognesi, C.; Zinker, B.A.; Garleb, K.A.; Firkins, J.L. Glycemic Response to a food starch esterified by 1-octenyl succinic anhydride in humans. J. Agric. Food Chem. 2001, 49, 2674–2678. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, J.; Zhang, G. Slowly digestible waxy maize starch prepared by octenyl succinic anhydride esterification and heat−moisture treatment: Glycemic response and mechanism. Biomacromolecules 2008, 9, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Heacock, P.M.; Hertzler, S.R.; Wolf, B. The glycemic, insulinemic, and breath hydrogen responses in humans to a food starch esterified by 1-octenyl succinic anhydride. Nutr. Res. 2004, 24, 581–592. [Google Scholar] [CrossRef]
- Huang, J.; Chang, R.; Ma, R.; Zhan, J.; Lu, X.; Tian, Y. Effects of structure and physical chemistry of resistant starch on short-term satiety. Food Hydrocoll. 2022, 132, 107828. [Google Scholar] [CrossRef]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F. Intestinal microbiota, diet and health. Br. J. Nutr. 2013, 111, 387–402. [Google Scholar] [CrossRef] [Green Version]
- Rogero, M.M.; Calder, P.C. Obesity, inflammation, toll-Like receptor 4 and fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef]
- Araújo, J.R.; Tomas, J.; Brenner, C.; Sansonetti, P.J. Impact of high-fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie 2017, 141, 97–106. [Google Scholar] [CrossRef]
- Lee, K.Y.; Yoo, S.-H.; Lee, H.G. The effect of chemically-modified resistant starch, RS type-4, on body weight and blood lipid profiles of high fat diet-induced obese mice. Starch-Starke 2012, 64, 78–85. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Hu, M.; Kim, J.J.; Lin, R.; Xu, J.; Fan, L.; Qi, Y.; Wang, L.; Liu, W.; et al. Dietary type 2 resistant starch improves systemic inflammation and intestinal permeability by modulating microbiota and metabolites in aged mice on high-fat diet. Aging 2020, 12, 9173–9187. [Google Scholar] [CrossRef]
- Si, X.; Strappe, P.; Blanchard, C.; Zhou, Z. Enhanced anti-obesity effects of complex of resistant starch and chitosan in high fat diet fed rats. Carbohydr. Polym. 2017, 157, 834–841. [Google Scholar] [CrossRef]
- Rosado, C.P.; Rosa, V.H.C.; Martins, B.C.; Soares, A.C.; Santos, I.B.; Monteiro, E.B.; Moura-Nunes, N.; da Costa, C.A.; Mulder, A.D.R.P.; Daleprane, J.B. Resistant starch from green banana (Musa sp.) attenuates non-alcoholic fat liver accumulation and increases short-chain fatty acids production in high-fat diet-induced obesity in mice. Int. J. Biol. Macromol. 2020, 145, 1066–1072. [Google Scholar] [CrossRef]
- Polakof, S.; Díaz-Rubio, M.E.; Dardevet, D.; Martin, J.-F.; Pujos-Guillot, E.; Scalbert, A.; Sebedio, J.-L.; Mazur, A.; Comte, B. Resistant starch intake partly restores metabolic and inflammatory alterations in the liver of high-fat-diet-fed rats. J. Nutr. Biochem. 2013, 24, 1920–1930. [Google Scholar] [CrossRef]
- Xu, J.; Ma, Z.; Li, X.; Liu, L.; Hu, X. A more pronounced effect of type III resistant starch vs. type II resistant starch on ameliorating hyperlipidemia in high fat diet-fed mice is associated with its supramolecular structural characteristics. Food Funct. 2020, 11, 1982–1995. [Google Scholar] [CrossRef]
- Shen, R.-L.; Zhang, W.-L.; Dong, J.-L.; Ren, G.-X.; Chen, M. Sorghum resistant starch reduces adiposity in high-fat diet-induced overweight and obese rats via mechanisms involving adipokines and intestinal flora. Food Agric. Immunol. 2015, 26, 120–130. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Y.; Tan, S.; Wang, J. Effects of banana resistant starch on the biochemical indexes and intestinal flora of obese rats induced by a high-fat diet and their correlation analysis. Front. Bioeng. Biotechnol. 2021, 9, 575724. [Google Scholar] [CrossRef]
- Zaman, S.A.; Sarbini, S.R. The potential of resistant starch as a prebiotic. Crit. Rev. Biotechnol. 2016, 36, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Zaragoza, E.; Sánchez-Zapata, E.; Sendra, E.; Sayas, E.; Navarro, C.; Fernández-López, J.; Pérez-Alvarez, J.A. Resistant starch as prebiotic: A review. Starch-Stärke 2011, 63, 406–415. [Google Scholar] [CrossRef]
- Shang, W.; Si, X.; Zhou, Z.; Li, Y.; Strappe, P.; Blanchard, C. Characterization of fecal fat composition and gut derived fecal microbiota in high-fat diet fed rats following intervention with chito-oligosaccharide and resistant starch complexes. Food Funct. 2017, 8, 4374–4383. [Google Scholar] [CrossRef] [PubMed]
- Shou, D.; Cao, C.; Xu, H.; Huang, H.; Xia, Y.; Mei, Q.; Quan, Y.; Chen, H.; Zhao, C.; Tang, W.; et al. Type 2 resistant starch improves liver steatosis induced by high-fat diet relating to gut microbiota regulation and concentration of propionic acid in portal vein blood in C57BL/6J mice. Gut 2021, 70, A13–A14. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, S.; Jiang, Y.; Wei, Y.; Zhou, X. Regulatory function of buckwheat-resistant starch supplementation on lipid profile and gut microbiota in mice fed with a high-fat diet. J. Food Sci. 2019, 84, 2674–2681. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Nelson, B.; Birt, D.; Jane, J.-L. In vitro and in vivo digestion of octenyl succinic starch. Carbohydr. Polym. 2013, 98, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Mei, J.-Q.; Chen, B.; Chen, H.-Q. Digestibility, physicochemical and structural properties of octenyl succinic anhydride-modified cassava starches with different degree of substitution. Food Chem. 2017, 229, 136–141. [Google Scholar] [CrossRef]
- Wolf, B.W.; Bauer, L.L.; Fahey, G.C. Effects of chemical modification on in vitro rate and extent of food starch digestion: An attempt to discover a slowly digested starch. J. Agric. Food Chem. 1999, 47, 4178–4183. [Google Scholar] [CrossRef]
- Han, J.-A.; BeMiller, J.N. Preparation and physical characteristics of slowly digesting modified food starches. Carbohydr. Polym. 2007, 67, 366–374. [Google Scholar] [CrossRef]
- Liang, S.; Hong, Y.; Gu, Z.; Cheng, L.; Li, C.; Li, Z. Effect of debranching on the structure and digestibility of octenyl succinic anhydride starch nanoparticles. LWT 2021, 141, 111076. [Google Scholar] [CrossRef]
- Olawoye, B.; Fagbohun, O.F.; Popoola, O.O.; Gbadamosi, S.O.; Akanbi, C.T. Understanding how different modification processes affect the physiochemical, functional, thermal, morphological structures and digestibility of cardaba banana starch. Int. J. Biol. Macromol. 2022, 201, 158–172. [Google Scholar] [CrossRef]
- Remya, R.; Jyothi, A.N.; Sreekumar, J. Comparative study of RS4 type resistant starches derived from cassava and potato starches via octenyl succinylation. Starch-Stärke 2017, 69, 1600264. [Google Scholar] [CrossRef]
- No, J.; Mun, S.; Shin, M. Properties and digestibility of octenyl succinic anhydride-modified Japonica-type waxy and non-waxy rice starches. Molecules 2019, 24, 765. [Google Scholar] [CrossRef]
- Lan, X.; Huang, B.; Wu, J.; Wang, Z. The effect of octenylsuccinylation on morphological, rheological, and in vitro digestibility properties of Canna edulis Ker starch. Starch-Stärke 2015, 67, 846–853. [Google Scholar] [CrossRef]
- Quintero-Castaño, V.D.; Castellanos-Galeano, F.J.; Álvarez-Barreto, C.I.; Bello-Pérez, L.A.; Alvarez-Ramirez, J. In vitro digestibility of octenyl succinic anhydride-starch from the fruit of three Colombian Musa. Food Hydrocoll. 2020, 101, 105566. [Google Scholar] [CrossRef]
- Lu, K.; Miao, M.; Ye, F.; Cui, S.W.; Li, X.; Jiang, B. Impact of dual-enzyme treatment on the octenylsuccinic anhydride esterification of soluble starch nanoparticle. Carbohydr. Polym. 2016, 147, 392–400. [Google Scholar] [CrossRef]
- Simsek, S.; Ovando-Martínez, M.; Marefati, A.; Sjöö, M.; Rayner, M. Chemical composition, digestibility and emulsification properties of octenyl succinic esters of various starches. Food Res. Int. 2015, 75, 41–49. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, A.K.; Yadav, D.N.; Arora, S.; Vishwakarma, R. Impact of octenyl succinylation on rheological, pasting, thermal and physicochemical properties of pearl millet (Pennisetum typhoides) starch. LWT 2016, 73, 52–59. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, L.; Ding, N.; Liu, P.; Yao, C.; Zhang, H. Physicochemical and structural characteristics of the octenyl succinic ester of ginkgo starch. Int. J. Biol. Macromol. 2017, 94, 566–570. [Google Scholar] [CrossRef]
- Lv, Q.-Q.; Li, G.-Y.; Xie, Q.-T.; Zhang, B.; Li, X.-M.; Pan, Y.; Chen, H.-Q. Evaluation studies on the combined effect of hydrothermal treatment and octenyl succinylation on the physic-chemical, structural and digestibility characteristics of sweet potato starch. Food Chem. 2018, 256, 413–418. [Google Scholar] [CrossRef]
- Ferguson, M.J.; Jones, G.P. Production of short-chain fatty acids following in vitro fermentation of saccharides, saccharide esters, fructo-oligosaccharides, starches, modified starches and non-starch polysaccharides. J. Sci. Food Agric. 2000, 80, 166–170. [Google Scholar] [CrossRef]
- Shi, Y.C.; Bai, Y. Starch esters and method of preparation. U.S. Patent 9458252B2, 4 October 2016. [Google Scholar]
- Englyst, K.N.; Englyst, H.N.; Hudson, G.J.; Cole, T.J.; Cummings, J.H. Rapidly available glucose in foods: An in vitro measurement that reflects the glycemic response. Am. J. Clin. Nutr. 1999, 69, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Seib, P.A. Resistant starches from amylose mutants of corn by simultaneous heat-moisture treatment and phosphorylation. Carbohydr. Polym. 2006, 63, 167–175. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, J.; Wang, Z.; Cheng, H.; Zhang, Y.; Lin, B.; Qin, L.; Bai, Y. Effects of reaction condition on glycosidic linkage structure, physical–chemical properties and in vitro digestibility of pyrodextrins prepared from native waxy maize starch. Food Chem. 2020, 320, 126491. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Jenkins, D.J.; Jenkins, A.L.; Josse, R.G. The glycemic index: Methodology and clinical implications. Am. J. Clin. Nutr. 1991, 54, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Kritchevsky, D.; Tepper, S.A. Influence of a fiber mixture on serum and liver lipids and on fecal fat excretion in rats. Nutr. Res. 2005, 25, 485–489. [Google Scholar] [CrossRef]
- García-Villalba, R.; Giménez-Bastida, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; Carlos Espín, J.; Larrosa, M. Alternative method for gas chromatography-mass spectrometry analysis of short-chain fatty acids in faecal samples. J. Sep. Sci. 2012, 35, 1906–1913. [Google Scholar] [CrossRef]
- Deming, L.; Qian, H.; Huanhuan, Y.; Yafang, D.; Kangqing, Y.; Jing, Y.; Xing, T.; Yaxin, G.; Jiaying, X.; Liqiang, Q. Daily dose of bovine lactoferrin prevents ethanol-induced liver injury and death in male mice by regulating hepatic alcohol metabolism and modulating gut microbiota. Mol. Nutr. Food Res. 2021, 65, 2100253. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Li, D.; Cui, Y.; Li, X. Apple polyphenols extract alleviated dextran sulfate sodium-induced ulcerative colitis in C57BL/6 male mice by restoring bile acid metabolism disorder and gut microbiota dysbiosis. Phytother. Res. 2021, 35, 1468–1485. [Google Scholar] [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant starch: Promise for improving human health. Adv. Nutr. Int. Rev. J. 2013, 4, 587–601. [Google Scholar] [CrossRef] [Green Version]
- Keenan, M.J.; Zhou, J.; Hegsted, M.; Pelkman, C.; Durham, H.A.; Coulon, D.B.; Martin, R.J. Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv. Nutr. 2015, 6, 198–205. [Google Scholar] [CrossRef]
- Aziz, A.A.; Kenney, L.S.; Goulet, B.; Abdel-Aal, E.-S. Dietary starch type affects body weight and glycemic control in freely fed but not energy-restricted obese rats. J. Nutr. 2009, 139, 1881–1889. [Google Scholar] [CrossRef]
- Shen, L.; Keenan, M.J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Zhou, J. Dietary resistant starch increases hypothalamic POMC expression in rats. Obesity 2009, 17, 40–45. [Google Scholar] [CrossRef]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Shen, L.; Danna, S.C.; Tripathy, S.; Hegsted, M.; Keenan, M.J. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol. Metab. 2008, 295, E1160–E1166. [Google Scholar] [CrossRef]
- Keenan, M.J.; Martin, R.J.; Raggio, A.M.; McCutcheon, K.L.; Brown, I.L.; Birkett, A.; Newman, S.S.; Skaf, J.; Hegsted, M.; Tulley, R.T.; et al. High-Amylose resistant starch increases hormones and improves structure and function of the gastrointestinal tract: A microarray study. Lifestyle Genom. 2012, 5, 26–44. [Google Scholar] [CrossRef]
- Zhou, J.; Martin, R.J.; Raggio, A.M.; Shen, L.; McCutcheon, K.; Keenan, M.J. The importance of GLP-1 and PYY in resistant starch’s effect on body fat in mice. Mol. Nutr. Food Res. 2015, 59, 1000–1003. [Google Scholar] [CrossRef]
- Lehmann, U.; Robin, F. Slowly digestible starch - its structure and health implications: A review. Trends Food Sci. Technol. 2007, 18, 346–355. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Wang, F.; Strappe, P.; Liu, W.; Zheng, J.; Zhou, Z.; Zhang, Y. Microbiota fermentation characteristics of acylated starches and the regulation mechanism of short-chain fatty acids on hepatic steatosis. Food Funct. 2021, 12, 8659–8668. [Google Scholar] [CrossRef]
- Jiminez, J.A.; Uwiera, T.C.; Abbott, D.W.; Uwiera, R.R.E.; Inglis, G.D. Impacts of resistant starch and wheat bran consumption on enteric inflammation in relation to colonic bacterial community structures and short-chain fatty acid concentrations in mice. Gut Pathog. 2016, 8, 67. [Google Scholar] [CrossRef] [Green Version]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; King, R.A.; Christophersen, C.T.; Bird, A.R. Dietary resistant starch dose-dependently reduces adiposity in obesity-prone and obesity-resistant male rats. Nutr. Metab. 2012, 9, 93. [Google Scholar] [CrossRef]
- Wan, J.; Wu, Y.; Pham, Q.; Yu, L. Effects of rice with different amounts of resistant starch on mice fed a high-fat diet: Attenuation of adipose weight gain. J. Agric. Food Chem. 2020, 68, 13046–13055. [Google Scholar] [CrossRef]
- Liang, D.; Zhang, L.; Chen, H.; Zhang, H.; Hu, H.; Dai, X. Potato resistant starch inhibits diet-induced obesity by modifying the composition of intestinal microbiota and their metabolites in obese mice. Int. J. Biol. Macromol. 2021, 180, 458–469. [Google Scholar] [CrossRef]
- Chang, D.; Hu, X.; Ma, Z. Pea-Resistant starch with different multi-scale structural features attenuates the obesity-related physiological changes in high-fat diet mice. J. Agric. Food Chem. 2022, 70, 11377–11390. [Google Scholar] [CrossRef]
- Van der Beek, C.M.; Canfora, E.E.; Lenaerts, K.; Troost, F.J.; Damink, S.W.M.O.; Holst, J.J.; Masclee, A.A.M.; Dejong, C.H.C.; Blaak, E.E. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin. Sci. 2016, 130, 2073–2082. [Google Scholar] [CrossRef]
- Besten, G.d.; Bleeker, A.; Gerding, A.; Eunen, K.v.; Havinga, R.; Dijk, T.H.v.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J.; et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating g protein-coupled receptors and gut microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut microbiota and short chain fatty acids: Implications in glucose homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110–119. [Google Scholar] [CrossRef]
- Lim, S.; Choo, J.; Li, H.; O’Rielly, R.; Carragher, J.; Rogers, G.; Searle, I.; Robertson, S.; Page, A.; Muhlhausler, B. A high amylose wheat diet improves gastrointestinal health parameters and gut microbiota in male and female mice. Foods 2021, 10, 220. [Google Scholar] [CrossRef]
- Martínez, I.; Kim, J.; Duffy, P.R.; Schlegel, V.L.; Walter, J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE 2010, 5, e15046. [Google Scholar] [CrossRef]
- Herrmann, E.; Young, W.; Rosendale, D.; Conrad, R.; Riedel, C.U.; Egert, M. Determination of resistant starch assimilating bacteria in fecal samples of mice by in vitro RNA-based stable isotope probing. Front. Microbiol. 2017, 8, 1331. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Jo, J.-K.; Seo, S.-H.; Park, S.-E.; Kim, H.-W.; Kim, E.-J.; Kim, J.-S.; Pyo, J.-Y.; Cho, K.-M.; Kwon, S.-J.; Park, D.-H.; et al. Gut microbiome and metabolome profiles associated with high-fat diet in mice. Metabolites 2021, 11, 482. [Google Scholar] [CrossRef]
- Wang, B.; Yu, H.; He, Y.; Wen, L.; Gu, J.; Wang, X.; Miao, X.; Qiu, G.; Wang, H. Effect of soybean insoluble dietary fiber on prevention of obesity in high-fat diet fed mice via regulation of the gut microbiota. Food Funct. 2021, 12, 7923–7937. [Google Scholar] [CrossRef]
- Cai, H.; Wen, Z.; Li, X.; Meng, K.; Yang, P. Lactobacillus plantarum FRT10 alleviated high-fat diet–induced obesity in mice through regulating the PPARα signal pathway and gut microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 5959–5972. [Google Scholar] [CrossRef]
- Murphy, E.F.; Cotter, P.D.; Healy, S.; Marques, T.M.; O’Sullivan, O.; Fouhy, F.; Clarke, S.F.; O’Toole, P.W.; Quigley, E.M.; Stanton, C.; et al. Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut 2010, 59, 1635–1642. [Google Scholar] [CrossRef]
- Li, Z.-T.; Hu, G.-A.; Zhu, L.; Zhao, Z.-C.; Jiang, Y.; Gao, M.-J.; Zhan, X.-B. In vitro digestion and fecal fermentation of highly resistant starch rice and its effect on the gut microbiota. Food Chem. 2021, 361, 130095. [Google Scholar] [CrossRef]
- Maharshak, N.; Packey, C.D.; Ellermann, M.; Manick, S.; Siddle, J.P.; Huh, E.Y.; Plevy, S.E.; Sartor, R.B.; Carroll, I.M. Altered enteric microbiota ecology in interleukin 10-deficient mice during development and progression of intestinal inflammation. Gut Microbes 2013, 4, 316–324. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Pang, X.; Zhao, Y.; Wang, L.; Zhao, L. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 2012, 6, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, T.; Alim, A.; Ren, D.; Zhao, Y.; Yang, X. Regulatory effects of stachyose on colonic and hepatic inflammation, gut microbiota dysbiosis, and peripheral CD4+ T cell distribution abnormality in high-fat diet-fed mice. J. Agric. Food Chem. 2019, 67, 11665–11674. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Xing, Y.; Xing, R.; Liu, Y.; Xu, Y. Changes of gut microbiota during silybin-mediated treatment of high-fat diet-induced non-alcoholic fatty liver disease in mice. Hepatol. Res. 2020, 50, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The controversial role of human gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Rainey, F.A.; Family, V. Lachnospiraceae fam. nov. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; De Vos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A., Schleifer, K.-H., Whitman, W.B., Eds.; Springer: New York, NY, USA, 2009; Volume 3, pp. 921–968. [Google Scholar] [CrossRef]
- Guo, M.; Li, Z. Polysaccharides isolated from Nostoc commune Vaucher inhibit colitis-associated colon tumorigenesis in mice and modulate gut microbiota. Food Funct. 2019, 10, 6873–6881. [Google Scholar] [CrossRef]
- Yang, D.; Lyu, W.; Hu, Z.; Gao, J.; Zheng, Z.; Wang, W.; Firrman, J.; Ren, D. Probiotic effects of lactobacillus fermentum zjuids06 and lactobacillus plantarum zy08 on hypercholesteremic golden hamsters. Front. Nutr. 2021, 8, 705763. [Google Scholar] [CrossRef]
- Hu, Y.; Le Leu, R.K.; Christophersen, C.T.; Somashekar, R.; Conlon, M.A.; Meng, X.Q.; Winter, J.M.; Woodman, R.J.; McKinnon, R.; Young, G.P. Manipulation of the gut microbiota using resistant starch is associated with protection against colitis-associated colorectal cancer in rats. Carcinogenesis 2016, 37, 366–375. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The genus Alistipes: Gut bacteria with emerging implications to inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Dziarski, R.; Park, S.Y.; Kashyap, D.; Dowd, S.; Gupta, D. Pglyrp-regulated gut microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii Enhance and Alistipes finegoldii attenuates colitis in mice. PLoS ONE 2016, 11, e0146162. [Google Scholar] [CrossRef]
- Schubert, A.M.; Sinani, H.; Schloss, P.D. Antibiotic-induced alterations of the murine gut microbiota and subsequent effects on colonization resistance against Clostridium difficile. mBio 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; DeRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, D.; He, Y.; Li, Y.; Yang, Z.; Zhao, X.; Liu, Y.; Wang, Y.; Sun, J.; Feng, X.; et al. Discrepant gut microbiota markers for the classification of obesity-related metabolic abnormalities. Sci. Rep. 2019, 9, 13424. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef]
- Lennon, G.; Balfe, Á.; Bambury, N.; Lavelle, A.; Maguire, A.; Docherty, N.G.; Coffey, J.C.; Winter, D.C.; Sheahan, K.; O’Connell, P.R. Correlations between colonic crypt mucin chemotype, inflammatory grade and Desulfovibrio species in ulcerative colitis. Colorectal Dis. 2014, 16, O161–O169. [Google Scholar] [CrossRef]
- Rowan, F.; Docherty, N.G.; Murphy, M.; Murphy, B.; Coffey, J.C.; O’Connell, P.R. Desulfovibrio bacterial species are increased in ulcerative colitis. Dis. Colon Rectum 2010, 53, 1530–1536. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Liu, H.; Sui, X.; Liu, Y.; Wei, X.; Liu, C.; Cheng, Y.; Ye, W.; Gao, B.; et al. The anti-inflammatory effect and mucosal barrier protection of Clostridium butyricum RH2 in ceftriaxone-induced intestinal dysbacteriosis. Front. Cell. Infect. Microbiol. 2021, 11, 647048. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-S.; Li, W.-B.; Wang, H.-Y.; Ma, Y.-M.; Zhao, X.-H.; Yang, H.; Qian, J.-M.; Li, J.-N. VSL#3 can prevent ulcerative colitis-associated carcinogenesis in mice. World J. Gastroenterol. 2018, 24, 4254–4262. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, P.; An, Y.; Ren, J.; Yan, D.; Cui, J.; Li, D.; Li, M.; Wang, M.; Zhong, G. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol. Res. 2019, 150, 104489. [Google Scholar] [CrossRef] [PubMed]
- Bailén, M.; Bressa, C.; Martínez-López, S.; González-Soltero, R.; Lominchar, M.G.M.; Juan, C.S.; Larrosa, M. Microbiota features associated with a high-fat/low-fiber diet in healthy adults. Front. Nutr. 2020, 7, 583608. [Google Scholar] [CrossRef]
- Kelly, T.N.; Bazzano, L.A.; Ajami, N.J.; He, H.; Zhao, J.; Petrosino, J.F.; Correa, A.; He, J. Gut microbiome associates with lifetime cardiovascular disease risk profile among bogalusa heart study participants. Circ. Res. 2016, 119, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.M.; Clemente, J.C.; Giannarelli, C. Physical activity, immune system, and the microbiome in cardiovascular disease. Front. Physiol. 2018, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-L.; Pan, Y.-Y.; Li, L.; Li, T.-T.; Liu, B.; Lv, X.-C. Ethanol extract of Ganoderma lucidum ameliorates lipid metabolic disorders and modulates the gut microbiota composition in high-fat diet fed rats. Food Funct. 2018, 9, 3419–3431. [Google Scholar] [CrossRef]
- Li, T.-T.; Huang, Z.-R.; Jia, R.-B.; Lv, X.-C.; Zhao, C.; Liu, B. Spirulina platensis polysaccharides attenuate lipid and carbohydrate metabolism disorder in high-sucrose and high-fat diet-fed rats in association with intestinal microbiota. Food Res. Int. 2021, 147, 110530. [Google Scholar] [CrossRef]
- Wan, X.; Li, T.; Liu, D.; Chen, Y.; Liu, Y.; Liu, B.; Zhang, H.; Zhao, C. Effect of marine microalga Chlorella pyrenoidosa ethanol extract on lipid metabolism and gut microbiota composition in high-fat diet-fed rats. Mar. Drugs 2018, 16, 498. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Dong, L.; Huang, L.; Shi, Z.; Dong, J.; Yao, Y.; Shen, R. Effects of oat β-glucan, oat resistant starch, and the whole oat flour on insulin resistance, inflammation, and gut microbiota in high-fat-diet-induced type 2 diabetic rats. J. Funct. Foods 2020, 69, 103939. [Google Scholar] [CrossRef]
- Flint, H.J. The microbiology of resistant starch fermentation in the human large intestine: A host of unanswered questions. In Resistant Starch; Shi, Y.-C., Maningat, C.C., Eds.; 2013; pp. 251–266. [Google Scholar] [CrossRef]

| Ingredient | Regular-Fat (RF) Diet (g) | High-Fat (HF) Diet (g) | High-Fat Diet Supplemented with OS Starch (HFOSS) 1 (g) |
|---|---|---|---|
| Casein | 200.0 | 200.0 | 200.0 |
| Sucrose | 68.8 | 68.8 | 56.2 |
| Corn starch | 506.2 | 0.0 | 0.0 |
| Maltodextrin | 125.0 | 125.0 | 0.0 |
| Soy oil | 25.0 | 25.0 | 25.0 |
| Lard | 20.0 | 245.0 | 245.0 |
| Cellulose | 50.0 | 50.0 | 0.0 |
| OS starch 1 | 0.0 | 0.0 | 215.0 |
| Mineral Mix S10026 | 10.0 | 10.0 | 10.0 |
| Dicalcium phosphate | 13.0 | 13.0 | 13.0 |
| Calcium carbonate | 5.5 | 5.5 | 5.5 |
| Potassium citrate | 16.5 | 16.5 | 16.5 |
| Vitamin mix V10001 | 10.0 | 10.0 | 10.0 |
| Choline bitartrate | 2.0 | 2.0 | 2.0 |
| L-Cysteine | 3.0 | 3.0 | 3.0 |
| Ethoxyquin | 0.035 | 0.035 | 0.035 |
| Energy density (kcal/g) | 3.8 | 5.2 | 5.1 |
| Percentage of energy provided by macronutrients (%) | |||
| Fat | 10.1 | 60.5 | 60.6 |
| Carbohydrate | 69.7 | 19.3 | 19.1 |
| Protein | 20.2 | 20.2 | 20.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-P.; Maierheba, K.; Zhang, Y.; Cheng, H.; Lin, B.; Yue, P.; Wang, L.-H.; Liu, F.-Z.; Shi, J.-W.; Wan, Z.-X.; et al. Octenyl Succinic Anhydride-Modified Starch Attenuates Body Weight Gain and Changes Intestinal Environment of High-Fat Diet-Fed Mice. Foods 2022, 11, 2980. https://doi.org/10.3390/foods11192980
Chen J-P, Maierheba K, Zhang Y, Cheng H, Lin B, Yue P, Wang L-H, Liu F-Z, Shi J-W, Wan Z-X, et al. Octenyl Succinic Anhydride-Modified Starch Attenuates Body Weight Gain and Changes Intestinal Environment of High-Fat Diet-Fed Mice. Foods. 2022; 11(19):2980. https://doi.org/10.3390/foods11192980
Chicago/Turabian StyleChen, Jia-Ping, Kuerbanjiang Maierheba, Ying Zhang, Hui Cheng, Binbin Lin, Pan Yue, Le-Hua Wang, Feng-Zhi Liu, Jun-Wen Shi, Zhong-Xiao Wan, and et al. 2022. "Octenyl Succinic Anhydride-Modified Starch Attenuates Body Weight Gain and Changes Intestinal Environment of High-Fat Diet-Fed Mice" Foods 11, no. 19: 2980. https://doi.org/10.3390/foods11192980
APA StyleChen, J.-P., Maierheba, K., Zhang, Y., Cheng, H., Lin, B., Yue, P., Wang, L.-H., Liu, F.-Z., Shi, J.-W., Wan, Z.-X., Wang, X.-P., Xu, J., Qin, L.-Q., & Bai, Y. (2022). Octenyl Succinic Anhydride-Modified Starch Attenuates Body Weight Gain and Changes Intestinal Environment of High-Fat Diet-Fed Mice. Foods, 11(19), 2980. https://doi.org/10.3390/foods11192980