Effects of Germinated Lentil Flour on Dough Rheological Behavior and Bread Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dough Fundamental Rheological Properties
2.3. Dough Microstructure
2.4. Bread Making
2.5. Bread Quality Evaluation
2.5.1. Bread Physical Characteristics
2.5.2. Color Parameters
2.5.3. Texture Profile Analysis
2.5.4. Crumb Structure
2.5.5. Sensory Analysis
2.6. Statistical Analysis
3. Results
3.1. Flour Characteristics
3.2. Dough Fundamental Rheological Properties
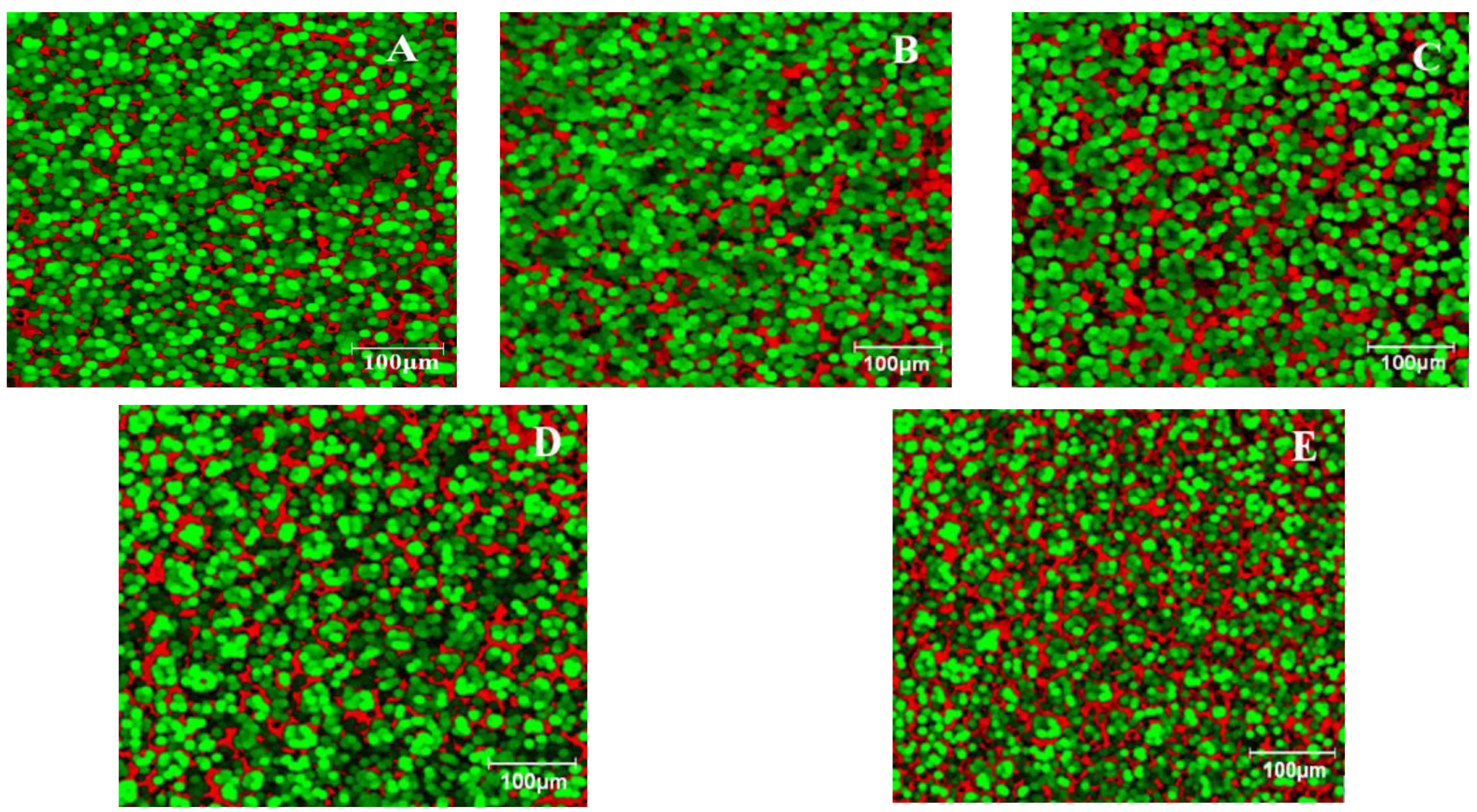
3.3. Dough Microstructure
3.4. Bread Quality Evaluation
3.4.1. Bread Physical Characteristics
3.4.2. Color Parameters of Bread Samples
3.4.3. Texture Profile Analysis of Bread Samples
3.4.4. Crumb Structure of Bread Samples
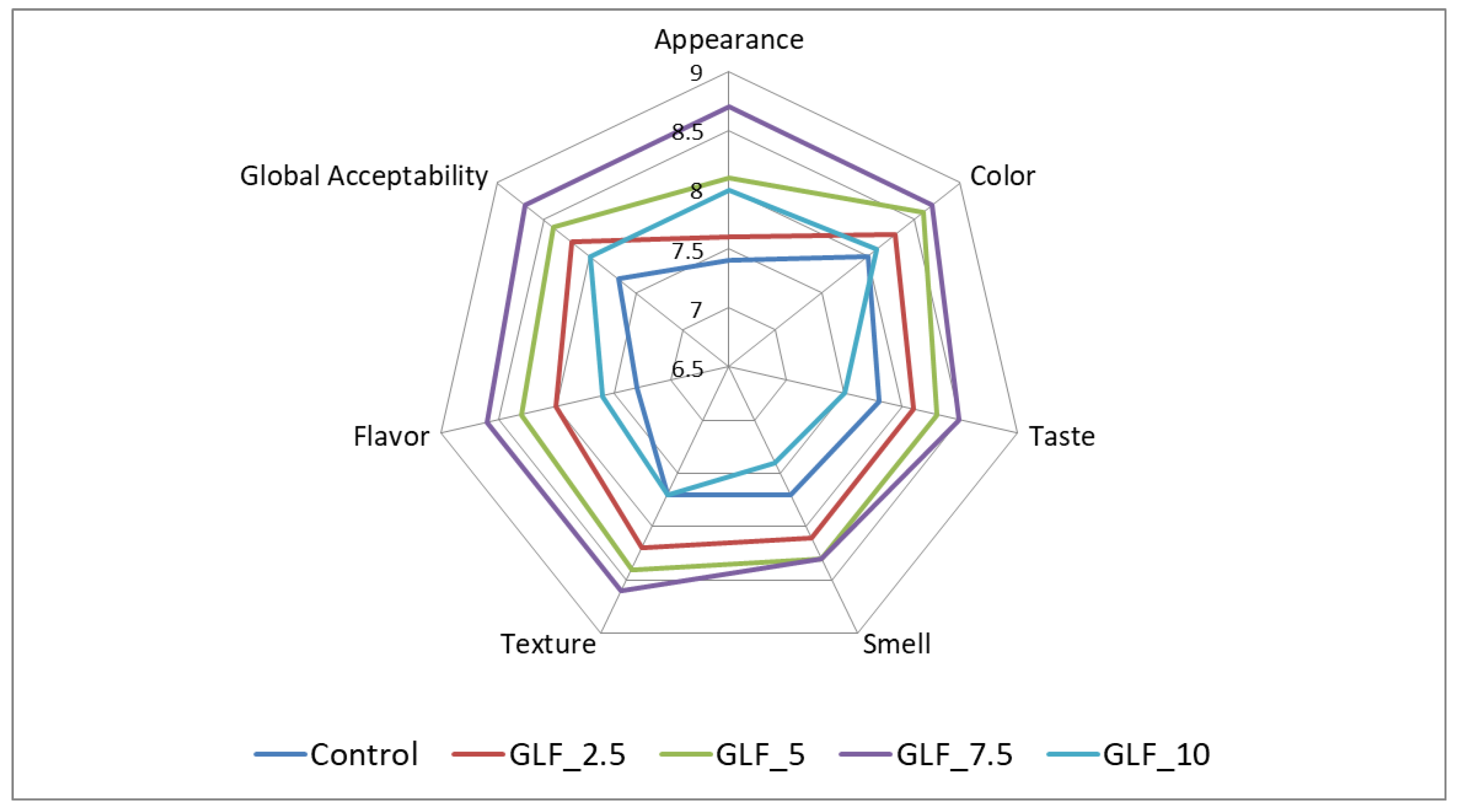
3.4.5. Sensory Analysis of Bread Samples
4. Discussion
4.1. Dough Fundamental Rheological Properties
4.2. Dough Microstructure
4.3. Bread Quality Evaluation
4.3.1. Bread Physical Characteristics
4.3.2. Color Analysis of Bread Samples
4.3.3. Texture Profile Analysis of Bread Samples
4.3.4. Crumb Microstructure of Bread Samples
4.3.5. Sensory Analysis of the Bread Samples
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Chillón, M.T.; Carazo-Díaz, C.; Prieto-Merino, D.; Zafrilla, P.; Moreno, D.A.; Villaño, D. Effects of long-term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clin. Nutr. 2019, 38, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.; Benton, T.G.; Beddington, J.; Flynn, D.; Kelly, N.M.; Thomas, S.M. The urgency of food system transformation is now irrefutable. Nat. Food 2020, 1, 584–585. [Google Scholar] [CrossRef]
- Viaggi, D.; Barrera, C.; Castelló, M.L.; Dalla Rosa, M.; Heredia, A.; Hobley, T.J.; Knöbl, C.F.; Materia, V.C.; Xu, S.M.; Romanova, G.; et al. Education for innovation and entrepreneurship in the food system: The Erasmus+ BoostEdu approach and results. Curr. Opin. Food Sci. 2021, 42, 157–166. [Google Scholar] [CrossRef]
- Pedersen, H.A.; Laursen, B.; Mortensen, A.; Fomsgaard, I.S. Bread from common cereal cultivars contains an important array of neglected bioactive benzoxazinoids. Food Chem. 2011, 127, 1814–1820. [Google Scholar] [CrossRef]
- Gu, M.; Hong, T.; Ma, Y.; Xi, J.; Zhao, J.; Xu, D.; Jin, Y.; Wu, F.; Xu, X. Effects of a commercial peptidase on rheology, microstructure, gluten properties of wheat dough and bread quality. LWT 2022, 160, 113266. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, G. Biofortification of pulses and legumes to enhance nutrition. Heliyon 2020, 6, e03682. [Google Scholar] [CrossRef]
- Gostin, A.I. Effects of substituting refined wheat flour with wholemeal and quinoa flour on the technological and sensory characteristics of salt-reduced breads. LWT Food Sci. Technol. 2019, 114, 108412. [Google Scholar] [CrossRef]
- Geng, P.; Harnly, J.M.; Chen, P. Differentiation of bread made with whole grain and refined wheat (T. aestivum) flour using LC/MS-based chromatographic fingerprinting and chemometric approaches. J. Food Compost. Anal. 2016, 47, 92–100. [Google Scholar] [CrossRef]
- Anaemene, D.; Fadupin, G. Anti-nutrient reduction and nutrient retention capacity of fermentation, germination and combined germination-fermentation in legume processing. Appl. Food Biotechnol. 2022, 2, 100059. [Google Scholar] [CrossRef]
- Atudorei, D.; Codină, G.G. Perspectives on the Use of Germinated Legumes in the Bread Making Process, A Review. Appl. Sci. 2020, 10, 6244. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Lu, H.; Shu, Q.; Zhang, Y.; Chen, Q. New perspectives on physiological, biochemical and bioactive components during germination of edible seeds: A review. Trends Food Sci. Technol. 2022, 123, 187–197. [Google Scholar] [CrossRef]
- Xing, B.; Teng, C.; Sun, M.; Zhang, Q.; Zhou, B.; Cui, H.; Ren, G.; Yang, X.; Qin, P. Effect of germination treatment on the structural and physicochemical properties of quinoa starch. Food Hydrocoll. 2021, 115, 106604. [Google Scholar] [CrossRef]
- Sharma, N.; Sahu, J.K.; Joshi, S.; Khubber, S.; Bansal, V.; Bhardwaj, A.; Bangar, S.P.; Bal, L.M. Modulation of lentil antinutritional properties using non-thermal mediated processing techniques—A review. J. Food Compos. Anal. 2022, 109, 104498. [Google Scholar] [CrossRef]
- FAOSTAT. Database. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 18 September 2022).
- Wilson, S.; Peterson, E.; Gaston, M.; McMilin, C.; Kuo, W.; Miles, M. 8 Weeks of Lentil Consumption Improves Insulin Sensitivity in Overweight and Obese Adults—A Randomized Controlled Trial. J. Acad. Nutr. Diet 2020, 120, A-73. [Google Scholar] [CrossRef]
- Zahradka, P.; Hanson, M.G.; Wu, Y.; Taylor, C.G. Consumption of lentils improves arterial elasticity. Atherosclerosis 2018, 32, 160. [Google Scholar] [CrossRef]
- Papandreou, C.; Becerra-Tomás, N.; Bulló, M.; Martínez-González, M.Á.; Corella, D.; Estruch, R.; Ros, E.; Arós, F.; Schroder, H.; Fitó, M.; et al. Legume consumption and risk of all-cause, cardiovascular, and cancer mortality in the PREDIMED study. Clin. Nutr. 2019, 38, 348–356. [Google Scholar] [CrossRef]
- Romano, A.; Gallo, V.; Ferranti, P.; Masi, P. Lentil flour: Nutritional and technological properties, in vitro digestibility and perspectives for use in the food industry. Curr. Opin. Food Sci. 2021, 40, 157–167. [Google Scholar] [CrossRef]
- Atudorei, D.; Stroe, S.G.; Codină, G.G. Physical, Physiological and Minerals Changes of Different Legumes Types during the Germination Process. Ukr. Food J. 2020, 9, 844–863. [Google Scholar] [CrossRef]
- Gallo, V.; Romano, A.; Miralles, B.; Ferranti, P.; Masi, P.; Santos-Hernández, M.; Recio, I. Physicochemical properties, structure and digestibility in simulated gastrointestinal environment of bread added with green lentil flour. LWT 2022, 154, 112713. [Google Scholar] [CrossRef]
- Ungureanu-Iuga, M.; Atudorei, D.; Codină, G.G.; Mironeasa, S. Rheological Approaches of Wheat Flour Dough Enriched with Germinated Soybean and Lentil. Appl. Sci 2021, 11, 11706. [Google Scholar] [CrossRef]
- Razavi, S.N.; Hojjatoleslamy, M.; Molavi, H.; Boroujeni, L.S. The Effect of Germinated Lentil Flour on the Physicochemical and Organoleptic Characteristics of Sangak Bread. J. Culin. Sci. Technol. 2022, 20, 253–265. [Google Scholar] [CrossRef]
- Hernandez-Aguilar, C.; Dominguez-Pacheco, A.; Palma Tenango, M.; Valderrama-Bravo, C.; Hernández, M.S.; Cruz-Orea, A.; Ordonez-Miranda, J. Lentil sprouts: A nutraceutical alternative for the elaboration of bread. J. Food Sci. Technol. 2020, 57, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.; Cardone, G.; Nicolodi, A.; Quaglia, L.; Pagani, M.A. Sprouted wheat as an alternative to conventional flour improvers in bread-making. LWT 2017, 80, 230–236. [Google Scholar] [CrossRef]
- ICC. Standard Methods of the International Association for Cereal Chemistry: Methods 104/1, 110/1, 136, 105/2, 171, 121, 107/1; International Association for Cereal Chemistry: Vienna, Austria, 2010. [Google Scholar]
- Mironeasa, S.; Codină, G.G. Dough Rheological Behavior and Microstructure Characterization of Composite Dough with Wheat and Tomato Seed Flours. Foods 2019, 8, 626. [Google Scholar] [CrossRef] [PubMed]
- AACC (American Association of Cereal Chemists). AACC official method. In Approved Methods of American Association of Cereal Chemists, 10th ed.; AACC (American Association of Cereal Chemists): St. Paul, MN, USA, 2000; pp. 14–50. [Google Scholar]
- Feili, R.; Zzaman, W.; Abdullah, T.W.; Yang, A. Physical and Sensory Analysis of High Fiber Bread Incorporated with Jackfruit Rind Flour. Food Sci. Technol. 2013, 1, 30–36. [Google Scholar]
- Sirbu, A.; Arghire, C. Functional bread: Effect of inulin-type products addition on dough rheology and bread quality. J. Cereal Sci. 2017, 75, 220–227. [Google Scholar] [CrossRef]
- ASRO. Romanian Standards Catalog for Cereal and Milling Products Analysis; SR 91:2007; ASRO: Bucharest, Romania, 2008. [Google Scholar]
- Codină, G.G.; Dabija, A.; Oroian, M. Prediction of Pasting Properties of Dough from Mixolab Measurements Using Artificial Neuronal Networks. Foods 2019, 8, 447. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Dadpour, M.R.; Dokouhaki, M. Application of epifluorescence light microscopy (EFLM) to study the microstructure of wheat dough: A comparison with confocal scanning laser microscopy (CSLM) technique. J. Cereal Sci. 2010, 51, 21–27. [Google Scholar] [CrossRef]
- Kotsiou, K.; Sacharidis, D.D.; Matsakidou, A.; Biliaderis, C.G. Physicochemical and functional aspects of composite wheat-roasted chickpea flours in relation to dough rheology, bread quality and staling phenomena. Food Hydrocoll. 2022, 124, 107322. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Torres, M.D. Effect of chia (Sativa hispanica L.) and hydrocolloids on the rheology of gluten-free doughs based on chestnut flour. LWT Food Sci. Technol. 2013, 50, 160–166. [Google Scholar] [CrossRef]
- Gallo, V.; Romano, A.; Ferranti, P.; D’Auria, G.; Masi, P. Properties and in vitro digestibility of a bread enriched with lentil flour at different leavening times. Food Struct. 2022, 33, 100284. [Google Scholar] [CrossRef]
- Atudorei, D.; Stroe, S.G.; Codină, G.G. Impact of Germination on the Microstructural and Physicochemical Properties of Different Legume Types. Foods 2021, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Bajka, B.H.; Pinto, A.M.; Ahn-Jarvis, J.; Ryden, P.; Perez-Moral, N.; van der Schoot, A.; Stocchi, C.; Bland, C.; Berry, S.E.; Ellis, P.R.; et al. The impact of replacing wheat flour with cellular legume powder on starch bioaccessibility, glycaemic response and bread roll quality: A double-blind randomised controlled trial in healthy participants. Food Hydrocoll. 2021, 114, 106565. [Google Scholar] [CrossRef] [PubMed]
- Ouazib, M.; Dura, A.; Zaidi, F.; Rosell, C.M. Effect of partial substitution of wheat flour by processed (germinated, toasted, cooked) chickpea on bread quality. Int. J. Agric. Sci. Technol. 2016, 4, 8–18. [Google Scholar] [CrossRef][Green Version]
- Suárez-Estrella, D. Germination as a Bio-Technological Process to Enhance the Use of Quinoa (Chenopodium Quinoa Willd.) in Cereal-Based Products. Ph.D. Thesis, Universitá degli Studi di Milano, Milan, Italy, 2019. [Google Scholar]
- Suárez-Estrella, D.; Cardone, G.; Buratti, S.; Pagani, M.A.; Marti, A. Sprouting as a pre-processing for producing quinoa-enriched bread. J. Cereal Sci. 2020, 96, 103111. [Google Scholar] [CrossRef]
- Goesaert, H.; Slade, L.; Levine, H.; Delcour, J.A. Amylases and bread firming—An integrated view. J. Cereal Sci. 2009, 50, 345–352. [Google Scholar] [CrossRef]
- Goesaert, H.; Gebruers, K.; Courtin, C.M.; Brijs, K.; Delcour, J.A. Enzymes in breadmaking. In Bakery Products, Science and Technology; Hui, Y.H., Ed.; Blackwell Publishing: Ames, IA, USA, 2006; pp. 337–364. [Google Scholar]
- Cardone, G.; D’Incecco, P.; Pagani, M.A.; Marti, A. Sprouting improves the bread-making performance of whole wheat flour (Triticum aestivum L.). J. Sci. Food Agric. 2020, 100, 2453–2459. [Google Scholar] [CrossRef] [PubMed]
- Cardone, G.; Grassi, S.; Scipioni, A.; Marti, A. Bread-making performance of durum wheat as affected by sprouting. LWT 2020, 134, 110021. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Qiao, Y.; Zhang, Y.; Zheng, W.; Zhao, Y.; Huang, Y.; Cui, Z. Improvement of the quality and shelf life of wheat bread by a maltohexaose producing α-amylase. J. Cereal Sci. 2019, 87, 165–171. [Google Scholar] [CrossRef]
- Xing, Q.; Kyriakopoulou, K.; Zhang, L.; Boom, R.M.; Schutyser, M.A.I. Protein fortification of wheat bread using dry fractionated chickpea protein-enriched fraction or its sourdough. LWT Food Sci. Technol. 2021, 130, 110931. [Google Scholar] [CrossRef]
- Mohammed, I.; Ahmed, A.R.; Senge, B. Dough rheology and bread quality of wheat–chickpea flour blends. Ind. Crops Prod. 2012, 36, 196–202. [Google Scholar] [CrossRef]
- Özcan, M.M. The effect of ginger (Zingiber officinale) powders at different concentrations on bioactive compounds, antioxidant activity, phenolic constituents, nutrients and sensory characteristics of wheat bread. Int. J. Gastron Food Sci. 2022, 28, 100532. [Google Scholar] [CrossRef]
- Popoola, O.O. Phenolic compounds composition and in vitro antioxidant activity of Nigerian Amaranthus viridis seed as affected by autoclaving and germination. Meas. Food 2022, 6, 100028. [Google Scholar] [CrossRef]
- Jaballah, S.B.; Zribi, I.; Haouala, R. Physiological and biochemical responses of two lentil varieties to chickpea (Cicer arietinum L.) aqueous extracts. Sci. Hortic. 2017, 225, 74–80. [Google Scholar] [CrossRef]
- Troadec, R.; Nestora, S.; Niquet-Léridon, C.; Marier, D.; Jacolot, P.; Sarron, E.; Regnault, S.; Anton, P.M.; Jouquand, C. Effect of leavening agent on Maillard reaction and the bifidogenic effect of traditional French bread. Food Chem. 2022, 393, 133387. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, R.C.; Fogliano, V. Bread crust melanoidins as potential prebiotic ingredients. Mol. Nutr. Food Res. 2005, 49, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Yaver, E.; Bilgiçli, N. Ultrasound-treated lupin (Lupinus albus L.) flour: Protein- and fiber-rich ingredient to improve physical and textural quality of bread with a reduced glycemic index. LWT 2021, 148, 111767. [Google Scholar] [CrossRef]
- Dueñas, M.; Sarmento, T.; Aguilera, Y.; Benitez, V.; Mollá, E.; Esteban, R.M.; Martín-Cabrejas, M.A. Impact of cooking and germination on phenolic composition and dietary fibre fractions in dark beans (Phaseolus vulgaris L.) and lentils (Lens culinaris L.). LWT Food Sci. Technol. 2016, 66, 72–78. [Google Scholar] [CrossRef]
- Guemes-Vera, N.; Pena-Bautista, R.J.; Jimenez-Martinez, C.; Davila Ortiz, G.; Calderon-Dominguez, G. Effective detoxification and decoloration of Lupinus mutabilis seed derivatives, and effect of these derivatives on bread quality and acceptance. J. Sci. Food Agric. 2008, 88, 1135–1143. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Zhao, Y.; Wang, D.; Wang, W. Influence of antioxidant dietary fiber on dough properties and bread qualities: A review. J. Funct. Foods 2021, 80, 104434. [Google Scholar] [CrossRef]
- Gao, K.; Liu, Y.X.; Tan, B.; Tian, X.H.; Zhang, D.Q.; Wang, L.P. An insight into the rheology and texture assessment: The influence of sprouting treatment on the whole wheat flour. Food Hydrocoll. 2022, 125, 107248. [Google Scholar] [CrossRef]
- Codina, G.G.; Marineac, A.R.; Todosi-Sanduleac, E. The influence of lupin flour addition on bread quality. Food Environ. Saf. 2016, 17, 216–226. [Google Scholar]
- Xin, T.; Tang, S.; Su, T.; Huang, Z.; Huang, F.; Zhang, R.; Dong, L.; Deng, M.; Shen, Y.; Su, D. Impact of replacing wheat flour with lychee juice by-products on bread quality characteristics and microstructure. LWT 2022, 165, 113696. [Google Scholar] [CrossRef]
- Ünal, A.; Subaşı, A.S.; Malkoç, S.; Ocak, I.; Korcan, S.E.; Yetilmezer, E.; Yurdugül, S.; Yaman, H.; Şanal, T.; Keçeli, A. Potential of fungal thermostable alpha amylase enzyme isolated from Hot springs of Central Anatolia (Turkey) in wheat bread quality. Food Biosci. 2022, 45, 101492. [Google Scholar] [CrossRef]
- Yıltırak, S.; Kocadağli, T.; Çelik, E.E.; Kanmaz, E.Ö.; Gökmen, V. Effects of sprouting and fermentation on the formation of Maillard reaction products in different cereals heated as wholemeal. Food Chem. 2022, 389, 133075. [Google Scholar] [CrossRef]
- Lemmens, E.; Deleu, L.J.; De Brier, N.; Smolders, E.; Delcour, J.A. Mineral bio-accessibility and intrinsic saccharides in breakfast flakes manufactured from sprouted wheat. LWT 2021, 143, 111079. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Investigation of the nutritional, functional and technological effects of the sourdough fermentation of sprouted flours. Int. J. Food Microbiol. 2019, 302, 47–58. [Google Scholar] [CrossRef]

| Samples | Creep Phase | Recovery Phase | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| JCo—105 (Pa−1) | JCm—105 (Pa−1) | λC (s) | μCo—10−6 (Pa—s) | Jmax—105 (Pa−1) | JRo—105 (Pa−1) | JRm—105 (Pa−1) | λR (s) | Jr—105 (Pa−1) | Jr/Jmax (%) | |
| Control | 6.93 e (0.02) | 20.00 b (0.01) | 34.99 a (0.05) | 0.57 a (0.00) | 24.76 a (0.02) | 8.66 c (0.04) | 8.32 d (0.00) | 34.36 a (0.00) | 16.98 d (0.04) | 68.57 a (0.14) |
| LGF_2.5 | 5.86 c (0.02) | 20.00 b (0.00) | 35.33 a (0.01) | 0.56 a (0.00) | 21.78 c (0.02) | 7.93 b (0.02) | 7.34 c (0.00) | 38.20 b (0.58) | 15.27 c (0.02) | 70.09 ab (0.01) |
| LGF_5.0 | 5.13 b (0.03) | 20.01 b (0.01) | 39.73 d (0.09) | 0.64 b (0.00) | 20.47 b (0.03) | 8.78 c (0.03) | 6.11 a (0.00) | 31.94 a (0.00) | 14.89 b (0.03) | 72.77 c (0.04) |
| LGF_7.5 | 4.45 a (0.26) | 10.00 a (0.00) | 37.30 c (0.25) | 0.75 c (0.02) | 16.49 a (0.03) | 5.29 a (0.30) | 6.24 b (0.06) | 40.22 b (2.01) | 11.52 a (0.24) | 69.90 ab (1.55) |
| LGF_10 | 6.60 d (0.02) | 20.00 b (0.00) | 36.45 b (0.16) | 0.58 a (0.01) | 24.49 d (0.03) | 10.00 d (0.00) | 7.35 c (0.00) | 34.01 a (0.00) | 17.35 e (0.00) | 70.85 b (0.09) |
| Bread Samples | Specific Volume (cm3/100 g) | Porosity (%) | Elasticity (%) |
|---|---|---|---|
| Control | 331.5 ± 0.74 a | 67.4 ± 0.86 a | 91.3 ± 0.57 b |
| LGF_2.5 | 351.2 ± 1.02 b | 72.8 ± 1.31 b | 93.5 ± 0.37 c |
| LGF_5.0 | 366.2 ± 0.98 c | 78.5 ± 0.66 d | 94.5 ± 0.45 c |
| LGF_7.5 | 375.0 ± 2.33 d | 79.8 ± 0.30 d | 95.8 ± 0.26 d |
| LGF_10 | 351.1 ± 1.05 b | 75.4 ± 0.53 b | 89.8 ± 0.58 a |
| Bread Samples | Crust Color | Crumb Color | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | L | a* | b* | |
| Control | 76.25 ± 0.94 c | 3.44 ± 0.27 a | 3.14 ± 0.43 a | 66.37 ± 0.88 c | −4.62 ± 0.32 d | 1.69 ± 0.22 a |
| LGF_2.5 | 59.08 ± 0.94 b | 12.15 ± 0.50 b | 4.35 ± 0.39 b | 59.01 ± 0.95 b | −3.30 ± 0.16 c | 1.75 ± 0.29 a |
| LGF_5.0 | 57.68 ± 0.50 b | 17.38 ± 0.08 c | 5.13 ± 0.04 b | 58.42 ± 0.53 b | −2.57 ± 0.43 b | 2.21 ± 0.11 a |
| LGF_7.5 | 53.91 ± 0.87 a | 18.08 ± 0.14 c | 6.43 ± 0.12 c | 54.48 ± 0.45 a | −1.18 ± 0.14 a | 3.18 ± 0.03 b |
| LGF_20 | 53.08 ± 0.62 a | 19.07 ± 0.22 d | 7.35 ± 0.37 d | 53.43 ± 0.50 a | −0.61 ± 0.07 a | 4.60 ± 0.23 c |
| Bread Samples | Firmness (N) | Gumminess (N) | Cohesiveness (Adimensional) | Resilience (Adimensional) |
|---|---|---|---|---|
| Control | 9.01 ± 3.06 a | 7.23 ± 1.73 a | 0.82 ± 0.03 c | 1.72 ± 0.04 c |
| LGF_2.5 | 12.60 ± 0.60 a | 9.31 ± 0.43 ab | 0.55 ± 0.13 b | 0.95 ± 0.09 b |
| LGF_5.0 | 14.63 ± 0.67 bc | 9.43 ± 0.11 b | 0.47 ± 0.07 ab | 0.89 ± 0.08 b |
| LGF_7.5 | 17.25 ± 0.44 c | 8.24 ± 0.11 ab | 0.38 ± 0.04 ab | 0.77 ± 0.15 ab |
| LGF_10 | 18.21 ± 0.17 c | 8.07 ± 0.08 ab | 0.34 ± 0.05 a | 0.54 ± 0.06 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atudorei, D.; Mironeasa, S.; Codină, G.G. Effects of Germinated Lentil Flour on Dough Rheological Behavior and Bread Quality. Foods 2022, 11, 2982. https://doi.org/10.3390/foods11192982
Atudorei D, Mironeasa S, Codină GG. Effects of Germinated Lentil Flour on Dough Rheological Behavior and Bread Quality. Foods. 2022; 11(19):2982. https://doi.org/10.3390/foods11192982
Chicago/Turabian StyleAtudorei, Denisa, Silvia Mironeasa, and Georgiana Gabriela Codină. 2022. "Effects of Germinated Lentil Flour on Dough Rheological Behavior and Bread Quality" Foods 11, no. 19: 2982. https://doi.org/10.3390/foods11192982
APA StyleAtudorei, D., Mironeasa, S., & Codină, G. G. (2022). Effects of Germinated Lentil Flour on Dough Rheological Behavior and Bread Quality. Foods, 11(19), 2982. https://doi.org/10.3390/foods11192982








