Effects of Hydroxypropyl Methylcellulose on Physicochemical Properties and Microstructure of κ-Carrageenan Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of κ-Carrageenan/HPMC Films
2.3. Rheological Measurement of Film-Forming Solutions
2.4. Water Resistance
2.5. Barrier Capacities
2.6. Optical Properties
2.7. Mechanical Properties
2.8. Thermogravimetric Analysis (TGA)
2.9. X-ray Diffraction (XRD) Analysis
2.10. ATR-FTIR Spectroscopy
2.11. Morphological Characterization
2.12. Statistical Analysis
3. Results and Discussion
3.1. Rheological Properties
3.2. Water Resistance
3.3. Barrier Properties
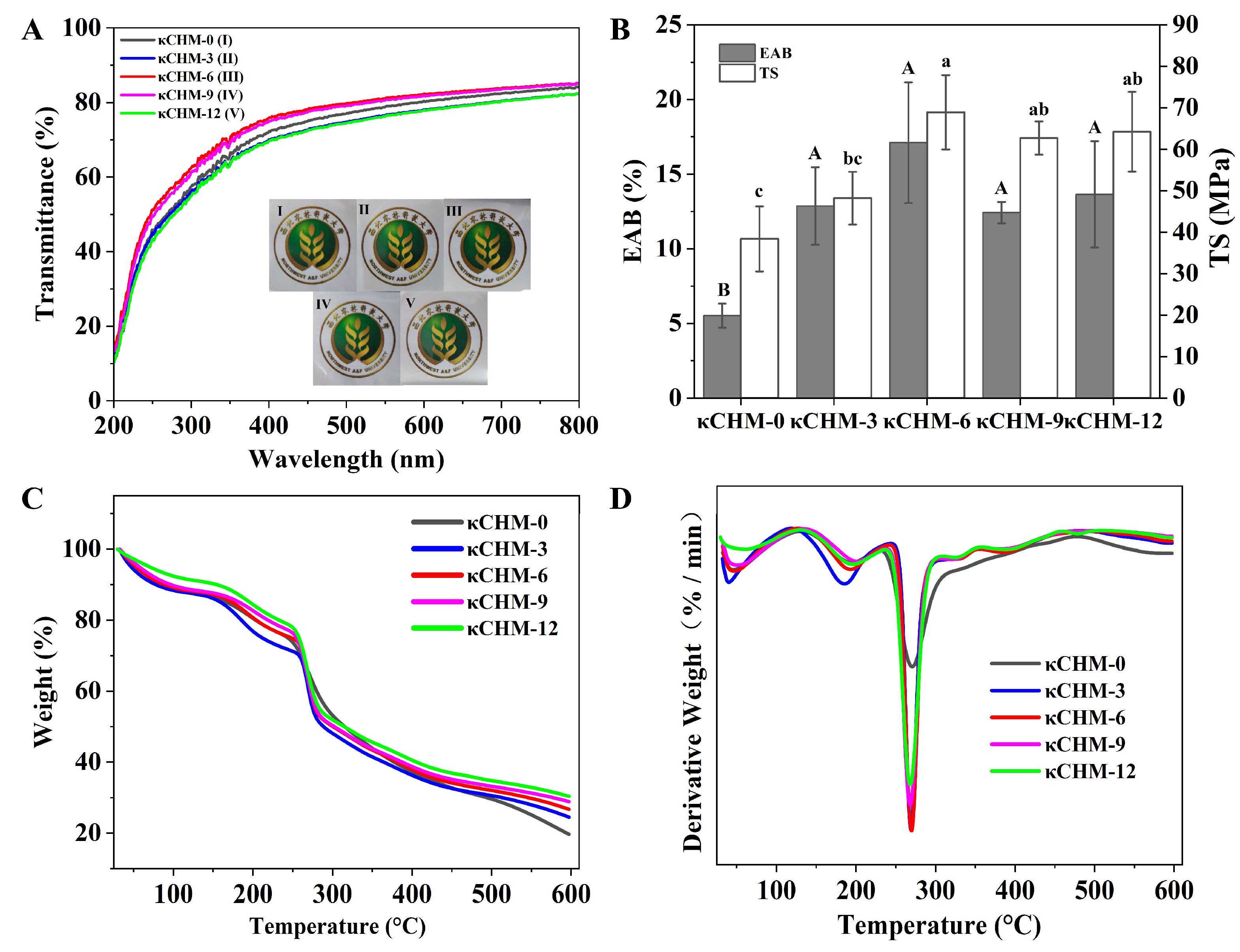
3.4. Optical Properties
3.5. Mechanical Properties
3.6. Thermal Stability
3.7. XRD Analysis
3.8. ATR-FTIR
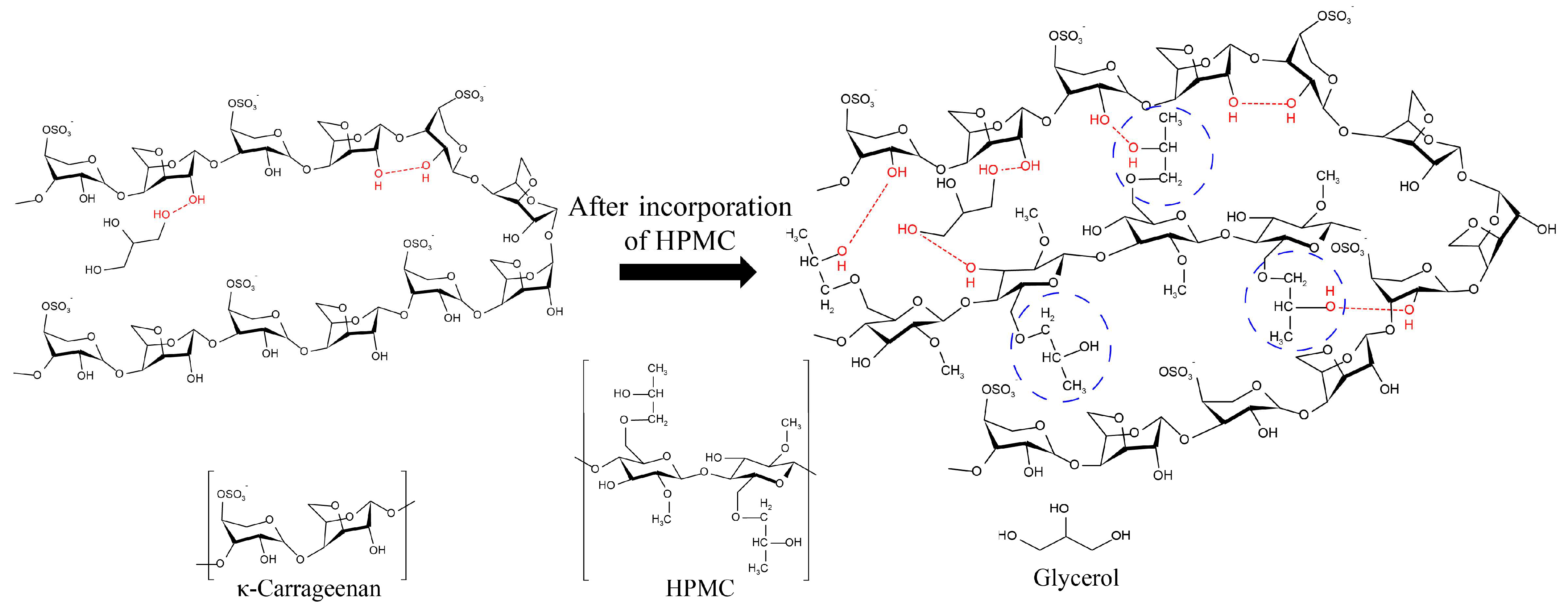
3.9. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basumatary, I.B.; Mukherjee, A.; Katiyar, V.; Kumar, S. Biopolymer-based nanocomposite films and coatings: Recent advances in shelf-life improvement of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2020, 62, 1912–1935. [Google Scholar] [CrossRef] [PubMed]
- Ünalan, İ.U.; Arcan, I.; Korel, F.; Yemenicioğlu, A. Application of active zein-based films with controlled release properties to control Listeria monocytogenes growth and lipid oxidation in fresh Kashar cheese. Innov. Food Sci. Emerg. Technol. 2013, 20, 208–214. [Google Scholar] [CrossRef]
- Wang, C.; Gong, C.; Qin, Y.; Hu, Y.; Jiao, A.; Jin, Z.; Qiu, C.; Wang, J. Bioactive and functional biodegradable packaging films reinforced with nanoparticles. J. Food Eng. 2022, 312, 110752. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive Review of Polysaccharide-Based Materials in Edible Packaging: A Sustainable Approach. Foods 2021, 10, 1845. [Google Scholar] [CrossRef] [PubMed]
- Nur Fatin Nazurah, R.; Nur Hanani, Z.A. Physicochemical characterization of kappa-carrageenan (Euchema cottoni) based films incorporated with various plant oils. Carbohydr. Polym. 2017, 157, 1479–1487. [Google Scholar] [CrossRef]
- Wibowo, A.H.; Listiyawati, O.; Purnawan, C. The Effects of plasticizers and palmitic acid toward the properties of the carrageenan Film. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012043. [Google Scholar] [CrossRef]
- Ye, Z.; Ma, P.; Tang, M.; Li, X.; Zhang, W.; Hong, X.; Chen, X.; Chen, D. Interactions between calcium alginate and carrageenan enhanced mechanical property of a natural composite film for general packaging application. Polym. Bull. 2017, 74, 3421–3429. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Hilliou, L.; Lagaron, J.M. Nanobiocomposites of Carrageenan, Zein, and Mica of Interest in Food Packaging and Coating Applications. J. Agric. Food Chem. 2010, 58, 6884–6894. [Google Scholar] [CrossRef]
- Soni, A.; Kandeepan, G.; Mendiratta, S.K.; Shukla, V.; Kumar, A. Development and characterization of essential oils incorporated carrageenan based edible film for packaging of chicken patties. Nutr. Food Sci. 2016, 46, 82–95. [Google Scholar] [CrossRef]
- Huang, J.; Chen, M.; Zhou, Y.; Li, Y.; Hu, Y. Functional characteristics improvement by structural modification of hydroxypropyl methylcellulose modified polyvinyl alcohol films incorporating roselle anthocyanins for shrimp freshness monitoring. Int. J. Biol. Macromol. 2020, 162, 1250–1261. [Google Scholar] [CrossRef]
- Brindle, L.P.; Krochta, J.M. Physical Properties of Whey Protein–Hydroxypropylmethylcellulose Blend Edible Films. J. Food Sci. 2008, 73, E446–E454. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Liang, T.; Tan, W.; Wang, L. Rheological behaviors and physical properties of plasticized hydrogel films developed from κ-carrageenan incorporating hydroxypropyl methylcellulose. Food Hydrocoll. 2018, 85, 61–68. [Google Scholar] [CrossRef]
- Sun, G.; Chi, W.; Xu, S.; Wang, L. Developing a simultaneously antioxidant and pH-responsive κ-carrageenan/hydroxypropyl methylcellulose film blended with Prunus maackii extract. Int. J. Biol. Macromol. 2020, 155, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Chi, W.; Zhang, C.; Xu, S.; Li, J.; Wang, L. Developing a green film with pH-sensitivity and antioxidant activity based on κ-carrageenan and hydroxypropyl methylcellulose incorporating Prunus maackii juice. Food Hydrocoll. 2019, 94, 345–353. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, G.; Li, J.; Wang, L. A green strategy for maintaining intelligent response and improving antioxidant properties of κ-carrageenan-based film via cork bark extractive addition. Food Hydrocoll. 2021, 113, 106470. [Google Scholar] [CrossRef]
- Chi, W.; Cao, L.; Sun, G.; Meng, F.; Zhang, C.; Li, J.; Wang, L. Developing a highly pH-sensitive ĸ-carrageenan-based intelligent film incorporating grape skin powder via a cleaner process. J. Clean. Prod. 2020, 244, 118862. [Google Scholar] [CrossRef]
- Jin, Y.; Li, F.; Lou, X.; Xiao, Y.; Wang, X.; Liu, F.; Wang, J.; Xu, H. Evaluation of the encapsulation capacity of nervous acid in nanoemulsions obtained with natural and ethoxylated surfactants. J. Mol. Liq. 2021, 343, 117632. [Google Scholar] [CrossRef]
- Jancikova, S.; Dordevic, D.; Jamroz, E.; Behalova, H.; Tremlova, B. Chemical and Physical Characteristics of Edible Films, Based on κ- and ι-Carrageenans with the Addition of Lapacho Tea Extract. Foods 2020, 9, 357. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Kang, S.; Wang, K.; Xu, H. Development and evaluation of soy protein isolate-based antibacterial nanocomposite films containing cellulose nanocrystals and zinc oxide nanoparticles. Food Hydrocoll. 2020, 106, 105898. [Google Scholar] [CrossRef]
- Ben Shalom, T.; Belsey, S.; Chasnitsky, M.; Shoseyov, O. Cellulose Nanocrystals and Corn Zein Oxygen and Water Vapor Barrier Biocomposite Films. Nanomaterials 2021, 11, 247. [Google Scholar] [CrossRef]
- GB/T 1038-2000; Plastics-Film and Sheeting-Determination of Gas Transmission-Differential Pressure Method. ISO: Geneva, Switzerland, 2000.
- Wu, J.; Sun, Q.; Huang, H.; Duan, Y.; Xiao, G.; Le, T. Enhanced physico-mechanical, barrier and antifungal properties of soy protein isolate film by incorporating both plant-sourced cinnamaldehyde and facile synthesized zinc oxide nanosheets. Colloids Surf. B 2019, 180, 31–38. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Kang, S.; Xu, H. Insight into the formation mechanism of soy protein isolate films improved by cellulose nanocrystals. Food Chem. 2021, 359, 129971. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Xiao, Y.; Guo, X.; Huang, A.; Xu, H. Development of gum arabic-based nanocomposite films reinforced with cellulose nanocrystals for strawberry preservation. Food Chem. 2021, 350, 129199. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, D.; Oltrogge, K.; Hamidi-Esfahani, Z. Preparation and characterization of blended edible films manufactured using gelatin, tragacanth gum and, Persian gum. LWT 2020, 117, 108617. [Google Scholar] [CrossRef]
- Ni, Y.; Nie, H.; Wang, J.; Lin, J.; Wang, Q.; Sun, J.; Zhang, W.; Wang, J. Enhanced functional properties of chitosan films incorporated with curcumin-loaded hollow graphitic carbon nitride nanoparticles for bananas preservation. Food Chem. 2022, 366, 130539. [Google Scholar] [CrossRef] [PubMed]
- da Nóbrega Santos, E.; Cesar de Albuquerque Sousa, T.; Cassiano de Santana Neto, D.; Brandão Grisi, C.V.; Cardoso da Silva Ferreira, V.; Pereira da Silva, F.A. Edible active film based on gelatin and Malpighia emarginata waste extract to inhibit lipid and protein oxidation in beef patties. LWT 2022, 154, 112837. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; Lopez-Sanchez, P.; Yang, X. Characterizations of bacterial cellulose nanofibers reinforced edible films based on konjac glucomannan. Int. J. Biol. Macromol. 2020, 145, 634–645. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Han, X.; Sun, Y.; Wang, X. Effect of ethanol content on rheology of film-forming solutions and properties of zein/chitosan film. Int. J. Biol. Macromol. 2019, 134, 807–814. [Google Scholar] [CrossRef]
- Ni, X.; Wang, K.; Wu, K.; Corke, H.; Nishinari, K.; Jiang, F. Stability, microstructure and rheological behavior of konjac glucomannan-zein mixed systems. Carbohydr. Polym. 2018, 188, 260–267. [Google Scholar] [CrossRef]
- Cao, L.; Ge, T.; Meng, F.; Xu, S.; Li, J.; Wang, L. An edible oil packaging film with improved barrier properties and heat sealability from cassia gum incorporating carboxylated cellulose nano crystal whisker. Food Hydrocoll. 2020, 98, 105251. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Zhang, L.; Wang, X.; Li, L. Effects of plasticizer type and concentration on rheological, physico-mechanical and structural properties of chitosan/zein film. Int. J. Biol. Macromol. 2020, 143, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yuan, C.; Cui, B.; Sha, H.; Liu, P.; Lu, L.; Wu, Z. High-Amylose Corn Starch/Konjac Glucomannan Composite Film: Reinforced by Incorporating beta-Cyclodextrin. J. Agric. Food Chem. 2021, 69, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Preparation of carrageenan-based functional nanocomposite films incorporated with melanin nanoparticles. Colloids Surf. B 2019, 176, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, R.; Kim, S.S.; Lee, J. Novel synergistic transparent k-Carrageenan/Xanthan gum/Gellan gum hydrogel film: Mechanical, thermal and water barrier properties. Int. J. Biol. Macromol. 2018, 118 Pt A, 561–568. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Wang, X.; Dong, S.; Sun, Y.; Zhao, Z. The properties of chitosan/zein blend film and effect of film on quality of mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2019, 155, 47–56. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Souza, B.W.S.; Vicente, A.A. Synergistic effects between κ-carrageenan and locust bean gum on physicochemical properties of edible films made thereof. Food Hydrocoll. 2012, 29, 280–289. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Carrageenan-based antimicrobial bionanocomposite films incorporated with ZnO nanoparticles stabilized by melanin. Food Hydrocoll. 2019, 90, 500–507. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Edible films and coatings to prevent the detrimental effect of oxygen on food quality: Possibilities and limitations. J. Food Eng. 2012, 110, 208–213. [Google Scholar] [CrossRef]
- Yoo, S.; Krochta, J.M. Whey protein–polysaccharide blended edible film formation and barrier, tensile, thermal and transparency properties. J. Sci. Food Agric. 2011, 91, 2628–2636. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Weng, Y.-M. Novel edible composite films fabricated with whey protein isolate and zein: Preparation and physicochemical property evaluation. LWT 2019, 101, 567–574. [Google Scholar] [CrossRef]
- Kassab, Z.; Aziz, F.; Hannache, H.; Ben Youcef, H.; El Achaby, M. Improved mechanical properties of k-carrageenan-based nanocomposite films reinforced with cellulose nanocrystals. Int. J. Biol. Macromol. 2019, 123, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, D.; Zhang, J.; Li, J.; Lai, D.; Lin, S.; Hu, J. Preparation and Characterization of Biodegradable κ-Carrageenan Based Anti-Bacterial Film Functionalized with Wells-Dawson Polyoxometalate. Foods 2022, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Rhim, J.-W. Development and characterization of carrageenan/grapefruit seed extract composite films for active packaging. Int. J. Biol. Macromol. 2014, 68, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Gu, C.; Zhu, D.; Huang, Y.; Luo, Y.; Zhou, Q. Development and characterization of an edible chitosan/zein-cinnamaldehyde nano-cellulose composite film and its effects on mango quality during storage. LWT 2021, 140, 110809. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Carrageenan-based hydrogels and films: Effect of ZnO and CuO nanoparticles on the physical, mechanical, and antimicrobial properties. Food Hydrocoll. 2017, 67, 45–53. [Google Scholar] [CrossRef]
- Avila, L.B.; Barreto, E.R.; Moraes, C.C.; Morais, M.M.; Rosa, G.S. Promising New Material for Food Packaging: An Active and Intelligent Carrageenan Film with Natural Jaboticaba Additive. Foods 2022, 11, 792. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Vasilieva, N.Y.; Borovkova, V.S.; Fetisova, O.Y.; Issaoui, N.; Malyar, Y.N.; Elsuf’ev, E.V.; Karacharov, A.A.; Skripnikov, A.M.; Miroshnikova, A.V.; et al. Food Xanthan Polysaccharide Sulfation Process with Sulfamic Acid. Foods 2021, 10, 2571. [Google Scholar] [CrossRef]
- Bayer, G.; Shayganpour, A.; Zia, J.; Bayer, I.S. Polyvinyl alcohol-based films plasticized with an edible sweetened gel enriched with antioxidant carminic acid. J. Food Eng. 2022, 323, 111000. [Google Scholar] [CrossRef]



| Samples | Thickness (μm) | MC (%) | WS (%) | WCA (°) | WVP (10−11g·Pa−1·s−1·m−1) | OP (cm3/m2·24 h·0.1·MPa) |
|---|---|---|---|---|---|---|
| κCHM-0 | 39 ± 4 a | 18.56 ± 2.90 a | 10.40 ± 1.25 a | 39.5 ± 1.9 c | 8.73 ± 0.16 b | 0.228 |
| κCHM-3 | 39 ± 4 a | 17.23 ± 3.55 a | 10.18 ± 0.94 ab | 43.4 ± 2.5 c | 9.41 ± 0.37 a | 0.122 |
| κCHM-6 | 38 ± 4 a | 16.51 ± 2.51 a | 8.43 ± 0.99 bc | 88.6 ± 2.8 a | 7.71 ± 0.12 c | 0.123 |
| κCHM-9 | 38 ± 5 a | 17.31 ± 2.37 a | 7.62 ± 0.48 c | 79.8 ± 8.1 ab | 8.48 ± 0.17 b | 0.131 |
| κCHM-12 | 41 ± 4 a | 16.44 ± 1.87 a | 6.82 ± 0.63 c | 69.6 ± 6.7 b | 9.52 ± 0.04 a | 0.164 |
| Samples | L* | a* | b* | ΔE | Opacity (A/mm) |
|---|---|---|---|---|---|
| κCHM-0 | 97.15 ± 0.11 ab | −0.02 ± 0.01 ab | 0.54 ± 0.04 b | 0.86 ± 0.08 abc | 2.39 ± 0.13 a |
| κCHM-3 | 97.36 ± 0.24 a | 0.00 ± 0.02 a | 0.54 ± 0.12 b | 1.04 ± 0.13 a | 2.58 ± 0.41 a |
| κCHM-6 | 96.93 ± 0.09 b | −0.03 ± 0.01 b | 0.59 ± 0.03 ab | 0.79 ± 0.01 c | 2.27 ± 0.35 a |
| κCHM-9 | 96.97 ± 0.12 b | −0.03 ± 0.00 b | 0.62 ± 0.04 ab | 0.83 ± 0.07 bc | 2.39 ± 0.15 a |
| κCHM-12 | 97.19 ± 0.08 ab | −0.02 ± 0.02 ab | 0.70 ± 0.07 a | 1.01 ± 0.10 ab | 2.69 ± 0.86 a |
| Samples | Rq (nm) | Ra (nm) |
|---|---|---|
| κCHM-0 | 32.33 ± 4.74 a | 25.40 ± 3.73 a |
| κCHM-3 | 23.67 ± 4.38 b | 18.90 ± 3.08 b |
| κCHM-6 | 21.20 ± 0.72 b | 17.10 ± 0.69 b |
| κCHM-9 | 19.97 ± 1.14 b | 13.93 ± 0.66 b |
| κCHM-12 | 7.78 ± 1.55 c | 6.04 ± 1.10 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Dong, S.; Ye, M.; Wu, X.; Lv, X.; Xu, H.; Li, M. Effects of Hydroxypropyl Methylcellulose on Physicochemical Properties and Microstructure of κ-Carrageenan Film. Foods 2022, 11, 3023. https://doi.org/10.3390/foods11193023
Guo J, Dong S, Ye M, Wu X, Lv X, Xu H, Li M. Effects of Hydroxypropyl Methylcellulose on Physicochemical Properties and Microstructure of κ-Carrageenan Film. Foods. 2022; 11(19):3023. https://doi.org/10.3390/foods11193023
Chicago/Turabian StyleGuo, Jintao, Shuting Dong, Mengyu Ye, Xuan Wu, Xin Lv, Huaide Xu, and Mei Li. 2022. "Effects of Hydroxypropyl Methylcellulose on Physicochemical Properties and Microstructure of κ-Carrageenan Film" Foods 11, no. 19: 3023. https://doi.org/10.3390/foods11193023
APA StyleGuo, J., Dong, S., Ye, M., Wu, X., Lv, X., Xu, H., & Li, M. (2022). Effects of Hydroxypropyl Methylcellulose on Physicochemical Properties and Microstructure of κ-Carrageenan Film. Foods, 11(19), 3023. https://doi.org/10.3390/foods11193023






