Incorporation of Blue Honeysuckle Juice into Fermented Goat Milk: Physicochemical, Sensory and Antioxidant Characteristics and In Vitro Gastrointestinal Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of FGM Supplemented with Blue Honeysuckle Juice
2.3. Determination of pH, WHC and Viscosity
2.4. Color Measurement
2.5. Total Polyphenol Contents and Antioxidant Properties
2.6. Microstructure Analysis
2.7. Sensory Evaluation
2.8. In Vitro Gastrointestinal Digestion
2.9. Statistical Analysis
3. Results and Discussions
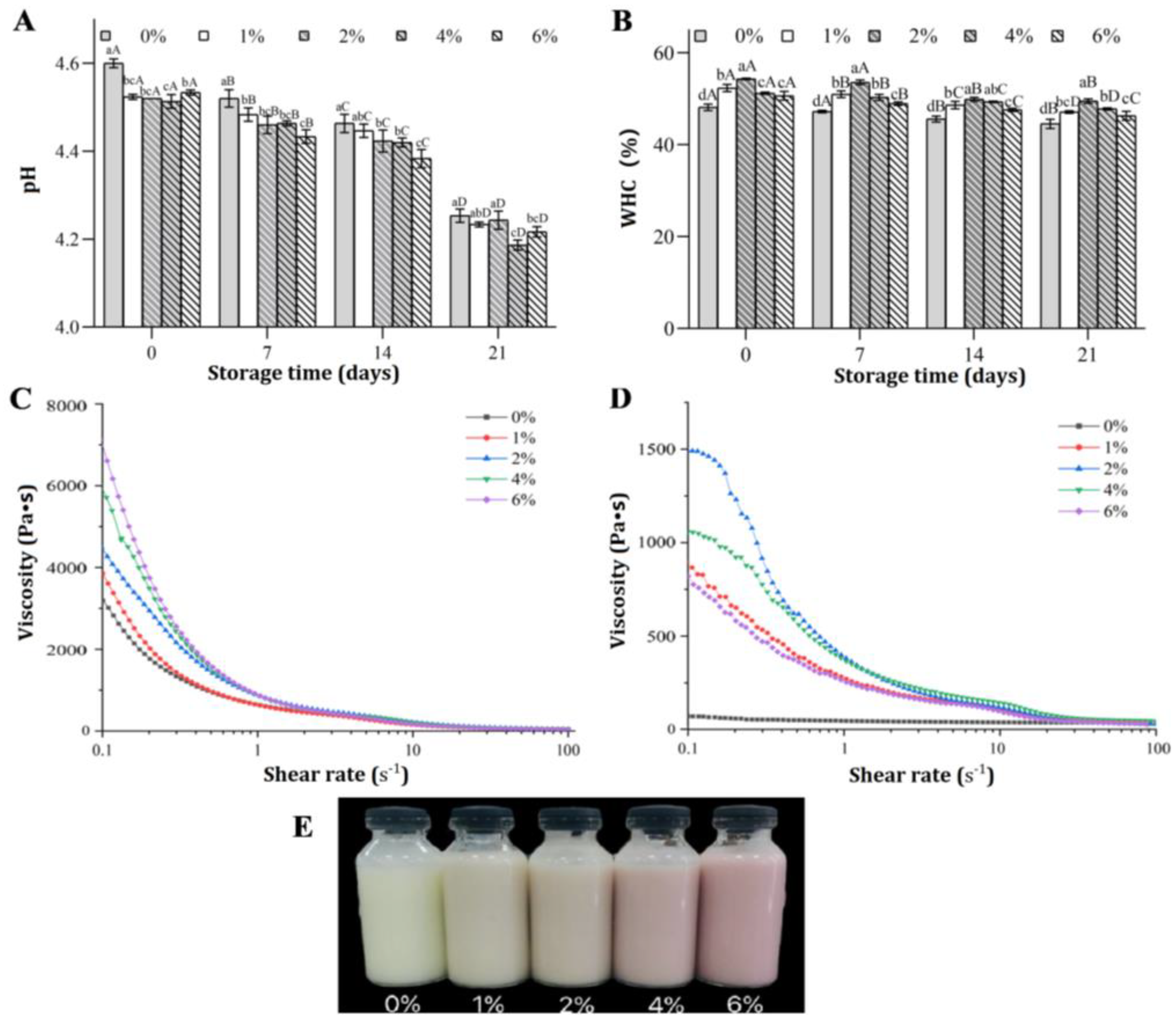
3.1. Physicochemical Properties of FGM Supplemented with BHJ during Storage
3.2. Color of FGM Supplemented with BHJ during Storage
3.3. Total Phenolic Content and Antioxidant Activity of FGM Supplemented with BHJ during Storage
3.4. Sensory Analysis of FGM Supplemented with BHJ
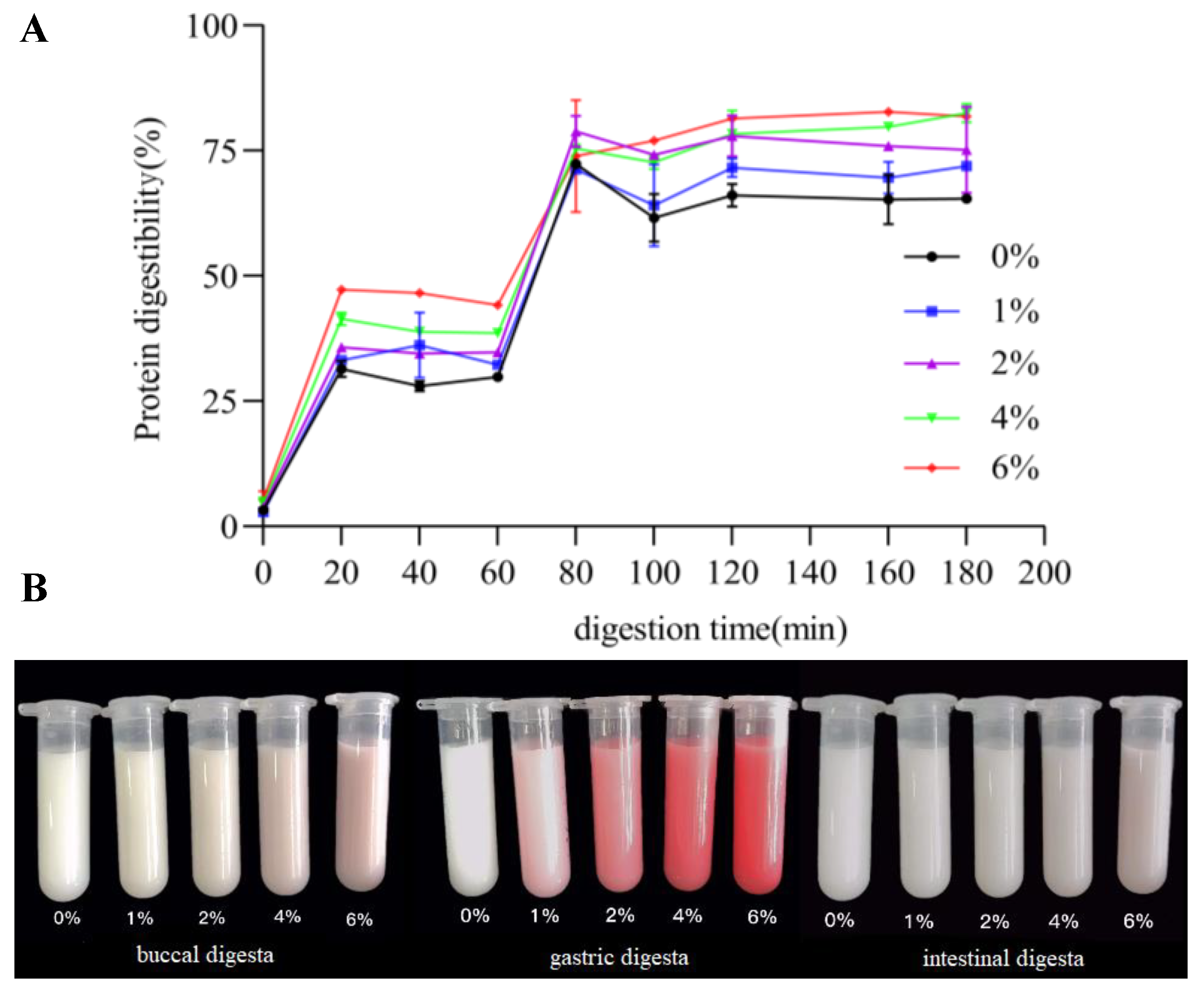
3.5. Protein Digestibility of FGM Supplemented with BHJ during Simulated In Vitro Digestion
3.6. Microstructure of FGM Supplemented with BHJ during Storage and In Vitro Digestion
3.7. TPC and Antioxidant Activity of FGM Supplemented with BHJ during In Vitro Digestion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, C.; Ma, J.; Liu, Z.; Wang, W.; Liu, X.; Qian, S.; Chen, L.; Gu, L.; Sun, C.; Hou, J.; et al. Preparation of shell-core fiber-encapsulated Lactobacillus rhamnosus 1.0320 using coaxial electrospinning. Food Chem. 2022, 402, 134253. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Du, A.; Fan, Z.; Shi, L. Novel insight into the transformation of peptides and potential benefits in brown fermented goat milk by mesoporous magnetic dispersive solid phase extraction-based peptidomics. Food Chem. 2022, 389, 133110. [Google Scholar] [CrossRef] [PubMed]
- Sakandar, H.A.; Zhang, H. Trends in Probiotic(s)-Fermented milks and their in vivo functionality: A review. Trends Food Sci. Technol. 2021, 110, 55–65. [Google Scholar] [CrossRef]
- Alqahtani, N.K.; Darwish, A.A.; El-Menawy, R.K.; Alnemr, T.M.; Aly, E. Textural and organoleptic attributes and antioxidant activity of goat milk yoghurt with added oat flour. Int. J. Food Prop. 2021, 24, 433–445. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.N.; Guo, M.R. Effects of addition of strawberry juice pre- or postfermentation on physiochemical and sensory properties of fermented goat milk. J. Dairy Sci. 2019, 102, 4978–4988. [Google Scholar] [CrossRef]
- Cais-Sokolinska, D.; Walkowiak-Tomczak, D. Consumer-perception, nutritional, and functional studies of a yogurt with restructured elderberry juice. J. Dairy Sci. 2021, 104, 1318–1335. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Mujumdar, A.S.; Chang, L. Effect of edible rose (Rosa rugosa cv. Plena) flower extract addition on the physicochemical, rheological, functional and sensory properties of set-type yogurt. Food Biosci. 2021, 43, 101249. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Huang, Z.; Enomoto, T.; Huang, L.; Li, L. Bioactive properties of probiotic set-yogurt supplemented with Siraitia grosvenorii fruit extract. Food Chem. 2020, 303, 125400. [Google Scholar] [CrossRef]
- Ma, C.; Bai, J.; Shao, C.; Liu, J.; Zhang, Y.; Li, X.; Yang, Y.; Xu, Y.; Wang, L. Degradation of blue honeysuckle polysaccharides, structural characteristics and antiglycation and hypoglycemic activities of degraded products. Food Res. Int. 2021, 143, 110281. [Google Scholar] [CrossRef]
- Oszmianski, J.; Kucharska, A.Z. Effect of pre-treatment of blue honeysuckle berries on bioactive iridoid content. Food Chem. 2018, 240, 1087–1091. [Google Scholar] [CrossRef]
- Li, Y.; He, D.; Li, B.; Lund, M.N.; Xing, Y.; Wang, Y.; Li, F.; Cao, X.; Liu, Y.; Chen, X.; et al. Engineering polyphenols with biological functions via polyphenol-protein interactions as additives for functional foods. Trends Food Sci. Technol. 2021, 110, 470–482. [Google Scholar] [CrossRef]
- Silva, F.A.; Queiroga, R.; de Souza, E.L.; Voss, G.B.; Borges, G.; Lima, M.D.S.; Pintado, M.M.E.; Vasconcelos, M. Incorporation of phenolic-rich ingredients from integral valorization of Isabel grape improves the nutritional, functional and sensory characteristics of probiotic goat milk yogurt. Food Chem. 2022, 369, 130957. [Google Scholar] [CrossRef] [PubMed]
- Szołtysik, M.; Kucharska, A.Z.; Dąbrowska, A.; Zięba, T.; Bobak, Ł.; Chrzanowska, J. Effect of two combined functional additives on yoghurt properties. Foods 2021, 10, 1159. [Google Scholar] [CrossRef] [PubMed]
- Segliņa, D.; Krasnova, I.; Alsiņa, S. Lonicera caerulea L. as a source of biologically active compounds for the enrichment of fermented milk product. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2021, 75, 449–456. [Google Scholar] [CrossRef]
- Li, J.; Fu, J.; Ma, Y.; He, Y.; Fu, R.; Qayum, A.; Jiang, Z.; Wang, L. Low temperature extrusion promotes transglutaminase cross-linking of whey protein isolate and enhances its emulsifying properties and water holding capacity. Food Hydrocoll. 2022, 125. [Google Scholar] [CrossRef]
- Ma, J.; Xu, C.; Yu, H.; Feng, Z.; Yu, W.; Gu, L.; Liu, Z.; Chen, L.; Jiang, Z.; Hou, J. Electro-encapsulation of probiotics in gum Arabic-pullulan blend nanofibres using electrospinning technology. Food Hydrocoll. 2021, 111, 106381. [Google Scholar] [CrossRef]
- Öztürk, H.İ.; Aydın, S.; Sözeri, D.; Demirci, T.; Sert, D.; Akın, N. Fortification of set-type yoghurts with Elaeagnus angustifolia L. flours: Effects on physicochemical, textural, and microstructural characteristics. LWT-Food Sci. Technol. 2018, 90, 620–626. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, J.; Zhao, X.; Sun, R.; Sun, C.; Hou, D.; Zhang, X.; Jiang, L.; Hou, J.; Jiang, Z. Oil bodies extracted from high-oil soybeans (Glycine max) exhibited higher oxidative and physical stability than oil bodies from high-protein soybeans. Food Funct. 2022, 13, 2042–6496. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, X.; Shi, L.; Ni, C.; Hou, J.; Cheng, J. Enhancement of soluble protein, polypeptide production and functional properties of heat-denatured soybean meal by fermentation of Monascus purpureus 04093. CyTA-J. Food 2019, 17, 1014–1022. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Zhao, J.; Yu, R.; Altaf Hussain, M.; Qayum, A.; Jiang, Z.; Qu, B. Glycosylated whey protein isolate enhances digestion behaviors and stabilities of conjugated linoleic acid oil in water emulsions. Food Chem. 2022, 383, 132402. [Google Scholar] [CrossRef]
- Wang, W.; Wang, M.; Xu, C.; Liu, Z.; Gu, L.; Ma, J.; Jiang, L.; Jiang, Z.; Hou, J. Effects of soybean oil body as a milk fat substitute on ice cream: Physicochemical, sensory and digestive properties. Foods 2022, 11, 1504. [Google Scholar] [CrossRef] [PubMed]
- Ardabilchi Marand, M.; Amjadi, S.; Ardabilchi Marand, M.; Roufegarinejad, L.; Jafari, S.M. Fortification of yogurt with flaxseed powder and evaluation of its fatty acid profile, physicochemical, antioxidant, and sensory properties. Powder Technol. 2020, 359, 76–84. [Google Scholar] [CrossRef]
- Helal, A.; Tagliazucchi, D. Impact of in-vitro gastro-pancreatic digestion on polyphenols and cinnamaldehyde bioaccessibility and antioxidant activity in stirred cinnamon-fortified yogurt. LWT-Food Sci. Technol. 2018, 89, 164–170. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Li, T.; Gantumur, M.-A.; Qayum, A.; Bilawal, A.; Jiang, Z.; Wang, L. Non-covalent interaction and digestive characteristics between α-lactalbumin and safflower yellow: Impacts of microwave heating temperature. LWT-Food Sci. Technol. 2022, 159, 113206. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, M. Physical and chemical modification of SPI as a potential means to enhance small peptide contents and antioxidant activity found in hydrolysates. Innov. Food Sci. Emerg. Technol. 2010, 11, 677–683. [Google Scholar] [CrossRef]
- Feng, C.; Wang, B.; Zhao, A.; Wei, L.; Shao, Y.; Wang, Y.; Cao, B.; Zhang, F. Quality characteristics and antioxidant activities of goat milk yogurt with added jujube pulp. Food Chem. 2019, 277, 238–245. [Google Scholar] [CrossRef]
- Kwon, H.C.; Bae, H.; Seo, H.G.; Han, S.G. Short communication: Chia seed extract enhances physiochemical and antioxidant properties of yogurt. J. Dairy Sci. 2019, 102, 4870–4876. [Google Scholar] [CrossRef]
- Trigueros, L.; Wojdylo, A.; Sendra, E. Antioxidant activity and protein-polyphenol interactions in a pomegranate (Punica granatum L.) yogurt. J. Agric. Food Chem. 2014, 62, 6417–6425. [Google Scholar] [CrossRef]
- Zannini, E.; Jeske, S.; Lynch, K.M.; Arendt, E.K. Development of novel quinoa-based yoghurt fermented with dextran producer Weissella cibaria MG1. Int. J. Food Microbiol. 2018, 268, 19–26. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Zhou, J.; Wadhwa, S.S. Drinking yoghurts with berry polyphenols added before and after fermentation. Food Control 2013, 32, 450–460. [Google Scholar] [CrossRef]
- Mohamed Ahmed, I.A.; Alqah, H.A.S.; Saleh, A.; Al-Juhaimi, F.Y.; Babiker, E.E.; Ghafoor, K.; Hassan, A.B.; Osman, M.A.; Fickak, A. Physicochemical quality attributes and antioxidant properties of set-type yogurt fortified with argel (Solenostemma argel Hayne) leaf extract. LWT-Food Sci. Technol. 2021, 137, 110389. [Google Scholar] [CrossRef]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Rawdkuen, S.; Faseha, A.; Benjakul, S.; Kaewprachu, P. Application of anthocyanin as a color indicator in gelatin films. Food Biosci. 2020, 36, 100603. [Google Scholar] [CrossRef]
- Anuyahong, T.; Chusak, C.; Adisakwattana, S. Incorporation of anthocyanin-rich riceberry rice in yogurts: Effect on physicochemical properties, antioxidant activity and in vitro gastrointestinal digestion. LWT-Food Sci. Technol. 2020, 129, 109571. [Google Scholar] [CrossRef]
- Gris, C.C.T.; Frota, E.G.; Guarienti, C.; Vargas, B.K.; Gutkoski, J.P.; Biduski, B.; Bertolin, T.E. In vitro digestibility and stability of encapsulated yerba mate extract and its impact on yogurt properties. J. Food Meas. Charact. 2021, 15, 2000–2009. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y.L. Interaction of whey proteins with phenolic derivatives under neutral and acidic pH conditions. J. Food Sci. 2017, 82, 409–419. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Z.; Zhao, J.; Liu, Y. The effect of non-covalent interaction of chlorogenic acid with whey protein and casein on physicochemical and radical-scavenging activity of in vitro protein digests. Food Chem. 2018, 268, 334–341. [Google Scholar] [CrossRef]
- Zhou, S.D.; Lin, Y.F.; Xu, X.; Meng, L.; Dong, M.S. Effect of non-covalent and covalent complexation of (-)-epigallocatechin gallate with soybean protein isolate on protein structure and in vitro digestion characteristics. Food Chem. 2020, 309, 125718. [Google Scholar] [CrossRef]
- Pelaes Vital, A.C.; Goto, P.A.; Hanai, L.N.; Gomes-da-Costa, S.M.; de Abreu Filho, B.A.; Nakamura, C.V.; Matumoto-Pintro, P.T. Microbiological, functional and rheological properties of low fat yogurt supplemented with Pleurotus ostreatus aqueous extract. LWT-Food Sci. Technol. 2015, 64, 1028–1035. [Google Scholar] [CrossRef]
- Peterson, R.B.; Rankin, S.A.; Ikeda, S. Short communication: Stabilization of milk proteins at pH 5.5 using pectic polysaccharides derived from potato tubers. J. Dairy Sci. 2019, 102, 8691–8695. [Google Scholar] [CrossRef]
- Krzeminski, A.; Großhable, K.; Hinrichs, J. Structural properties of stirred yoghurt as influenced by whey proteins. LWT-Food Sci. Technol. 2011, 44, 2134–2140. [Google Scholar] [CrossRef]
- Pan, L.; Liu, F.; Luo, S.; Luo, J. Pomegranate juice powder as sugar replacer enhanced quality and function of set yogurts: Structure, rheological property, antioxidant activity and in vitro bioaccessibility. LWT-Food Sci. Technol. 2019, 115, 108479. [Google Scholar] [CrossRef]
- Zygmantaitė, G.; Keršienė, M.; Jasutienė, I.; Šipailienė, A.; Venskutonis, P.R.; Leskauskaitė, D. Extract isolated from cranberry pomace as functional ingredient in yoghurt production: Technological properties and digestibility studies. LWT-Food Sci. Technol. 2021, 148, 111751. [Google Scholar] [CrossRef]
- Paz-Yépez, C.; Peinado, I.; Heredia, A.; Andrés, A. Lipids digestibility and polyphenols release under in vitro digestion of dark, milk and white chocolate. J. Funct. Foods 2019, 52, 196–203. [Google Scholar] [CrossRef]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Lafarga, T.; Rodriguez-Roque, M.J.; Bobo, G.; Villaro, S.; Aguilo-Aguayo, I. Effect of ultrasound processing on the bioaccessibility of phenolic compounds and antioxidant capacity of selected vegetables. Food Sci. Biotechnol. 2019, 28, 1713–1721. [Google Scholar] [CrossRef]



| Color Parameters | Storage Time (Days) | 0% (Control) | 1% BHJ | 2% BHJ | 4% BHJ | 6% BHJ |
|---|---|---|---|---|---|---|
| L* | 0 | 86.09 ± 0.17 aA | 82.49 ± 0.12 bA | 82.77 ± 0.21 cA | 81.68 ± 0.38 dA | 77.90 ± 0.33 eA |
| 7 | 86.33 ± 0.18 aA | 82.47 ± 0.19 bA | 82.41 ± 0.24 cA | 81.10 ± 0.76 dA | 77.45 ± 0.08 eA | |
| 14 | 86.47 ± 0.38 aA | 82.85 ± 0.79 bA | 82.81 ± 0.12 cA | 81.50 ± 0.11 dA | 77.90 ± 0.31 eA | |
| 21 | 86.32 ± 0.11 aA | 82.54 ± 0.08 bA | 82.26 ± 0.17 cA | 81.82 ± 0.12 dA | 77.84 ± 0.03 eA | |
| a* | 0 | −1.72 ± 0.19 eA | 2.43 ± 0.03 dA | 4.85 ± 0.05 cA | 8.30 ± 0.46 bA | 10.89 ± 0.04 aA |
| 7 | −1.73 ± 0.02 eA | 2.44 ± 0.07 dA | 4.87 ± 0.07 cA | 8.29 ± 0.18 bA | 10.88 ± 0.02 aA | |
| 14 | −1.72 ± 0.03 eA | 2.43 ± 0.05 dA | 4.86 ± 0.15 cA | 8.29 ± 0.13 bA | 10.89 ± 0.09 aA | |
| 21 | −1.72 ± 0.09 eA | 2.44 ± 0.33 dA | 4.85 ± 0.06 cA | 8.28 ± 0.05 bA | 10.88 ± 0.14 aA | |
| b* | 0 | 5.55 ± 0.22 aA | 4.82 ± 0.14 bA | 3.71 ± 0.03 cA | 2.65 ± 0.11 dA | 1.78 ± 0.02 eA |
| 7 | 5.53 ± 0.01 aA | 4.81 ± 0.05 bA | 3.73 ± 0.06 cA | 2.68 ± 0.21 dA | 1.76 ± 0.03 eA | |
| 14 | 5.54 ± 0.12 aA | 4.83 ± 0.06 bA | 3.70 ± 0.21 cA | 2.62 ± 0.33 dA | 1.79 ± 0.11 eA | |
| 21 | 5.55 ± 0.23 aA | 4.82 ± 0.05 bA | 3.70 ± 0.02 cA | 2.68 ± 0.02 dA | 1.77 ± 0.02 eA | |
| ΔE* | 0 | — | 5.54 | 7.59 | 11.33 | 15.50 |
| 7 | — | 5.73 | 7.88 | 11.66 | 15.88 | |
| 14 | — | 5.55 | 7.75 | 11.55 | 15.70 | |
| 21 | — | 5.67 | 7.94 | 11.34 | 15.65 |
| Attribute | 0% BHJ (Control) | 1% BHJ | 2% BHJ | 4% BHJ | 6% BHJ |
|---|---|---|---|---|---|
| Apparence | 4.04 ± 0.15 b | 4.35 ± 0.31 ab | 4.56 ± 0.17 a | 4.54 ± 0.22 a | 3.67 ± 0.24 c |
| Texture | 3.73 ± 0.32 b | 4.23 ± 0.55 ab | 4.63 ± 0.33 a | 4.51 ± 0.43 a | 3.02 ± 0.14 c |
| Flavor | 3.68 ± 0.28 c | 4.15 ± 0.47 bc | 4.50 ± 0.21 ab | 4.83 ± 0.16 a | 3.05 ± 0.27 d |
| Taste | 4.28 ± 0.30 a | 4.28 ± 0.19 a | 4.37 ± 0.43 a | 4.50 ± 0.41 a | 3.39 ± 0.48 b |
| Overall Acceptability | 3.93 ± 0.21 c | 4.25 ± 0.07 b | 4.51 ± 0.13 ab | 4.60 ± 0.17 a | 3.28 ± 0.23 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Miao, Y.; Li, J.; Ma, Y.; Wu, M.; Wang, W.; Xu, C.; Jiang, Z.; Hou, J. Incorporation of Blue Honeysuckle Juice into Fermented Goat Milk: Physicochemical, Sensory and Antioxidant Characteristics and In Vitro Gastrointestinal Digestion. Foods 2022, 11, 3065. https://doi.org/10.3390/foods11193065
Ma J, Miao Y, Li J, Ma Y, Wu M, Wang W, Xu C, Jiang Z, Hou J. Incorporation of Blue Honeysuckle Juice into Fermented Goat Milk: Physicochemical, Sensory and Antioxidant Characteristics and In Vitro Gastrointestinal Digestion. Foods. 2022; 11(19):3065. https://doi.org/10.3390/foods11193065
Chicago/Turabian StyleMa, Jiage, Yusi Miao, Jinzhe Li, Yue Ma, Mengguo Wu, Wan Wang, Cong Xu, Zhanmei Jiang, and Juncai Hou. 2022. "Incorporation of Blue Honeysuckle Juice into Fermented Goat Milk: Physicochemical, Sensory and Antioxidant Characteristics and In Vitro Gastrointestinal Digestion" Foods 11, no. 19: 3065. https://doi.org/10.3390/foods11193065
APA StyleMa, J., Miao, Y., Li, J., Ma, Y., Wu, M., Wang, W., Xu, C., Jiang, Z., & Hou, J. (2022). Incorporation of Blue Honeysuckle Juice into Fermented Goat Milk: Physicochemical, Sensory and Antioxidant Characteristics and In Vitro Gastrointestinal Digestion. Foods, 11(19), 3065. https://doi.org/10.3390/foods11193065






