Diversity of Volatile Aroma Compound Composition Produced by Non-Saccharomyces Yeasts in the Early Phase of Grape Must Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vinification
2.2. Preparation of the Yeasts
2.3. Analysis of Volatile Aroma Compounds by Headspace Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry
2.4. Statistical Data Elaboration
3. Results and Discussion
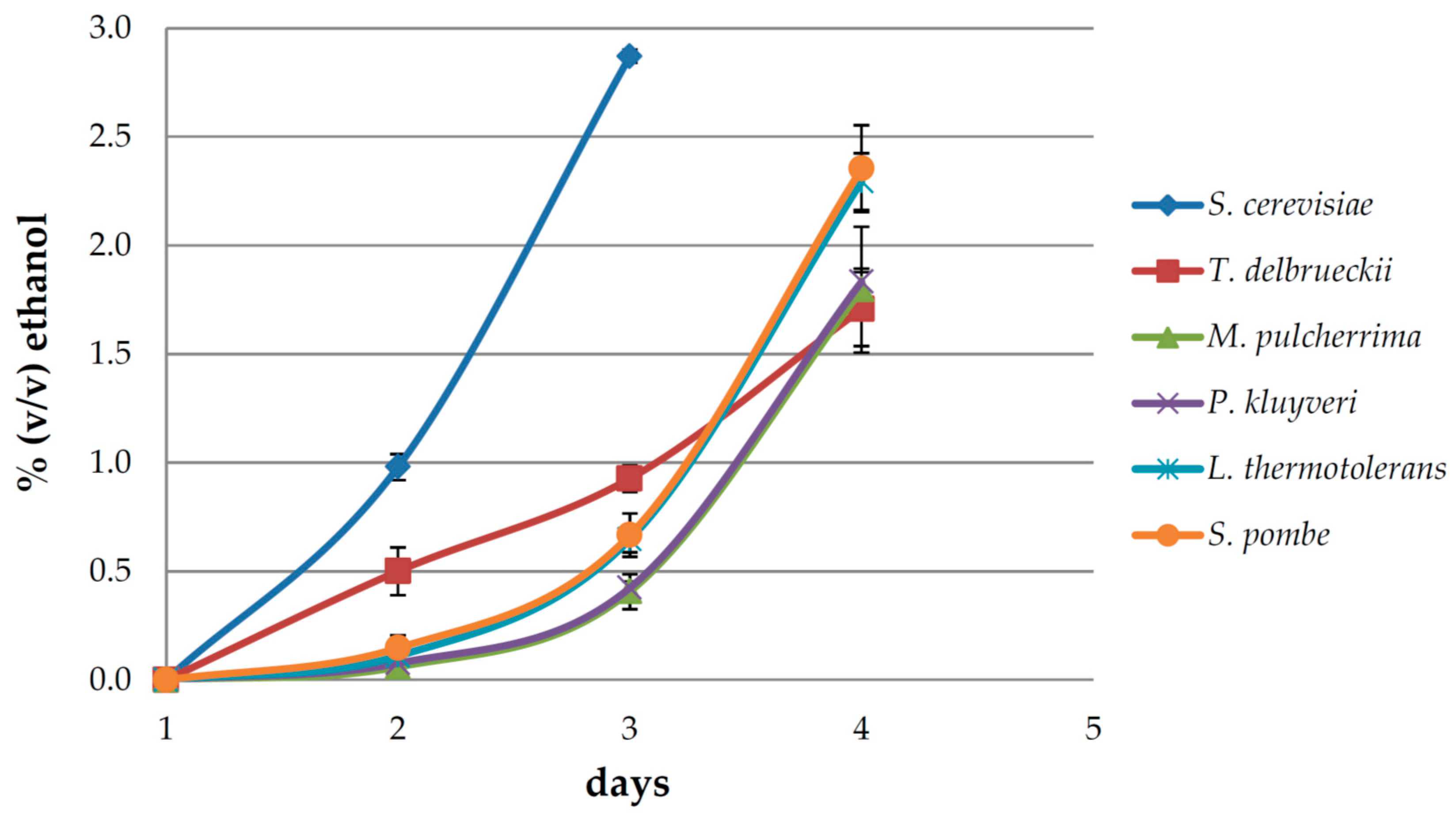
3.1. Fermentation Dynamics
3.2. Volatile Aroma Compounds
3.2.1. Terpenes
3.2.2. C13-Norisoprenoids
3.2.3. C6-Alcohols
3.2.4. Higher Alcohols
3.2.5. Volatile Acids
3.2.6. Esters
3.2.7. Miscellaneous Compounds
3.2.8. Multivariate Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zott, K.; Miot-Sertier, C.; Claisse, O.; Lonvaud-Funel, A.; Masneuf-Pomarede, I. Dynamics and diversity of non-Saccharomyces yeasts during the early stages in winemaking. Int. J. Food Microbiol. 2008, 125, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Contribution of Non-Saccharomyces Yeasts to Wine Freshness. Biomolecules 2020, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Lleixà, J.; Manzano, M.; Mas, A.; Portillo, M.D. Saccharomyces and non-Saccharomyces competition during microvinification under different sugar and nitrogen conditions. Front. Microbiol. 2016, 7, 1959. [Google Scholar] [CrossRef]
- Blanco, P.; Rabuñal, E.; Neira, N.; Castrillo, D. Dynamic of Lachancea thermotolerans population in monoculture and mixed fermentations: Impact on wine characteristics. Beverages 2020, 6, 36. [Google Scholar] [CrossRef]
- Benito, S. The impacts of Schizosaccharomyces on winemaking. Appl. Microbiol. Biotechnol. 2019, 103, 4291–4312. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Palomero, F.; González, C.; Suárez-Lepe, J.A. Schizosaccharomyces pombe: A Promising Biotechnology for Modulating Wine Composition. Fermentation 2018, 4, 70. [Google Scholar] [CrossRef] [Green Version]
- Vejarano, R.; Gil-Calderón, A. Commercially available non-Saccharomyces yeasts for winemaking: Current Market, Advantages over Saccharomyces, Biocompatibility and safety. Fermentation 2021, 7, 171. [Google Scholar] [CrossRef]
- Windholtz, S.; Redon, P.; Lacampagne, S.; Farris, L.; Lytra, G.; Cameleyre, M.; Barbe, J.C.; Coulon, J.; Thibon, J.; Masneuf-Pomarède, I. Non-Saccharomyces yeasts as bioprotection in the composition of red wine and in the reduction of sulfur dioxide. LWT—Food Sci. Technol. 2021, 149, 111781. [Google Scholar] [CrossRef]
- Jolly, N.; Pretorius, I.S.; Varela, C. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2013, 14, 215–237. [Google Scholar] [CrossRef] [Green Version]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Azzolini, M.; Fedrizzi, B.; Tosi, E.; Finato, F.; Vagnoli, P.; Scrinzi, C.; Zapparoli, G. Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae mixed cultures on fermentation and aroma of Amarone wine. Eur. Food Res. Technol. 2012, 235, 303–313. [Google Scholar] [CrossRef]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2015, 31, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Hranilovic, A.; Li, S.; Boss, P.K.; Bindon, K.; Ristic, R.; Grbin, P.R.; Van der Westhuizen, T.; Jiranek, V. Chemical and sensory profiling of Shiraz wines co-fermented with commercial non-Saccharomyces inocula. Aust. J. Grape Wine Res. 2018, 24, 166–180. [Google Scholar] [CrossRef]
- Beckner Whitener, M.E.; Stanstrup, J.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Effect of non-Saccharomyces yeasts on the volatile chemical profile of Shiraz wine. Aust. J. Grape Wine Res. 2017, 23, 179–192. [Google Scholar] [CrossRef]
- Dutraive, O.; Benito, S.; Fritsch, S.; Beisert, B.; Patz, C.-D.; Rauhut, D. Effect of Sequential inoculation with non-Saccharomyces and Saccharomyces yeasts on Riesling wine chemical composition. Fermentation 2019, 5, 79. [Google Scholar] [CrossRef] [Green Version]
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.C.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48. [Google Scholar] [CrossRef]
- Barbosa, C.; Mendes-Faia, A.; Lage, P.; Mira, N.P.; Mendes-Ferreira, A. Genomic expression program of Saccharomyces cerevisiae along a mixed-culture wine fermentation with Hanseniaspora guilliermondii. Microb. Cell Factories 2015, 14, 124. [Google Scholar] [CrossRef] [Green Version]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial Contribution to Wine Aroma and Its Intended Use for Wine Quality Improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef] [Green Version]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulospora delbrueckii in the brewing process: A new approach to enhance bioflavour and to reduce ethanol content. Food Microbiol. 2015, 56, 45–51. [Google Scholar] [CrossRef]
- Cioch-Skoneczny, M.; Grabowski, M.; Satora, P.; Skoneczny, S.; Klimczak, K. The use of yeast mixed cultures for deacidification and improvement of the composition of cold climate grape wines. Molecules 2021, 26, 2628. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Beckner Whitener, M.E.; Carlin, S.; Jacobson, D.; Weighill, D.; Divol, B.; Conterno, L.; Du Toit, M.; Vrhovsek, U. Early fermentation volatile metabolite profile of non-Saccharomyces yeasts in red and white grape must: A targeted approach. LWT—Food Sci. Technol. 2015, 64, 412–422. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology: The Microbiology of Wine and Vinifications; John Wiley and Sons, Ltd.: Bordeaux, France, 2016. [Google Scholar]
- Bubola, M.; Lukić, I.; Radeka, S.; Sivilotti, P.; Grozic, K.; Vanzo, A.; Bavčar, D.; Lisjak, K. Enhancement of Istrian Malvasia wine aroma and hydroxycinnamate composition by hand and mechanical leaf removal. J. Sci. Food Agric. 2019, 99, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sinelnikov, I.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Lukić, I.; Carlin, S.; Vrhovsek, U. Comprehensive 2D Gas Chromatography with TOF-MS Detection Confirms the Matchless Discriminatory Power of Monoterpenes and Provides In-Depth Volatile Profile Information for Highly Efficient White Wine Varietal Differentiation. Foods 2020, 9, 1787. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, M.; Di Stefano, R.; Briones, A. Hydrolysis and transformation of terpene glycosides from muscat must by different yeast species. Food Microbiol. 2003, 20, 35–41. [Google Scholar] [CrossRef]
- King, A.; Dickinson, J.R. Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. Yeast 2020, 16, 499–506. [Google Scholar] [CrossRef]
- Escribano, R.; González-Arenzana, L.; Garijo, P.; Berlanas, C.; López-Alfaro, I.; López, R.; Gutiérrez, A.R.; Santamaría, P. Screening of enzymatic activities within different enological non-Saccharomyces yeasts. J. Food Sci. Technol. 2017, 54, 1555–1564. [Google Scholar] [CrossRef] [Green Version]
- Bisotto, A.; Julien, A.; Rigou, P.; Schneider, R.; Salmon, J.M. Evaluation of the inherent capacity of commercial yeast strains to release glycosidic aroma precursors from Muscat grape must. Aust. J. Grape Wine Res. 2015, 21, 194–199. [Google Scholar] [CrossRef]
- Čuš, F.; Jenko, M. The influence of yeast strains on the composition and sensory quality of Gewürztraminer wine. Food Technol. Biotechnol. 2013, 51, 547–553. [Google Scholar]
- Beckner Whitener, M.E.; Stanstrup, J.; Panzeri, V.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Untangling the wine metabolome by combining untargeted SPME-GCxGC-TOF-MS and sensory analysis to profile Sauvignon blanc co-fermented with seven different yeasts. Metabolomics 2016, 12, 53. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M. Carotenoid breakdown products—The norisoprenoids—In wine aroma. Arch. Biochem. Biophys. 2009, 483, 236–245. [Google Scholar] [CrossRef]
- Lloyd, N.D.R.; Capone, D.L.; Ugliano, M.; Taylor, D.K.; Skouroumounis, G.K.; Sefton, M.A.; Elsey, G.M. Formation of Damascenone under both Commercial and Model Fermentation Conditions. J. Agric. Food Chem. 2011, 59, 1338–1343. [Google Scholar] [CrossRef]
- Ruiz, J.; Belda, I.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Santos, A.; Benito, S. Analytical impact of Metschnikowia pulcherrima in the volatile profile of Verdejo white wines. Appl. Microbiol. Biotechnol. 2018, 102, 8501–8509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela, C.; Barker, A.; Tran, T.; Borneman, A.; Curtin, C. Sensory profile and volatile aroma composition of reduced alcohol Merlot wines fermented with Metschnikowia pulcherrima and Saccharomyces uvarum. Int. Food Microbiol. 2017, 252, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic analysis of physiological properties of Torulospora delbrueckii in wine fermentation and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar] [CrossRef] [Green Version]
- Mylona, A.E.; Del Fresno, J.M.; Palomero, F.; Loira, I.; Bañuelos, M.A.; Morata, A.; Calderón, F.; Benito, S.; Suárez-Lepe, J.A. Use of Schizosaccharomyces strains for wine fermentation-Effect on the wine composition and food safety. Int. Int. J. Food Microbiol. 2016, 232, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.M.; Zamora, F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2015, 240, 999–1012. [Google Scholar] [CrossRef]
- Stribny, J.; Querol, A.; Pérez-Torrado, R. Differences in enzymatic properties of the Saccharomyces kudriavzevii and Saccharomyces uvarum alcohol acetyltransferases and their impact on aroma-active compounds production. Front. Microbiol. 2016, 7, 897. [Google Scholar] [CrossRef] [Green Version]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar] [CrossRef]
- Iranzo, J.U.; Perez, A.B.; Canas, P.I. Study of the oenological characteristics and enzymatic activities of wine yeasts. Food Microbiol. 1998, 15, 399–406. [Google Scholar] [CrossRef]


| Volatile Compound | ID | LRIexp | LRIlit | Yeast Species | |||||
|---|---|---|---|---|---|---|---|---|---|
| Saccharomyces cerevisiae | Torulaspora delbrueckii | Metschnikowia pulcherrima | Pichia kluyveri | Lachancea thermotolerans | Schizosacchaomyces pombe | ||||
| Terpenes | |||||||||
| Camphene | MS, LRI | 1056 | 1056 | 0.024 ± 0.006 b | 0.024 ± 0.005 b | 0.023 ± 0.006 b | 0.039 ± 0.019 b | 0.041 ± 0.015 b | 0.093 ± 0.051 a |
| β-Pinene | MS, LRI | 1146 | 1145 | 0.16 ± 0.01 bc | 0.13 ± 0.00 d | 0.14 ± 0.01 cd | 0.16 ± 0.01 bc | 0.16 ± 0.00 ab | 0.17 ± 0.01 a |
| Limonene | MS, LRI | 1191 | 1196 | 0.30 ± 0.04 | 0.35 ± 0.08 | 0.39 ± 0.18 | 0.28 ± 0.03 | 0.25 ± 0.02 | 0.27 ± 0.02 |
| β-Phellandrene | MS, LRI | 1208 | 1218 | 0.068 ± 0.004 | 0.070 ± 0.012 | 0.064 ± 0.002 | 0.064 ± 0.008 | 0.061 ± 0.015 | 0.064 ± 0.003 |
| Eucalyptol | MS, LRI | 1216 | 1224 | 0.061 ± 0.004 b | 0.092 ± 0.028 a | 0.071 ± 0.014 ab | 0.067 ± 0.018 ab | 0.055 ± 0.018 b | 0.067 ± 0.002 ab |
| Menthol | MS, LRI | 1637 | 1641 | 1.55 ± 0.10 b | 1.48 ± 0.23 b | 1.30 ± 0.20 b | 2.55 ± 1.42 ab | 2.01 ± 0.64 b | 4.97 ± 3.02 a |
| 6,10-Dihydromyrcenol | MS, LRI | 1473 | 1475 | 0.09 ± 0.01 c | 0.12 ± 0.01 bc | 0.12 ± 0.02 bc | 0.16 ± 0.06 ab | 0.11 ± 0.05 bc | 0.18 ± 0.03 a |
| Linalool | S, MS, LRI | 1542 | 1542 | 3.76 ± 0.08 c | 3.90 ± 0.07 bc | 4.15 ± 0.22 bc | 4.75 ± 0.24 a | 4.05 ± 0.12 bc | 4.27 ± 0.39 b |
| α-Farnesene | MS, LRI | 1752 | 1762 | 0.037 ± 0.011 | 0.032 ± 0.004 | 0.031 ± 0.007 | 0.022 ± 0.013 | 0.033 ± 0.010 | 0.030 ± 0.016 |
| Geranyl acetate | MS, LRI | 1764 | 1768 | 0.099 ± 0.029 ab | 0.049 ± 0.007 c | 0.067 ± 0.011 bc | 0.079 ± 0.028 abc | 0.108 ± 0.021 a | 0.111 ± 0.027 a |
| Geranyl acetone | MS, LRI | 1849 | 1845 | 0.17 ± 0.04 | 0.40 ± 0.18 | 0.39 ± 0.33 | 0.34 ± 0.14 | 0.22 ± 0.02 | 0.41 ± 0.25 |
| C13-norisoprenoids | |||||||||
| β-Damascenone | MS, LRI | 1809 | 1809 | 0.35 ± 0.00 d | 0.50 ± 0.03 c | 0.54 ± 0.04 bc | 0.61 ± 0.01 a | 0.57 ± 0.05 ab | 0.58 ± 0.03 ab |
| α-Isomethylionone | MS, LRI | 1835 | 1848 | 0.24 ± 0.03 ab | 0.20 ± 0.01 b | 0.23 ± 0.02 ab | 0.68 ± 0.45 ab | 0.43 ± 0.32 ab | 0.70 ± 0.42 a |
| β-Ionone | MS, LRI | 1916 | 1915 | 0.20 ± 0.02 ab | 0.18 ± 0.01 b | 0.20 ± 0.01 ab | 0.52 ± 0.33 ab | 0.33 ± 0.22 ab | 0.54 ± 0.28 a |
| β-Methylionone | MS, LRI | 2012 | 1988 | 1.87 ± 0.11 | 1.40 ± 0.14 | 1.46 ± 0.19 | 4.89 ± 3.52 | 2.89 ± 2.12 | 4.83 ± 2.56 |
| 6-Methylionone | MS | 2098 | n/a | 0.19 ± 0.02 abc | 0.15 ± 0.01 c | 0.16 ± 0.02 bc | 0.38 ± 0.21 ab | 0.24 ± 0.15 abc | 0.41 ± 0.18 a |
| Alcohols | |||||||||
| 3-Buten-2-ol | MS, LRI | 1051 | NA | 0.19 ± 0.01 c | 0.23 ± 0.01 c | 0.43 ± 0.22 ab | 0.58 ± 0.05 a | 0.26 ± 0.06 bc | 0.35 ± 0.04 bc |
| Isobutanol | MS, LRI | 1090 | 1098 | 5.50 ± 0.52 abc | 4.71 ± 0.11 c | 4.94 ± 0.45 bc | 5.54 ± 1.23 abc | 6.30 ± 0.67 a | 6.19 ± 1.00 ab |
| Isoamyl alcohol | MS, LRI | 1229 | 1229 | 328.6 ± 9.9 a | 213.5 ± 3.2 d | 269.6 ± 14.01 c | 307.9 ± 24.1 ab | 313.1 ± 13.0 ab | 281.1 ± 35.4 bc |
| 1-Hexanol | S, MS, LRI | 1357 | 1357 | 1107.0 ± 33.5 b | 1336.0 ± 35.3 a | 1359.0 ± 179.6 a | 1213.8 ± 94.3 ab | 1273.7 ± 62.9 a | 1072.2 ± 20.3 b |
| trans-3-Hexen-1-ol | S, MS, LRI | 1366 | 1361 | 63.02 ± 1.19 bc | 78.85 ± 1.78 a | 68.22 ± 11.29 b | 64.91 ± 2.90 bc | 65.22 ± 3.25 bc | 57.13 ± 3.42 c |
| cis-3-Hexen-1-ol | S, MS, LRI | 1389 | 1389 | 70.51 ± 5.11 ab | 75.03 ± 1.69 a | 75.09 ± 6.97 a | 69.15 ± 7.52 ab | 66.13 ± 5.80 ab | 61.48 ± 2.78 b |
| cis-2-Hexen-1-ol | MS, LRI | 1416 | 1413 | 0.10 ± 0.02 b | 0.08 ± 0.01 b | 0.11 ± 0.01 ab | 0.09 ± 0.01 b | 0.08 ± 0.01 b | 0.13 ± 0.03 a |
| 6-Methyl-5-hepten-2-ol | MS, LRI | 1463 | 1466 | 0.22 ± 0.03 bc | 0.26 ± 0.01 a | 0.23 ± 0.02 abc | 0.25 ± 0.02 ab | 0.23 ± 0.02 abc | 0.21 ± 0.01 c |
| 2-Phenylethanol | S, MS, LRI | 1891 | 1893 | 5053.5 ± 743.7 a | 3371.6 ± 517.4 b | 3278.1 ± 663.2 b | 2924.3 ± 438.0 b | 3004.9 ± 670.3 b | 2791.9 ± 354.7 b |
| Volatile acids | |||||||||
| Acetic acid | MS, LRI | 1445 | 1439 | 10.42 ± 2.17 ab | 8.12 ± 1.94 b | 8.76 ± 0.60 ab | 11.44 ± 2.16 a | 10.66 ± 2.10 ab | 8.89 ± 1.12 ab |
| Butyric acid | S, MS, LRI | 1617 | 1612 | 970.6 ± 21.8 a | 499.1 ± 38.1 c | 570.6 ± 33.8 bc | 560.8 ± 79.1 c | 645.4 ± 19.8 b | 530.4 ± 56.3 c |
| Hexanoic acid | S, MS, LRI | 1824 | 1828 | 3070.9 ± 24.4 b | 1879.9 ± 72.1 d | 2609.9 ± 105.9 c | 2991.7 ± 487.6 bc | 3298.9 ± 219.4 b | 3768.8 ± 272.6 a |
| Octanoic acid | S, MS, LRI | 2043 | 2042 | 3878.1 ± 187.6 a | 2347.1 ± 120.0 c | 3213.4 ± 23.8 b | 3092.8 ± 304.0 b | 3312.5 ± 88.4 b | 3847.4 ± 288.5 a |
| Nonanoic acid | MS, LRI | 2155 | 2119 | 63.60 ± 15.87 a | 59.29 ± 6.65 ab | 58.67 ± 8.51 ab | 26.12 ± 29.93 bc | 42.46 ± 31.64 ab | 7.74 ± 0.54 c |
| Decanoic acid | S, MS, LRI | 2257 | 2258 | 1874.6 ± 64.6 a | 1349.3 ± 220.0 bc | 1506.3 ± 94.3 b | 1322.4 ± 261.6 bc | 1107.6 ± 134.7 c | 1622.8 ± 277.3 ab |
| Ethyl esters | |||||||||
| Ethyl acetate | MS, LRI | <1000 | 885 | 96.29 ± 4.21 a | 96.16 ± 7.77 a | 44.00 ± 7.12 c | 77.64 ± 5.72 b | 85.32 ± 7.35 ab | 79.22 ± 15.74 b |
| Ethyl propanoate | MS, LRI | <1000 | 949 | 0.20 ± 0.01 b | 0.87 ± 0.01 a | 0.07 ± 0.01 e | 0.10 ± 0.02 d | 0.14 ± 0.01 c | 0.07 ± 0.01 e |
| Ethyl isobutyrate | MS, LRI | <1000 | 965 | 0.01 ± 0.00 b | 0.02 ± 0.00 a | 0.01 ± 0.00 b | 0.01 ± 0.01 ab | 0.01 ± 0.00 b | 0.01 ± 0.00 b |
| Ethyl butyrate | S, MS, LRI | 1030 | 1030 | 81.37 ± 7.02 a | 30.04 ± 3.65 c | 48.17 ± 10.95 b | 48.58 ± 6.44 b | 70.07 ± 7.51 a | 79.58 ± 14.44 a |
| Ethyl hexanoate | S, MS, LRI | 1242 | 1236 | 678.4 ± 49.5 b | 363.9 ± 35.8 c | 706.2 ± 134.6 b | 756.6 ± 143.3 b | 921.6 ± 39.2 a | 924.4 ± 75.5 a |
| Ethyl octanoate | S, MS, LRI | 1435 | 1435 | 1744.0 ± 132.2 b | 779.2 ± 193.8 c | 1981.0 ± 525.5 b | 2738.0 ± 432.3 a | 2599.5 ± 83.1 a | 2265.1 ± 400.8 ab |
| Ethyl nonanoate | MS, LRI | 1530 | 1535 | 6.14 ± 0.54 a | 4.54 ± 0.67 b | 5.37 ± 0.27 ab | 5.84 ± 0.56 a | 5.44 ± 0.83 ab | 5.93 ± 0.56 a |
| Ethyl 2-furoate | MS, LRI | 1609 | 1606 | 0.022 ± 0.002 ab | 0.021 ± 0.005 ab | 0.025 ± 0.006 a | 0.014 ± 0.012 b | 0.018 ± 0.004 ab | 0.022 ± 0.004 ab |
| Ethyl decanoate | S, MS, LRI | 1645 | 1638 | 1192.0 ± 167.1 ab | 1038.2 ± 132.9 b | 1467.3 ± 336.7 a | 1499.1 ± 199.3 a | 1571.7 ± 244.7 a | 1563.4 ± 269.3 a |
| Ethyl 9-decenoate | MS, LRI | 1694 | 1688 | 0.64 ± 0.32 d | 1.09 ± 0.35 cd | 1.64 ± 0.48 cd | 2.12 ± 0.61 bc | 3.89 ± 1.52 a | 3.19 ± 0.53 ab |
| Ethyl dodecanoate | MS, LRI | 1843 | 1843 | 56.14 ± 9.48 b | 49.71 ± 14.43 b | 95.08 ± 17.94 a | 94.53 ± 11.58 a | 98.13 ± 12.49 a | 91.20 ± 16.02 a |
| Acetate esters | |||||||||
| Methyl acetate | MS, LRI | <1000 | 813 | 0.14 ± 0.02 b | 0.15 ± 0.00 b | 0.15 ± 0.01 b | 0.22 ± 0.04 a | 0.18 ± 0.02 ab | 0.20 ± 0.03 a |
| Propyl acetate | MS, LRI | <1000 | 982 | 1.34 ± 0.09 a | 0.99 ± 0.05 bc | 0.69 ± 0.11 d | 0.94 ± 0.14 c | 1.13 ± 0.05 b | 1.12 ± 0.12 b |
| Isobutyl acetate | S, MS, LRI | 1015 | 1009 | 89.76 ± 2.57 a | 60.13 ± 5.31 cd | 52.15 ± 5.24 d | 66.99 ± 3.82 bc | 77.25 ± 6.30 ab | 65.71 ± 14.23 bc |
| Butyl acetate | MS, LRI | 1062 | 1064 | 0.19 ± 0.05 ab | 0.15 ± 0.01 b | 0.14 ± 0.02 b | 0.19 ± 0.03 ab | 0.22 ± 0.01 a | 0.23 ± 0.05 a |
| Isoamyl acetate | S, MS, LRI | 1133 | 1133 | 1893.4 ± 50.2 b | 1188.6 ± 91.2 d | 1494.3 ± 219.2 c | 2231.5 ± 223.6 a | 2084.3 ± 112.1 ab | 2028.5 ± 142.8 ab |
| Hexyl acetate | S, MS, LRI | 1272 | 1272 | 486.0 ± 29.5 b | 340.1 ± 17.4 c | 452.3 ± 41.1 b | 556.3 ± 45.0 a | 459.1 ± 23.2 b | 554.4 ± 24.03 a |
| cis-3-Hexen-1-yl acetate | MS, LRI | 1304 | 1300 | 2.73 ± 0.09 c | 1.66 ± 0.09 d | 2.04 ± 0.24 d | 4.20 ± 0.63 a | 2.78 ± 0.21 bc | 3.30 ± 0.31 b |
| trans-3-Hexen-1-yl acetate | MS, LRI | 1313 | 1316 | 2.78 ± 0.09 b | 1.53 ± 0.18 d | 2.0 ± 0.16 cd | 5.21 ± 0.86 a | 2.59 ± 0.20 bc | 3.13 ± 0.28 b |
| Heptyl acetate | MS, LRI | 1374 | 1374 | 0.085 ± 0.009 b | 0.043 ± 0.014 c | 0.094 ± 0.011 ab | 0.097 ± 0.027 ab | 0.075 ± 0.007 b | 0.119 ± 0.013 a |
| Octyl acetate | MS, LRI | 1481 | 1483 | 0.21 ± 0.07 a | 0.02 ± 0.00 d | 0.17 ± 0.06 ab | 0.12 ± 0.06 bc | 0.08 ± 0.01 cd | 0.11 ± 0.02 bc |
| Isobornyl acetate | MS, LRI | 1570 | 1571 | 1.65 ± 0.10 b | 1.43 ± 0.30 b | 1.40 ± 0.32 b | 2.71 ± 1.43 b | 2.66 ± 0.67 b | 6.74 ± 4.54 a |
| 2-Phenethyl acetate | S, MS, LRI | 1803 | 1801 | 101.80 ± 10.66 b | 74.36 ± 12.22 bc | 57.37 ± 15.66 c | 187.70 ± 24.84 a | 58.27 ± 13.48 c | 79.33 ± 13.59 bc |
| Other esters | |||||||||
| Methyl hexanoate | MS, LRI | 1170 | 1172 | 0.60 ± 0.04 b | 0.47 ± 0.02 c | 0.79 ± 0.03 a | 0.79 ± 0.12 a | 0.79 ± 0.12 a | 0.76 ± 0.04 a |
| Isoamyl propanoate | MS, LRI | 1179 | 1181 | 0.020 ± 0.017 ab | 0.040 ± 0.003 a | 0.015 ± 0.021 b | 0.013 ± 0.003 b | 0.005 ± 0.001 b | 0.014 ± 0.020 b |
| Isoamyl butyrate | MS, LRI | 1262 | 1266 | 0.050 ± 0.021 ab | 0.036 ± 0.004 b | 0.043 ± 0.005 ab | 0.057 ± 0.026 ab | 0.064 ± 0.024 ab | 0.073 ± 0.004 a |
| Propyl hexanoate | MS, LRI | 1324 | 1319 | 0.107 ± 0.037 a | 0.033 ± 0.008 b | 0.079 ± 0.011 a | 0.088 ± 0.024 a | 0.110 ± 0.010 a | 0.095 ± 0.012 a |
| Methyl 2-methyloctanoate | MS, LRI | 1399 | 1380 | 11.27 ± 0.77 | 10.53 ± 0.86 | 10.52 ± 0.28 | 10.66 ± 0.24 | 10.16 ± 1.42 | 11.56 ± 1.00 |
| Methyl octanoate | MS, LRI | 1407 | 1404 | 1.27 ± 0.50 c | 1.13 ± 0.34 c | 2.78 ± 0.68 b | 3.79 ± 0.72 a | 3.22 ± 0.15 ab | 2.85 ± 0.50 b |
| Isoamyl hexanoate | MS, LRI | 1457 | 1458 | 0.33 ± 0.15 b | 0.30 ± 0.05 b | 0.82 ± 0.20 a | 1.02 ± 0.46 a | 1.11 ± 0.08 a | 0.80 ± 0.11 a |
| Propyl octanoate | MS, LRI | 1520 | 1510 | 0.16 ± 0.07 a | 0.07 ± 0.02 b | 0.16 ± 0.04 a | 0.18 ± 0.08 a | 0.20 ± 0.02 a | 0.17 ± 0.03 a |
| Isobutyl octanoate | MS, LRI | 1550 | 1551 | 0.030 ± 0.008 b | 0.037 ± 0.005 b | 0.082 ± 0.019 a | 0.100 ± 0.034 a | 0.090 ± 0.020 a | 0.082 ± 0.005 a |
| Methyl decanoate | MS, LRI | 1594 | 1593 | 0.34 ± 0.07 b | 0.33 ± 0.05 b | 0.57 ± 0.11 a | 0.58 ± 0.15 a | 0.60 ± 0.07 a | 0.56 ± 0.09 a |
| Diethyl succinate | MS, LRI | 1677 | 1669 | 3.00 ± 0.31 b | 6.98 ± 1.61 a | 7.11 ± 3.29 a | 5.08 ± 0.47 ab | 6.90 ± 2.65 a | 5.61 ± 0.90 ab |
| Ester m/z 131, 43, 70, 113 | n.i. | 1713 | n/a | 1.66 ± 0.11 a | 1.21 ± 0.21 b | 1.45 ± 0.15 ab | 1.53 ± 0.09 a | 1.43 ± 0.30 ab | 1.69 ± 0.08 a |
| Isobutyl decanoate | MS, LRI | 1774 | 1756 | 0.007 ± 0.005 c | 0.014 ± 0.003 bc | 0.022 ± 0.005 ab | 0.023 ± 0.003 a | 0.017 ± 0.006 ab | 0.020 ± 0.005 ab |
| Isobutyl 4-ethylbenzoate | MS, LRI | 1788 | n/a | 0.38 ± 0.07 ab | 0.14 ± 0.12 c | 0.39 ± 0.16 ab | 0.54 ± 0.19 a | 0.40 ± 0.13 ab | 0.29 ± 0.08 bc |
| Isoamyl decanoate | MS, LRI | 1859 | 1856 | 2.15 ± 0.51 | 1.86 ± 0.18 | 2.19 ± 0.15 | 2.28 ± 0.13 | 2.19 ± 0.31 | 2.17 ± 0.24 |
| 2-Phenethyl propanoate | MS, LRI | 1872 | 1880 | n.d. | 0.930 ± 0.084 a | 0.233 ± 0.131 b | 0.057 ± 0.042 c | 0.023 ± 0.013 c | 0.018 ± 0.004 c |
| Hexyl salicylate | MS, LRI | 2186 | 2206 | 0.43 ± 0.06 ab | 0.26 ± 0.02 b | 0.27 ± 0.02 b | 0.47 ± 0.22 a | 0.41 ± 0.07 ab | 0.59 ± 0.10 a |
| Miscellaneous | |||||||||
| 3-Methylbutanal | MS, LRI | <1000 | 901 | 0.099 ± 0.005 b | 0.024 ± 0.002 d | 0.157 ± 0.022 a | 0.147 ± 0.006 a | 0.083 ± 0.009 bc | 0.071 ± 0.003 c |
| Hexanal | MS, LRI | 1068 | 1070 | 9.39 ± 0.55 b | 5.73 ± 0.53 c | 12.08 ± 2.75 a | 7.02 ± 1.24 bc | 5.19 ± 0.99 c | 4.87 ± 0.33 c |
| 2-Octanone | MS, LRI | 1279 | 1284 | 0.33 ± 0.01 a | 0.34 ± 0.03 a | 0.34 ± 0.04 a | 0.34 ± 0.06 a | 0.31 ± 0.01 ab | 0.26 ± 0.02 b |
| Benzaldehyde | S, MS, LRI | 1500 | 1505 | 1.94 ± 0.35 a | 0.88 ± 0.16 bc | 0.94 ± 0.12 b | 0.80 ± 0.12 bc | 0.61 ± 0.09 c | 0.71 ± 0.02 bc |
| Dihydro-2-methyl-3(2H)-thiophenone | MS, LRI | 1512 | 1506 | 0.41 ± 0.01 e | 1.11 ± 0.04 a | 0.79 ± 0.02 c | 0.80 ± 0.05 c | 0.96 ± 0.10 b | 0.61 ± 0.08 d |
| Benzothiazole | MS, LRI | 1930 | 1937 | 0.36 ± 0.06 b | 0.38 ± 0.03 ab | 0.40 ± 0.02 ab | 0.42 ± 0.07 ab | 0.49 ± 0.08 a | 0.46 ± 0.06 ab |
| 2-(Methylmercapto) benzothiazole | MS, LRI | 2433 | 2422 | 1.82 ± 0.50 bc | 0.85 ± 0.06 c | 0.88 ± 0.14 c | 2.06 ± 1.59 bc | 3.77 ± 1.10 a | 2.90 ± 0.70 ab |
| Homosalate | MS | 2577 | n/a | 0.054 ± 0.010 | 0.070 ± 0.013 | 0.075 ± 0.026 | 0.103 ± 0.042 | 0.065 ± 0.043 | 0.098 ± 0.039 |
| p-tert-Amylphenol | MS | 2776 | n/a | 1.18 ± 0.18 b | 1.51 ± 0.26 ab | 1.71 ± 0.18 a | 1.16 ± 0.42 b | 1.14 ± 0.12 b | 1.07 ± 0.27 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delač Salopek, D.; Horvat, I.; Hranilović, A.; Plavša, T.; Radeka, S.; Pasković, I.; Lukić, I. Diversity of Volatile Aroma Compound Composition Produced by Non-Saccharomyces Yeasts in the Early Phase of Grape Must Fermentation. Foods 2022, 11, 3088. https://doi.org/10.3390/foods11193088
Delač Salopek D, Horvat I, Hranilović A, Plavša T, Radeka S, Pasković I, Lukić I. Diversity of Volatile Aroma Compound Composition Produced by Non-Saccharomyces Yeasts in the Early Phase of Grape Must Fermentation. Foods. 2022; 11(19):3088. https://doi.org/10.3390/foods11193088
Chicago/Turabian StyleDelač Salopek, Doris, Ivana Horvat, Ana Hranilović, Tomislav Plavša, Sanja Radeka, Igor Pasković, and Igor Lukić. 2022. "Diversity of Volatile Aroma Compound Composition Produced by Non-Saccharomyces Yeasts in the Early Phase of Grape Must Fermentation" Foods 11, no. 19: 3088. https://doi.org/10.3390/foods11193088
APA StyleDelač Salopek, D., Horvat, I., Hranilović, A., Plavša, T., Radeka, S., Pasković, I., & Lukić, I. (2022). Diversity of Volatile Aroma Compound Composition Produced by Non-Saccharomyces Yeasts in the Early Phase of Grape Must Fermentation. Foods, 11(19), 3088. https://doi.org/10.3390/foods11193088








