Seaweed Slurry Improved Gel Properties and Enhanced Protein Structure of Silver Carp (Hypophthalmichthys molitrix) Surimi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Seaweed Blends Samples
2.3. Surimi Gel Preparation
2.4. Gel Strength
2.5. Texture Profile Analysis
2.6. Color Measurement
2.7. Water Holding Capacity (WHC) and Cooking Loss
2.8. Dynamic Rheology Measurement
2.9. Measurement of Structural-Related Changes of the MP
Amino Acid Residue Side-Chain Groups
2.10. MP Secondary Structure
2.11. Fluorescence Spectrum
2.12. Surface Hydrophobicity
2.13. Scanning Electron Microscopy (SEM)
2.14. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.15. Statistical Analysis
3. Results and Discussion
3.1. Gel Strength and TPA
3.2. Color
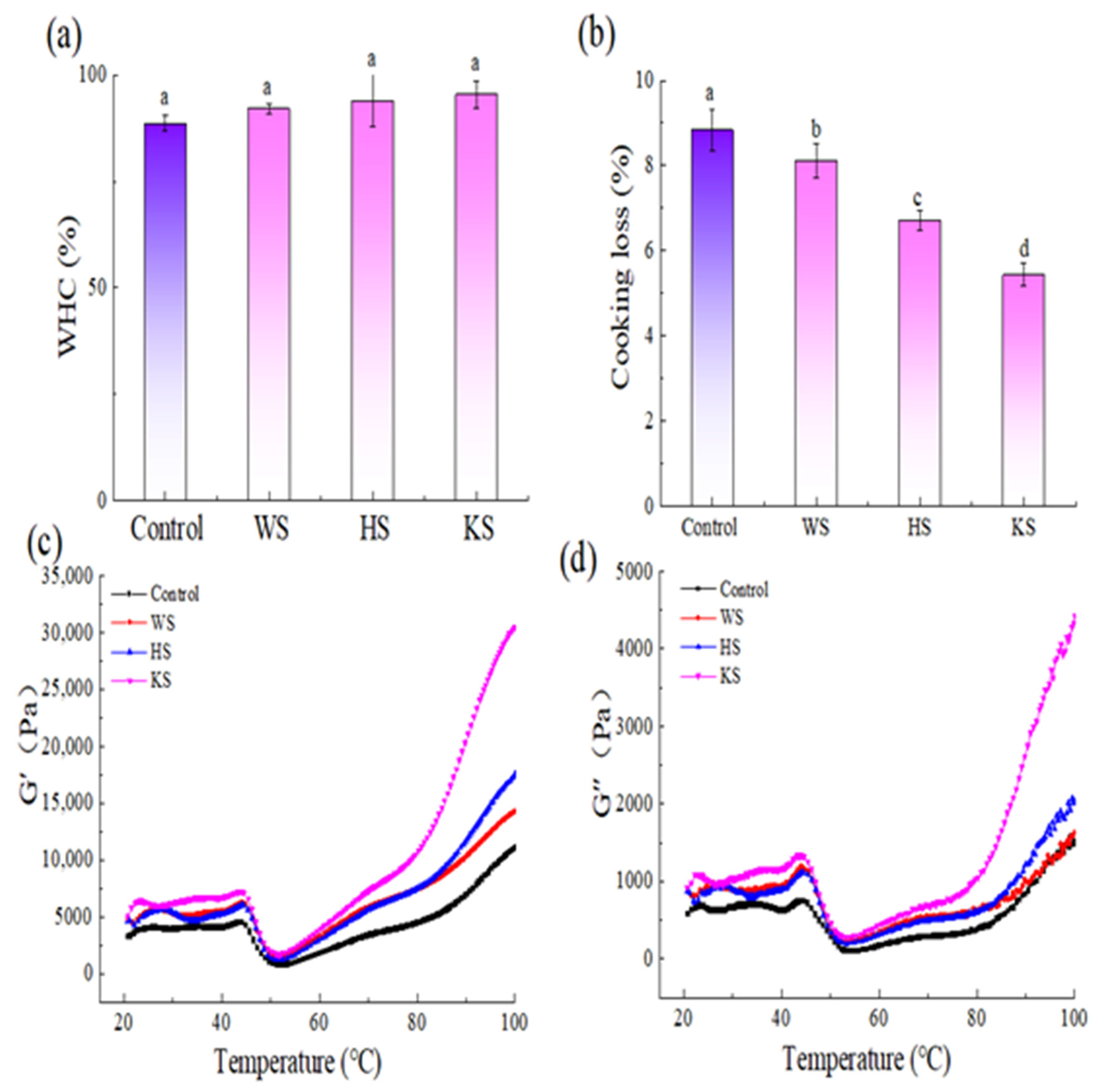
3.3. WHC and Cooking Loss
3.4. Dynamic Rheological Measurements
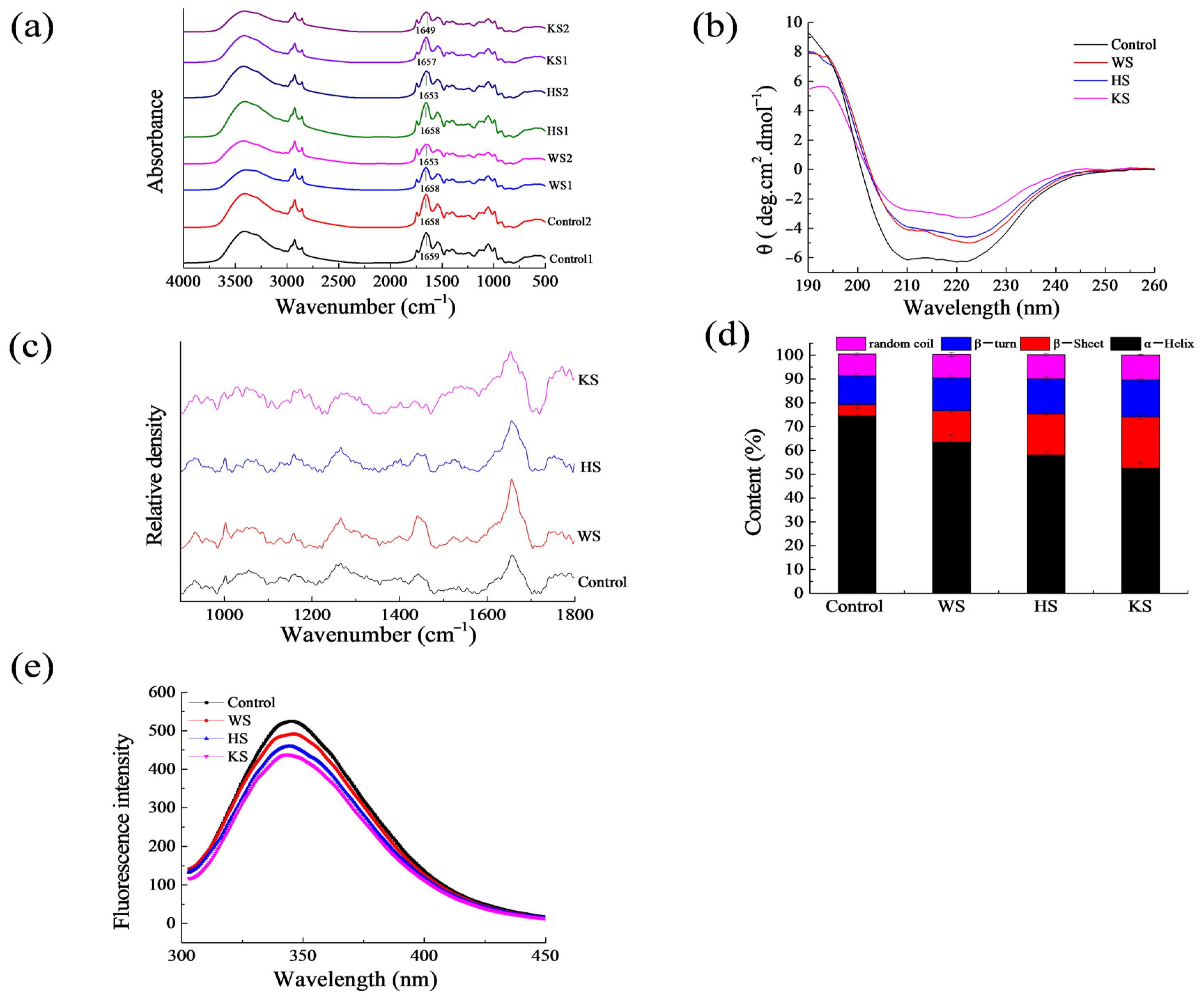
3.5. Structural Changes
Amino Acid Residue Side-Chain Groups
3.6. Secondary Structure
3.7. Fluorescence Spectrum
3.8. Surface Hydrophobicity
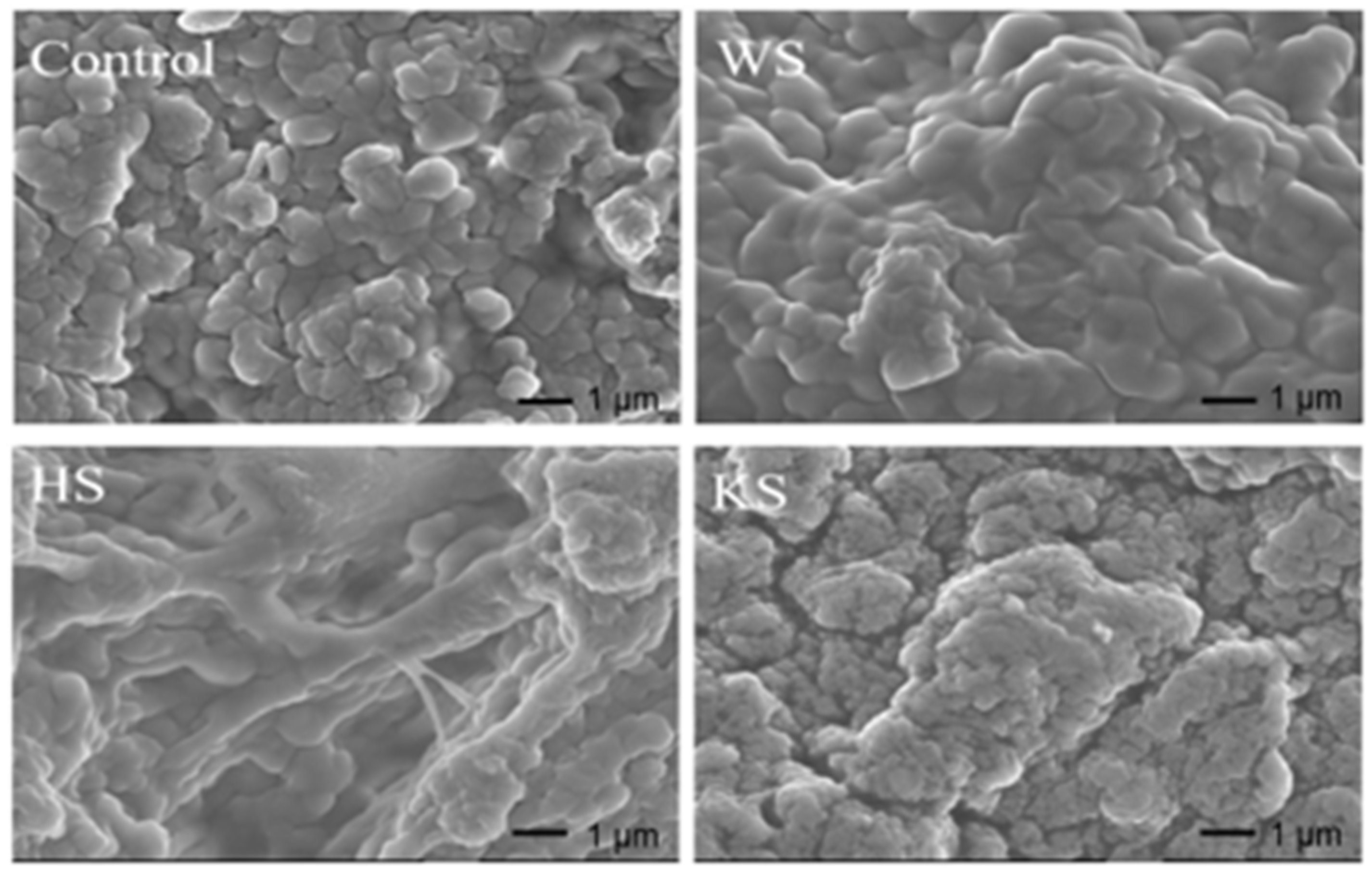
3.9. Microstructure of Surimi Gel
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, X.; Shi, L.; Xiong, S.; Hu, Y.; You, J.; Huang, Q.; Yin, T. Gelling properties of vacuum-freeze dried surimi powder as influenced by heating method and microbial transglutaminase. LWT 2019, 99, 105–111. [Google Scholar] [CrossRef]
- Shi, L.; Wang, X.; Chang, T.; Wang, C.; Yang, H.; Cui, M. Effects of vegetable oils on gel properties of surimi gels. LWT-Food Sci. Technol. 2014, 57, 586–593. [Google Scholar] [CrossRef]
- Mi, H.; Li, Y.; Wang, C.; Yi, S.; Li, X.; Li, J. The interaction of starch-gums and their effect on gel properties and protein conformation of silver carp surimi. Food Hydrocoll. 2021, 112, 106290. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Cong, Q.; Chen, H.; Liao, W.; Xiao, F.; Wang, P.; Qin, Y.; Dong, Q.; Ding, K. Structural characterization and effect on anti-angiogenic activity of a fucoidan from Sargassum fusiforme. Carbohydr. Polym. 2016, 136, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydr. Res. 2006, 341, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Dong, X.F.; Zhao, Y.P.; Li, N.; Fu, H.; Feng, D.D.; Liu, L.; Yu, C.X. ROS production in homogenate from the body wall of sea cucumber Stichopus japonicus under UVA irradiation: ESR spin-trapping study. Food Chem. 2016, 192, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Hou, R.; Zeng, X.-A. Synergistic effects of ultrasound and soluble soybean polysaccharide on frozen surimi from grass carp. J. Food Eng. 2019, 240, 1–8. [Google Scholar] [CrossRef]
- Cofrades, S.; Lopez-Lopez, I.; Solas, M.T.; Bravo, L.; Jimenez-Colmenero, F. Influence of different types and proportions of added edible seaweeds on characteristics of low-salt gel/emulsion meat systems. Meat Sci. 2008, 79, 767–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eluvakkal, T.; Sivakumar, S.R.; Arunkumar, K. Fucoidan in Some Indian Brown Seaweeds Found along the Coast Gulf of Mannar. Int. J. Bot. 2010, 6, 176–181. [Google Scholar] [CrossRef]
- Jannat-Alipour, H.; Rezaei, M.; Shabanpour, B.; Tabarsa, M.; Rafipour, F. Addition of seaweed powder and sulphated polysaccharide on shelf_life extension of functional fish surimi restructured product. J. Food. Sci. Technol. 2019, 56, 3777–3789. [Google Scholar] [CrossRef] [PubMed]
- Jaswir, I.; Tawakalit Tope, A.-H.; Raus, R.A.; Ademola Monsur, H.; Ramli, N. Study on anti-bacterial potentials of some Malaysian brown seaweeds. Food Hydrocoll. 2014, 42, 275–279. [Google Scholar] [CrossRef]
- Vilar, E.G.; Ouyang, H.; O’Sullivan, M.G.; Kerry, J.P.; Hamill, R.M.; O’Grady, M.N.; Mohammed, H.O.; Kilcawley, K.N. Effect of salt reduction and inclusion of 1% edible seaweeds on the chemical, sensory and volatile component profile of reformulated frankfurters. Meat Sci. 2020, 161, 108001. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, S.; Zhao, D.; Zhang, J.; Gu, S.; Pan, Z.; Ding, Y. Changes in physicochemical properties and protein structure of surimi enhanced with camellia tea oil. LWT 2017, 84, 562–571. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y.L. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, T.; Wang, X.; Zou, Y.; Wang, D.; Xu, W. Effects of the structure and gel properties of myofibrillar protein on chicken breast quality treated with ultrasound-assisted potassium alginate. Food Chem. 2021, 358, 129873. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar]
- Liu, G.; Xiong, Y.L.; Butterfield, D.A. Chemical, Physical, and Gel-forming Properties of Oxidized Myofibrils and Whey- and Soy-protein Isolates. J. Food Sci. 2000, 65, 811–818. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, T.; Lin, H.; Chen, H.; Liu, J.; Lyu, F.; Ding, Y. Physicochemical properties and microstructure of surimi treated with egg white modified by tea polyphenols. Food Hydrocoll. 2019, 90, 82–89. [Google Scholar] [CrossRef]
- Chelh, I.; Gatellier, P.; Sante-Lhoutellier, V. Technical note: A simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006, 74, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Balange, A.K.; Benjakul, S. Effect of oxidised phenolic compounds on the gel property of mackerel (Rastrelliger kanagurta) surimi. LWT-Food Sci. Technol. 2009, 42, 1059–1064. [Google Scholar] [CrossRef]
- Leammli U, K. Cleavage of structural protein during the assembly of the head of bacferiophage. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Ci, S.X.; Huynh, T.H.; Louie, L.W.; Yang, A.; Beals, B.J.; Ron, N.; Tsang, W.G.; Soonshiong, P.; Desai, N.P. Molecular mass distribution of sodium alginate by high-performance size-exclusion chromatography. J. Chromatogr. A 1999, 864, 199–210. [Google Scholar] [CrossRef]
- He, B.; Ming, Y.; Pu, Y.; Sun, Y.; Jin, M.; Yu, C.; Qi, H. The dual effects of riboflavin and kelp polyphenol extracts on the gel properties of myofibrillar protein from Scomberomorus Niphonius under UVA irradiation. Food Chem. 2020, 332, 127373. [Google Scholar] [CrossRef]
- Kudre, T.; Benjakul, S.; Kishimura, H. Effects of protein isolates from black bean and mungbean on proteolysis and gel properties of surimi from sardine (Sardinella albella). LWT-Food Sci. Technol. 2013, 50, 511–518. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; et al. Safety and efficacy of sodium and potassium alginate for pets, other non food-producing animals and fish. EFSA J. 2017, 15, e04945. [Google Scholar]
- Li, J.; Tang, W.; Lei, Z.; Wang, Z.; Liu, J. Effect of polysaccharides on the gel characteristics of “Yu Dong” formed with fish (Cyprinus carpio L.) scale aqueous extract. Food Chem. 2021, 338, 127792. [Google Scholar] [CrossRef]
- Fernandez-Martin, F.; Lopez-Lopez, I.; Cofrades, S.; Colmenero, F.J. Influence of adding Sea Spaghetti seaweed and replacing the animal fat with olive oil or a konjac gel on pork meat batter gelation. Potential protein/alginate association. Meat Sci. 2009, 83, 209–217. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, Y.; Li, Z.; Wang, Y.; Xue, C. Effects of deacetylation of konjac glucomannan on Alaska Pollock surimi gels subjected to high-temperature (120 °C) treatment. Food Hydrocoll. 2015, 43, 125–131. [Google Scholar] [CrossRef]
- Duangmal, K.; Taluengphol, A. Effect of protein additives, sodium ascorbate, and microbial transglutaminase on the texture and colour of red tilapia surimi gel. Int. J. Food Sci. Technol. 2009, 45, 48–55. [Google Scholar] [CrossRef]
- Alipour, H.J.; Rezaei, M.; Shabanpour, B.; Tabarsa, M. Effects of sulfated polysaccharides from green alga Ulva intestinalis on physicochemical properties and microstructure of silver carp surimi. Food Hydrocoll. 2018, 74, 87–96. [Google Scholar] [CrossRef]
- Chaijan, M.; Benjakul, S.; Visessanguan, W.; Faustman, C. Physicochemical properties, gel-forming ability and myoglobin content of sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) surimi produced by conventional method and alkaline solubilisation process. Eur. Food Res. Technol. 2005, 222, 58–63. [Google Scholar] [CrossRef]
- Chen, X.; Li, P.-j.; Nishiumi, T.; Takumi, H.; Suzuki, A.; Chen, C.-g. Effects of High-Pressure Processing on the Cooking Loss and Gel Strength of Chicken Breast Actomyosin Containing Sodium Alginate. Food Bioprocess Technol. 2014, 7, 3608–3617. [Google Scholar] [CrossRef]
- Tao, L.; Tian, L.; Zhang, X.; Huang, X.; Long, H.; Chang, F.; Li, T.; Li, S. Effects of γ-polyglutamic acid on the physicochemical properties and microstructure of grass carp (Ctenopharyngodon idellus) surimi during frozen storage. LWT 2020, 134, 109960. [Google Scholar] [CrossRef]
- Utrera, M.; Estevez, M. Impact of trolox, quercetin, genistein and gallic acid on the oxidative damage to myofibrillar proteins: The carbonylation pathway. Food Chem. 2013, 141, 4000–4009. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Shen, P.; Pu, Y.; Jin, M.; Yu, C.; Qi, H. Enhancement of gel properties of Scomberomorus niphonius myofibrillar protein using phlorotannin extracts under UVA irradiation. J. Food Sci. 2020, 85, 2050–2059. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Tatsumi, E.; Kotwal, S. Influence of high pressure on conformational changes of soybean glycinin. Innov. Food Sci. Emerg. Technol. 2003, 4, 269–275. [Google Scholar] [CrossRef]
- Cao, Y.; Ai, N.; True, A.D.; Xiong, Y.L. Effects of (-)-epigallocatechin-3-gallate incorporation on the physicochemical and oxidative stability of myofibrillar protein-soybean oil emulsions. Food Chem. 2018, 245, 439–445. [Google Scholar] [CrossRef]
- Feng, X.; Chen, L.; Lei, N.; Wang, S.; Xu, X.; Zhou, G.; Li, Z. Emulsifying Properties of Oxidatively Stressed Myofibrillar Protein Emulsion Gels Prepared with (-)-Epigallocatechin-3-gallate and NaCl. J. Agric. Food Chem. 2017, 65, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhao, M.; Sun, W. Effect of protein oxidation on the in vitro digestibility of soy protein isolate. Food Chem. 2013, 141, 3224–3229. [Google Scholar] [CrossRef]
- Li, C.; Xiong, Y.L.; Chen, J. Oxidation-induced unfolding facilitates Myosin cross-linking in myofibrillar protein by microbial transglutaminase. J. Agric. Food Chem. 2012, 60, 8020–8027. [Google Scholar] [CrossRef]
- Carbonaro, M.; Nucara, A. Secondary structure of food proteins by Fourier transform spectroscopy in the mid-infrared region. Amino Acids 2010, 38, 679–690. [Google Scholar]
- Qi, R.; Guo, J.; Liu, Y.; Zhang, R.; Gan, Z. Effects of salt content on secondary structure of protein in sodium alginate/antarctic krill protein composite system and characterization of fiber properties. Dye. Pigment. 2019, 171, 107686. [Google Scholar] [CrossRef]
- Jia, J.; Gao, X.; Hao, M.; Tang, L. Comparison of binding interaction between beta-lactoglobulin and three common polyphenols using multi-spectroscopy and modeling methods. Food Chem. 2017, 228, 143–151. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Guo, L.; Ho, H.; Wang, B.; Sun, J.; Xu, X.; Huang, M. Effects of ultrafine comminution treatment on gelling properties of myofibrillar proteins from chicken breast. Food Hydrocoll. 2019, 97, 105199. [Google Scholar] [CrossRef]
- Alix, A.J.P.; Pedanou, G.; Berjot, M. Fast determination of the quantitative secondary structure of proteins by using some parameters of the Raman Amide I band. J. Mol. Struct. 1988, 174, 159–164. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.; Tang, Y.; Lei, L.; Zhao, J.; Zhang, Y.; Li, L.; Wang, Q.; Ming, J. Effects of ionic strength and (−)-epigallocatechin gallate on physicochemical characteristics of soybean 11S and 7S proteins. Food Hydrocoll. 2021, 119, 106836. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.-M.; Xie, B.-J.; Xiong, S.-B. Contribution of protein conformation and intermolecular bonds to fish and pork gelation properties. Food Hydrocoll. 2011, 25, 898–906. [Google Scholar] [CrossRef]
- Skrt, M.; Benedik, E.; Podlipnik, C.; Ulrih, N.P. Interactions of different polyphenols with bovine serum albumin using fluorescence quenching and molecular docking. Food Chem. 2012, 135, 2418–2424. [Google Scholar] [CrossRef]
- Parolia, S.; Maley, J.; Sammynaiken, R.; Green, R.; Nickerson, M.; Ghosh, S. Structure-Functionality of lentil protein-polyphenol conjugates. Food Chem. 2022, 367, 130603. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, Y.; Zhong, L.; Ma, N.; Zhao, L.; Ma, G.; Cheng, N.; Nakata, P.A.; Xu, J. In vitro digestion and cellular antioxidant activity of β-carotene-loaded emulsion stabilized by soy protein isolate-Pleurotus eryngii polysaccharide conjugates. Food Hydrocoll. 2021, 112, 106340. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Z.; Li, Y.; Meng, X.; Sui, X.; Qi, B.; Zhou, L. Relationship Between Surface Hydrophobicity and Structure of Soy Protein Isolate Subjected to Different Ionic Strength. Int. J. Food Prop. 2015, 18, 1059–1074. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.-g.; Zhou, Y.-z.; Li, P.-j.; Ma, F.; Nishiumi, T.; Suzuki, A. Effects of high pressure processing on the thermal gelling properties of chicken breast myosin containing κ-carrageenan. Food Hydrocoll. 2014, 40, 262–272. [Google Scholar] [CrossRef]
- Perez, A.A.; Carrara, C.R.; Sánchez, C.C.; Rodríguez Patino, J.M.; Santiago, L.G. Interactions between milk whey protein and polysaccharide in solution. Food Chem. 2009, 116, 104–113. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Fasolin, L.H.; Avelar, Z.; Petersen, S.B.; Vicente, A.A.; Pereira, R.N. Effects of moderate electric fields on cold-set gelation of whey proteins–From molecular interactions to functional properties. Food Hydrocoll. 2020, 101, 105505. [Google Scholar] [CrossRef]
| Control | WS | HS | KS | |
|---|---|---|---|---|
| Gel strength (g·mm) | 403.41 ± 51.82 d | 475.09 ± 42.89 c | 629.52 ± 64.84 b | 1047.25 ± 85.04 a |
| Breaking force (g) | 84.19 ± 8.89 c | 100.81 ± 6.04 bc | 119.59 ± 11.93 b | 162.36 ± 21.74 a |
| Breaking distance (mm) | 4.78 ± 0.16 b | 4.71 ± 0.15 b | 5.26 ± 0.03 b | 6.52 ± 0.97 a |
| Hardness (g) | 832.28 ± 50.06 c | 1121.73 ± 50.06 b | 1183.55 ± 51.09 b | 1712.34 ± 70.69 a |
| Springiness | 0.67 ± 0.07 b | 0.78 ± 0.05 a | 0.81 ± 0.03 a | 0.84 ± 0.01 a |
| Cohesiveness | 0.41 ± 0.05 c | 0.54 ± 0.06 b | 0.60 ± 0.01 ab | 0.69 ± 0.07 a |
| Chewiness | 316.51 ± 20.25 d | 508.06 ± 44.98 c | 588.31 ± 43.89 b | 863.60 ± 41.05 a |
| Lightness (L*) | 77.49 ± 1.29 a | 65.26 ± 2.74 c | 50.64 ± 2.37 d | 70.77 ± 1.51 b |
| Redness (a*) | −2.76 ± 0.02 b | −5.31 ± 0.27 c | 3.09 ± 0.76 a | −2.88 ± 0.10 b |
| Yellowness (b*) | 4.25 ± 0.33 c | 10.95 ± 0.50 a | 9.63 ± 0.66 b | 9.41 ± 0.89 b |
| Whiteness | 76.93 ± 1.31 a | 63.18 ± 2.68 c | 49.61 ± 2.38 d | 69.15 ± 1.40 b |
| Treatment | Carbonyl (nmol/mg Protein) | Total Sulfhydryl (nmol/mg Protein) | Free Amino (nmol NH2/mg Protein) | BPB Bound |
|---|---|---|---|---|
| Control | 0.93 ± 0.02 d | 32.72 ± 1.80 a | 0.118 ± 0.020 a | 7.48 ± 0.59 c |
| WS | 1.14 ± 0.02 c | 26.34 ± 2.81 b | 0.079 ± 0.007 b | 8.47 ± 0.56 b |
| HS | 1.56 ± 0.10 b | 23.07 ± 2.72 bc | 0.028 ± 0.003 c | 8.83 ± 0.25 b |
| KS | 1.79 ± 0.10 a | 19.07 ± 0.89 c | 0.024 ± 0.001 c | 11.1 ±0.51 a |
| Treatment | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random (%) |
|---|---|---|---|---|
| Control | 54.00 ± 1.4 a | 1.07 ± 0.21 b | 17.10 ± 0.82 b | 27.83 ± 1.78 b |
| WS | 53.00 ± 1.20 a | 0.00 ± 0.00 b | 17.83 ± 0.32 b | 29.17 ± 1.40 b |
| HS | 51.43 ± 0.26 a | 0.00 ± 0.00 b | 20.20 ± 0.44 a | 28.37 ± 0.15 b |
| KS | 36.37 ± 0.35 b | 12.93 ± 1.42 a | 15.97 ± 0.49 c | 34.73 ± 0.75 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, L.; Zou, H.; Wang, Y.; Yu, C.; Qi, H. Seaweed Slurry Improved Gel Properties and Enhanced Protein Structure of Silver Carp (Hypophthalmichthys molitrix) Surimi. Foods 2022, 11, 3115. https://doi.org/10.3390/foods11193115
Leng L, Zou H, Wang Y, Yu C, Qi H. Seaweed Slurry Improved Gel Properties and Enhanced Protein Structure of Silver Carp (Hypophthalmichthys molitrix) Surimi. Foods. 2022; 11(19):3115. https://doi.org/10.3390/foods11193115
Chicago/Turabian StyleLeng, Liping, Haotian Zou, Yingzhen Wang, Chenxu Yu, and Hang Qi. 2022. "Seaweed Slurry Improved Gel Properties and Enhanced Protein Structure of Silver Carp (Hypophthalmichthys molitrix) Surimi" Foods 11, no. 19: 3115. https://doi.org/10.3390/foods11193115






