Herbal Mixture of Carthamus tinctorius L. Seed and Taraxacum coreanum Attenuates Amyloid Beta-Induced Cognitive Dysfunction In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Aβ Peptide Preparation and Injection
2.3. Animals and Treatment
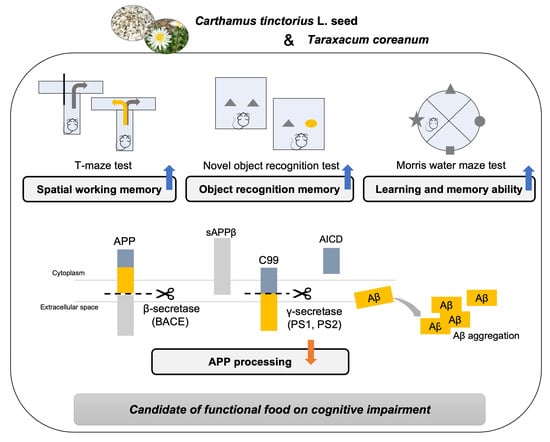
2.4. T-Maze Test
2.5. Novel Object Recognition Test
2.6. Morris Water Maze Test
2.7. Test for Aspartate Transaminase (AST) and Alanine Transaminase (ALT)
2.8. Western Blotting
2.9. Statistical Analyses
3. Results
3.1. Effect of CT on Body Weight Change and Liver Function in Aβ25-35-Infused Mice
3.2. Improvement of Aβ25-35-Induced Spatial Impairment in T-Maze Test in CT-Treated Mice
3.3. Improvement of Aβ25-35-Induced Object Recognition Impairment in Novel Object Recognition Test in CT-Treated Mice
3.4. Improvement of Aβ25-35-Induced Long-Term Spatial Memory Impairment in Morris Water Maze Test in CT-Treated Mice
3.5. Regulatory Effect of CT on Amyloidogenic Pathway in Aβ25-35-Infused Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–186. [Google Scholar] [CrossRef]
- Selkoe, D.J. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature 1999, 399, A23–A31. [Google Scholar] [CrossRef]
- Cole, S.L.; Vassar, R. BACE1 structure and function in health and Alzheimer’s disease. Curr. Alzheimer Res. 2008, 5, 100–120. [Google Scholar] [CrossRef]
- Kam, T.I.; Gwon, Y.; Jung, Y.K. Amyloid beta receptors responsible for neurotoxicity and cellular defects in Alzheimer’s disease. Cell. Mol. Life Sci. 2014, 71, 4803–4813. [Google Scholar] [CrossRef]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef]
- Kim, H.S.; Cho, J.Y.; Kim, D.H.; Yan, J.J.; Lee, H.K.; Suh, H.W.; Song, D.K. Inhibitory effects of long-term administration of ferulic acid on microglial activation induced by intracerebroventricular injection of β-amyloid peptide (1–42) in mice. Biol. Pharm. Bull. 2004, 27, 120–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, J.G.; Casadesus, G.; Gustaw-Rothenberg, K.; Siedlak, S.L.; Wang, X.; Zhu, X.; Perry, G.; Castellani, R.J.; Smith, M.A. Frontiers in Alzheimer’s disease therapeutics. Ther. Adv. Chronic Dis. 2011, 2, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Mangialasche, F.; Xu, W.; Kivipelto, M. Prevention of Alzheimer’s Disease: Intervention Studies. In Understanding Alzheimer’s Disease; Zerr, I., Ed.; IntechOpen: London, UK, 2013; Volume 17, pp. 451–484. [Google Scholar] [CrossRef] [Green Version]
- Barage, S.H.; Sonawane, K.D. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 2015, 52, 1–8. [Google Scholar] [CrossRef]
- Rogers, S.L.; Farlow, M.R.; Doody, R.S.; Mohs, R.; Friedhoff, L.T. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology 1998, 50, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Ekin, Z. Resurgence of safflower (Carthamus tinctorius L.) utilization: A global view. J. Agron. 2005, 4, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Yuk, T.H.; Kang, J.H.; Lee, S.R.; Yuk, S.W.; Lee, K.G.; Song, B.Y.; Kim, C.H.; Kim, D.W.; Kim, D.I.; Lee, T.K.; et al. Inhibitory effect of Carthamus tinctorius L. seed extracts on bone resorption mediated by tyrosine kinase, COX-2 (cyclooxygenase) and PG (prostaglandin) E2. Am. J. Chin. Med. 2002, 30, 95–108. [Google Scholar] [CrossRef]
- Kim, D.H.; Hwang, E.Y.; Son, J.H. Anti-inflammatory activity of Carthamus tinctorious seed extracts in Raw 264.7 cells. J. Life Sci. 2013, 23, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Hwang, E.Y.; Yu, M.H.; Jung, Y.S.; Lee, S.P.; Shon, J.H.; Lee, S.O. Defatted safflower seed extract inhibits adipogenesis in 3T3-L1 preadipocytes and improves lipid profiles in C57BL/6J ob/ob mice fed a high-fat diet. Nutr. Res. 2016, 36, 995–1003. [Google Scholar] [CrossRef]
- Yu, S.Y.; Lee, Y.J.; Kim, J.D.; Kang, S.N.; Lee, S.K.; Jang, J.Y.; Lee, H.K.; Lim, J.H.; Lee, O.H. Phenolic composition, antioxidant activity and anti-adipogenic effect of hot water extract from safflower (Carthamus tinctorius L.) seed. Nutrients 2013, 5, 4894–4907. [Google Scholar] [CrossRef]
- Im, D.Y.; Lee, K.I. Antioxidative and antibacterial activity and tyrosinase inhibitory activity of the extract and fractions from Taraxacum coreanum Nakai. Korean J. Med. Crop. Sci. 2011, 19, 238–245. [Google Scholar] [CrossRef]
- Lee, M.H.; Kang, H.; Lee, K.; Yang, G.; Ham, I.; Bu, Y.; Kim, H.; Choi, H.Y. The aerial part of Taraxacum coreanum extract has an anti-inflammatory effect on peritoneal macrophages in vitro and increases survival in a mouse model of septic shock. J. Ethnopharmacol. 2013, 146, 1–8. [Google Scholar] [CrossRef]
- Park, M.S.; So, J.S.; Bahk, G.J. Antioxidative and anticancer activities of water extracts from different parts of Taraxacum coreanum Nakai cultivated in Korea. J. Korean Soc. Food Sci. Nutr. 2015, 44, 1234–1240. [Google Scholar] [CrossRef]
- Lee, A.Y.; Choi, J.M.; Lee, S.; Kim, H.Y.; Lee, S.; Cho, E.J. The protective effects of the ethyl acetate fraction and flavonoids from Taraxacum coreanum against oxidative stress in neuronal cells induced by hydrogen peroxide and amyloid beta. Korean J. Pharmacogn. 2013, 44, 263–268. [Google Scholar]
- Lee, A.Y.; Yamabe, N.; Kang, K.S.; Kim, Y.H.; Lee, S.; Cho, E.J. Cognition and memory function of Taraxacum coreanum in an in vivo amyloid-β-induced mouse model of Alzheimer’s disease. Arch. Biol. Sci. 2014, 66, 1357–1366. [Google Scholar] [CrossRef]
- He, M.T.; Kim, J.H.; Kim, J.H.; Park, C.H.; Cho, E.J. Combination of Carthamus tinctorius L. seed and Taraxacum coreanum exerts synergistic effects on learning and memory function by regulating metabolism of amyloid beta in mice. J. Funct. Foods 2020, 72, 104048. [Google Scholar] [CrossRef]
- Maurice, T.; Lockhart, B.P.; Privat, A. Amnesia induced in mice by centrally administered β-amyloid peptides involves cholinergic dysfunction. Brain Res. 1996, 706, 181–193. [Google Scholar] [CrossRef]
- Franklin, K.B.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Lee, Y.W.; Kim, D.H.; Jeon, S.J.; Park, S.J.; Kim, J.M.; Jung, J.M.; Lee, H.E.; Bae, S.G.; Oh, H.K.; Son, K.H.; et al. Neuroprotective effects of salvianolic acid B on an Aβ25-35 peptide-induced mouse model of Alzheimer’s disease. Eur. J. Pharmacol. 2013, 704, 70–77. [Google Scholar] [CrossRef]
- Montgomery, K.C. A test of two explanations of spontaneous alternation. J. Comp. Physiol. Psychol. 1952, 45, 287. [Google Scholar] [CrossRef]
- Bevins, R.A.; Besheer, J. Object recognition in rats and mice: A one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat. Protoc. 2006, 1, 1306–1311. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Argmann, C.A.; Auwerx, J. Collection of blood and plasma from the mouse. Curr. Protoc. Mol. Biol. 2006, 75, 29A-3. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, A.Y.; Park, C.H.; Shin, Y.S.; Cho, E.J. Protective effect of Carthamus tinctorius L. seed on oxidative stress and cognitive impairment induced by chronic alcohol consumption in mice. Food Sci. Biortechnol. 2018, 27, 1475–1484. [Google Scholar] [CrossRef]
- Kim, J.H.; He, M.T.; Kim, M.J.; Yang, C.Y.; Shin, Y.S.; Yokozawa, T.; Park, C.H.; Cho, E.J. Safflower (Carthamus tinctorius L.) seed attenuates memory impairment induced by scopolamine in mice via regulation of cholinergic dysfunction and oxidative stress. Food Funct. 2019, 10, 3650–3659. [Google Scholar] [CrossRef]
- Sakamura, S.; Terayama, Y.; Kawakatsu, S.; Ichihara, A.; Saito, H. Conjugated serotonins and phenolic constituents in safflower seed (Carthamus tinctorius L.). Agric. Biol. Chem. 1980, 44, 2951–2954. [Google Scholar] [CrossRef]
- Roh, J.S.; Han, J.Y.; Kim, J.H.; Hwang, J.K. Inhibitory effects of active compounds isolated from safflower (Carthamus tinctorius L.) seeds for melanogenesis. Biol. Pharm. Bull. 2004, 27, 1976–1978. [Google Scholar] [CrossRef] [Green Version]
- Koyama, N.; Kuribayashi, K.; Seki, T.; Kobayashi, K.; Furuhata, Y.; Suzuki, K.; Arisaka, H.; Nakano, T.; Amino, Y.; Ishii, K. Serotonin derivatives, major safflower (Carthamus tinctorius L.) seed antioxidants, inhibit low-density lipoprotein (LDL) oxidation and atherosclerosis in apolipoprotein E-deficient mice. J. Agric. Food Chem. 2006, 54, 4970–4976. [Google Scholar] [CrossRef]
- Piga, R.; Naito, Y.; Kokura, S.; Handa, O.; Yoshikawa, T. Protective effect of serotonin derivatives on glucose-induced damage in PC12 rat pheochromocytoma cells. Br. J. Nutr. 2010, 103, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Yoon, C.S.; Ko, W.; Lee, D.S.; Kim, D.C.; Kim, J.; Choi, M.; Beom, J.S.; An, R.B.; Oh, H.; Kim, Y.C. Taraxacum coreanum protects against glutamate-induced neurotoxicity through heme oxygenase-1 expression in mouse hippocampal HT22 cells. Mol. Med. Rep. 2017, 15, 2347–2352. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Han, S.; Kim, H.M.; Lee, J.M.; Mok, S.Y.; Lee, S. Isolation and identification of phytochemical constituents from Taraxacum coreanum. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 73–78. [Google Scholar] [CrossRef]
- Lee, K.H.; Whang, W.K. Inhibitory effects of bioassay-guided isolation of anti-glycation components from Taraxacum coreanum and simultaneous quantification. Molecules 2018, 23, 2148. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Chen, Y.; Shen, C.; Xiao, Y.; Wang, Y.; Liu, Z.; Liu, X. Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-κB. FASEB J. 2017, 31, 1494–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, H. Multitarget therapy-the future of treatment for more than just functional dyspepsia. Phytomedicine 2006, 13, 122–129. [Google Scholar] [CrossRef]
- Qi, Y.; Cheng, X.; Jing, H.; Yan, T.; Xiao, F.; Wu, B.; Bi, K.; Jia, Y. Combination of schisandrin and nootkatone exerts neuroprotective effect in Alzheimer’s disease mice model. Metab. Brain Dis. 2019, 34, 1689–1703. [Google Scholar] [CrossRef]
- Lan, Z.; Xie, G.; Wei, M.; Wang, P.; Chen, L. The protective effect of Epimedii Folium and Curculiginis Rhizoma on Alzheimer’s disease by the inhibitions of NF-κB/MAPK pathway and NLRP3 inflammasome. Oncotarget 2017, 8, 43709. [Google Scholar] [CrossRef] [Green Version]
- Dudchenko, P.A. An overview of the tasks used to test working memory in rodents. Neurosci. Biobehav. Rev. 2004, 28, 699–709. [Google Scholar] [CrossRef]
- Huang, X.J.; Choi, Y.K.; Im, H.S.; Yarimaga, O.; Yoon, E.; Kim, H.S. Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors 2006, 6, 756–782. [Google Scholar] [CrossRef] [Green Version]
- Andreeva, T.V.; Lukiw, W.; Rogaev, E.I. Biological basis for amyloidogenesis in Alzheimer’s disease. Biochemistry 2017, 82, 122–139. [Google Scholar] [CrossRef]
- Sprecher, C.A.; Grant, F.J.; Grimm, G.; O’Hara, P.J.; Norris, F.; Norris, K.; Foster, D.C. Molecular cloning of the cDNA for a human amyloid precursor protein homolog: Evidence for a multigene family. Biochemistry 1993, 32, 4481–4486. [Google Scholar] [CrossRef]
- Tsuruma, K.; Tanaka, Y.; Shimazawa, M.; Hara, H. Induction of amyloid precursor protein by the neurotoxic peptide, amyloid-beta 25-35, causes retinal ganglion cell death. J. Neurochem. 2010, 113, 1545–1554. [Google Scholar] [CrossRef]
- Schmitt, T.L.; Steiner, E.; Trieb, K.; Grubeck-Loebenstein, B. Amyloid β-protein25-35 increases cellular APP and inhibits the secretion of APPs in human extraneuronal cells. Exp. Cell Res. 1997, 234, 336–340. [Google Scholar] [CrossRef]
- Koh, E.J.; Kim, K.J.; Song, J.H.; Choi, J.; Lee, H.Y.; Kang, D.H.; Heo, H.J.; Lee, B.Y. Spirulina maxima extract ameliorates learning and memory impairments via inhibiting GSK-3β phosphorylation induced by intracerebroventricular injection of amyloid-β 1-42 in mice. Int. J. Mol. Sci. 2017, 18, 2401. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.; Khan, A.; Lee, H.J.; Ur Rehman, I.; Khan, I.; Alam, S.I.; Kim, M.O. Lupeol, a plant-derived flavonoid, protects mice brains against Aβ-induced oxidative stress and neurodegeneration. Biomedicines 2020, 8, 380. [Google Scholar] [CrossRef]
- Cai, H.; Wang, Y.; McCarthy, D.; Wen, H.; Borchelt, D.R.; Price, D.L.; Wong, P.C. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat. Neurosci. 2001, 4, 233–234. [Google Scholar] [CrossRef]
- Krishnaswamy, S.; Verdile, G.; Groth, D.; Kanyenda, L.; Martins, R.N. The structure and function of Alzheimer’s gamma secretase enzyme complex. Crit. Rev. Clin. Lab. Sci. 2009, 46, 282–301. [Google Scholar] [CrossRef]
- Zussy, C.; Brureau, A.; Keller, E.; Marchal, S.; Blayo, C.; Delair, B.; Ixart, G.; Maurice, T.; Givalois, L. Alzheimer’s disease related markers, cellular toxicity and behavioral deficits induced six weeks after oligomeric amyloid-β peptide injection in rats. PLoS ONE 2013, 8, e53117. [Google Scholar] [CrossRef]
- Lu, C.D.; Ma, J.K.; Luo, Z.Y.; Tai, Q.X.; Wang, P.; Guan, P.P. Transferrin is responsible for mediating the effects of iron ions on the regulation of anterior pharynx-defective-1α/β and Presenilin 1 expression via PGE2 and PGD2 at the early stage of Alzheimer’s Disease. Aging (Albany NY) 2018, 10, 3117. [Google Scholar] [CrossRef]






| Groups | Body Weight (g) | |||||
|---|---|---|---|---|---|---|
| Stocked Day | Injection | Intragastric Administration | Behavioral Experiment | Dissection | Body Weight Gain | |
| Normal | 27.4 ± 1.3 NS | 32.3 ± 1.3 NS | 33.4 ± 1.1 NS | 33.6 ± 1.6 NS | 33.8 ± 1.3 NS | 6.4 ± 0.6 NS |
| Control | 27.6 ± 1.1 | 32.0 ± 0.3 | 33.4 ± 1.0 | 34.6 ± 1.6 | 34.2 ± 1.4 | 6.6 ± 0.5 |
| CT50 | 27.1 ± 1.1 | 32.1 ± 0.9 | 33.0 ± 1.1 | 33.6 ± 1.5 | 33.0 ± 1.6 | 6.0 ± 1.2 |
| CT100 | 27.5 ± 0.6 | 33.2 ± 1.1 | 33.9 ± 1.0 | 34.5 ± 1.7 | 33.5 ± 1.1 | 6.0 ± 1.1 |
| CT200 | 27.6 ± 1.3 | 32.4 ± 1.4 | 33.7 ± 1.6 | 33.7 ± 1.7 | 33.5 ± 2.2 | 5.9 ± 1.4 |
| DO | 26.8 ± 0.8 | 32.8 ± 0.8 | 32.8 ± 2.1 | 33.0 ± 1.2 | 32.3 ± 1.0 | 5.5 ± 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Kim, J.; Park, C.; Cho, E. Herbal Mixture of Carthamus tinctorius L. Seed and Taraxacum coreanum Attenuates Amyloid Beta-Induced Cognitive Dysfunction In Vivo. Foods 2022, 11, 142. https://doi.org/10.3390/foods11020142
He M, Kim J, Park C, Cho E. Herbal Mixture of Carthamus tinctorius L. Seed and Taraxacum coreanum Attenuates Amyloid Beta-Induced Cognitive Dysfunction In Vivo. Foods. 2022; 11(2):142. https://doi.org/10.3390/foods11020142
Chicago/Turabian StyleHe, Meitong, Jihyun Kim, Chanhum Park, and Eunju Cho. 2022. "Herbal Mixture of Carthamus tinctorius L. Seed and Taraxacum coreanum Attenuates Amyloid Beta-Induced Cognitive Dysfunction In Vivo" Foods 11, no. 2: 142. https://doi.org/10.3390/foods11020142
APA StyleHe, M., Kim, J., Park, C., & Cho, E. (2022). Herbal Mixture of Carthamus tinctorius L. Seed and Taraxacum coreanum Attenuates Amyloid Beta-Induced Cognitive Dysfunction In Vivo. Foods, 11(2), 142. https://doi.org/10.3390/foods11020142