Nutritional Changes and Early Warning of Moldy Rice under Different Relative Humidity and Storage Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Equipment
2.3. Experimental Methods
2.3.1. Rice Storage
2.3.2. Sample Preparation
2.3.3. Measurement of the Moisture Content
2.3.4. Measurement of Fatty Acid Content
2.3.5. Changes in the Total Starch Content
2.3.6. Changes in Peak Viscosity
2.3.7. Total Count of Mold Colonies
2.3.8. Scanning Electron Microscope Irradiation
2.3.9. Infrared Spectrum Measurement
2.4. Statistical Analysis
3. Results
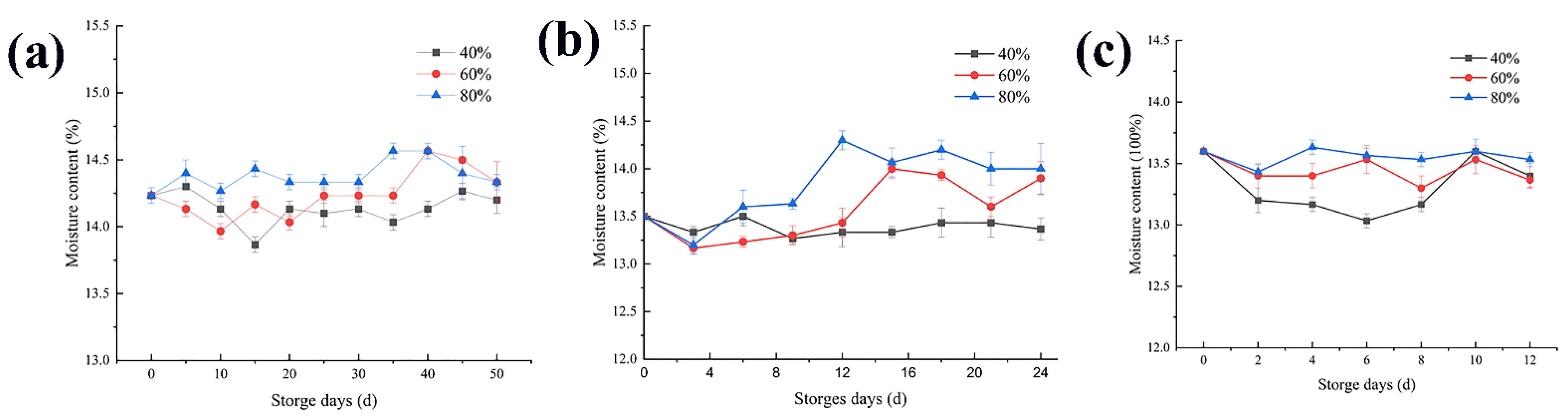
3.1. Changes in the Moisture Content
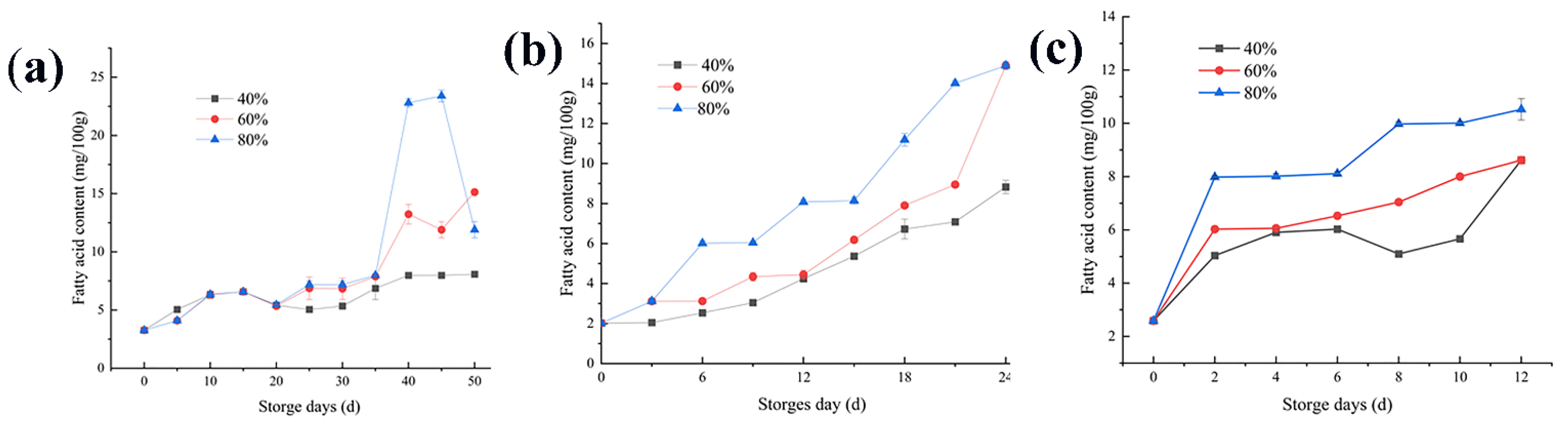
3.2. Changes in the Fatty Acid Content
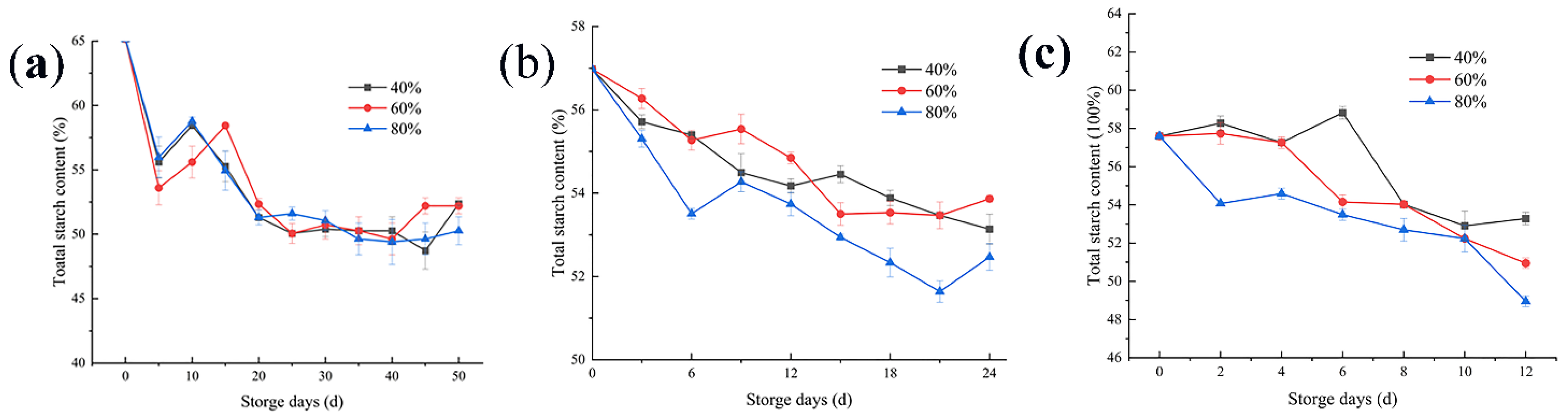
3.3. Changes in the Total Starch Content
3.4. Changes in Peak Viscosity
3.5. Changes in the Number of Mold Colonies
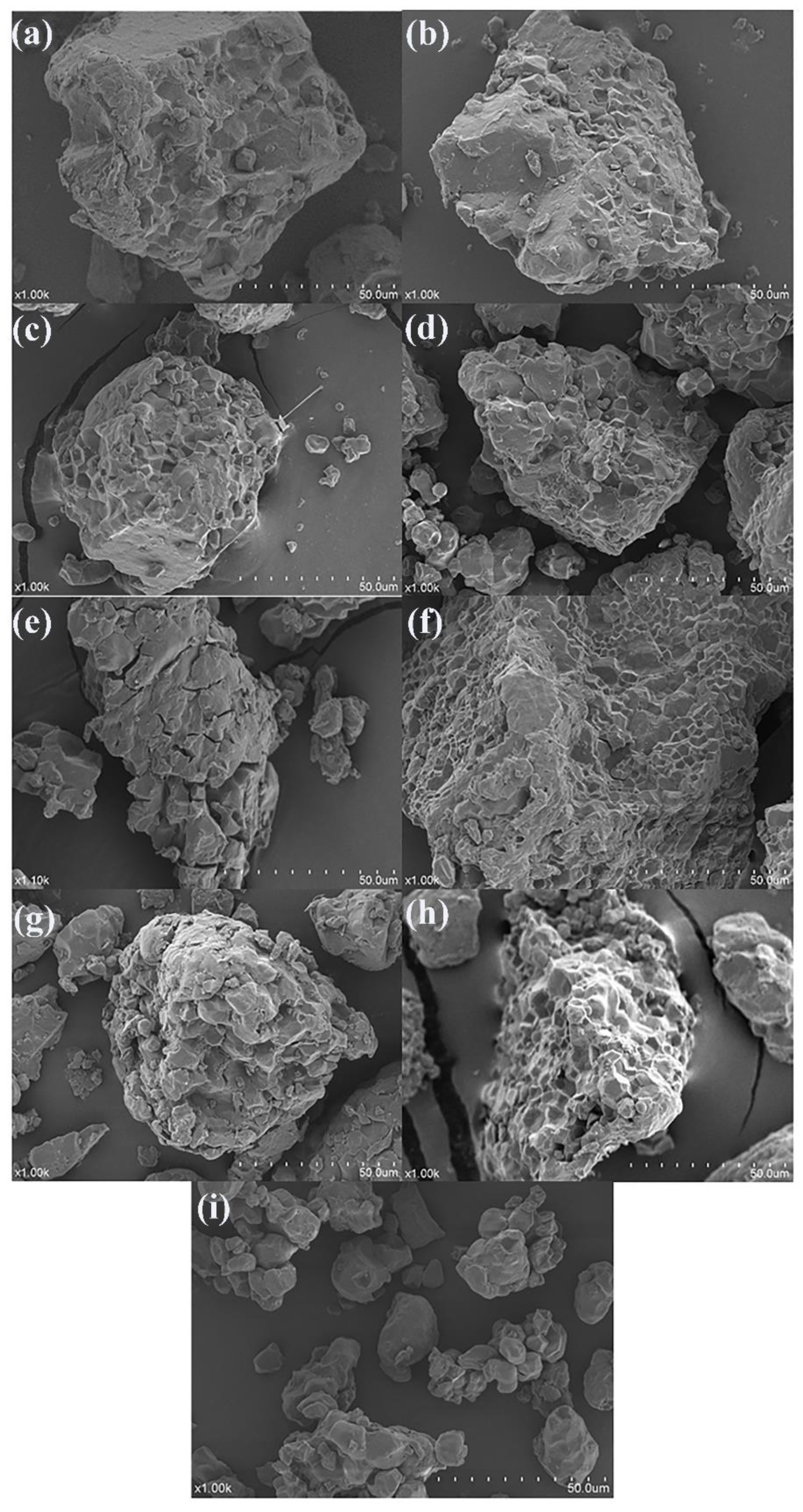
3.6. Analysis of the SEM
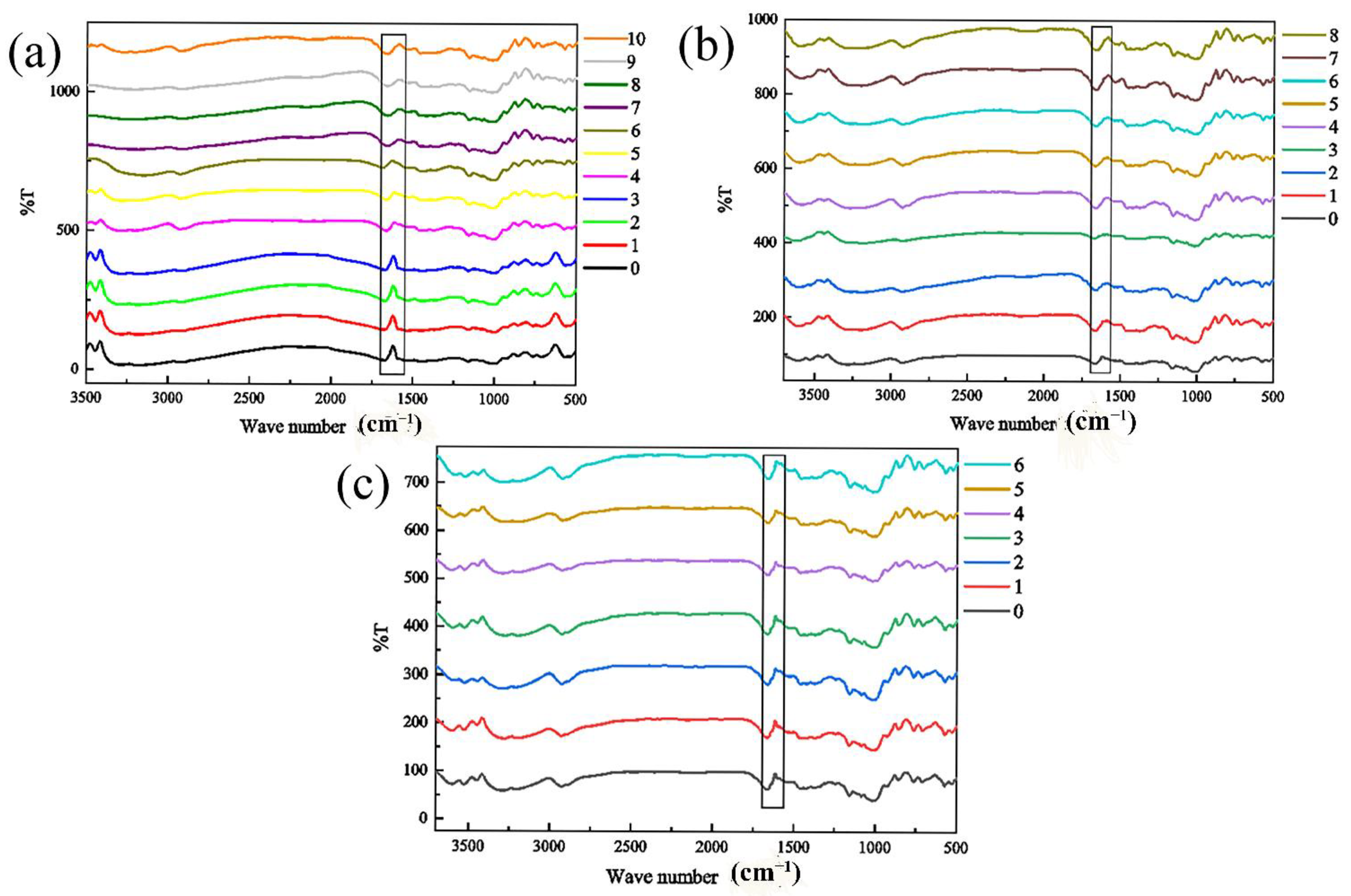
3.7. Analysis of Infrared Measurement
4. Discussion
4.1. Changes in the Moisture Content
4.2. Changes in the Fatty Acid Content
4.3. Changes in the Total Starch Content
4.4. Changes in the Peak Viscosity
4.5. Changes in the Number of Mold Colonies
4.6. Analysis of the SEM
4.7. Analysis of Infrared Measurement
4.8. Study on the Early Warning of the Mildew Temperature and Humidity of Rice
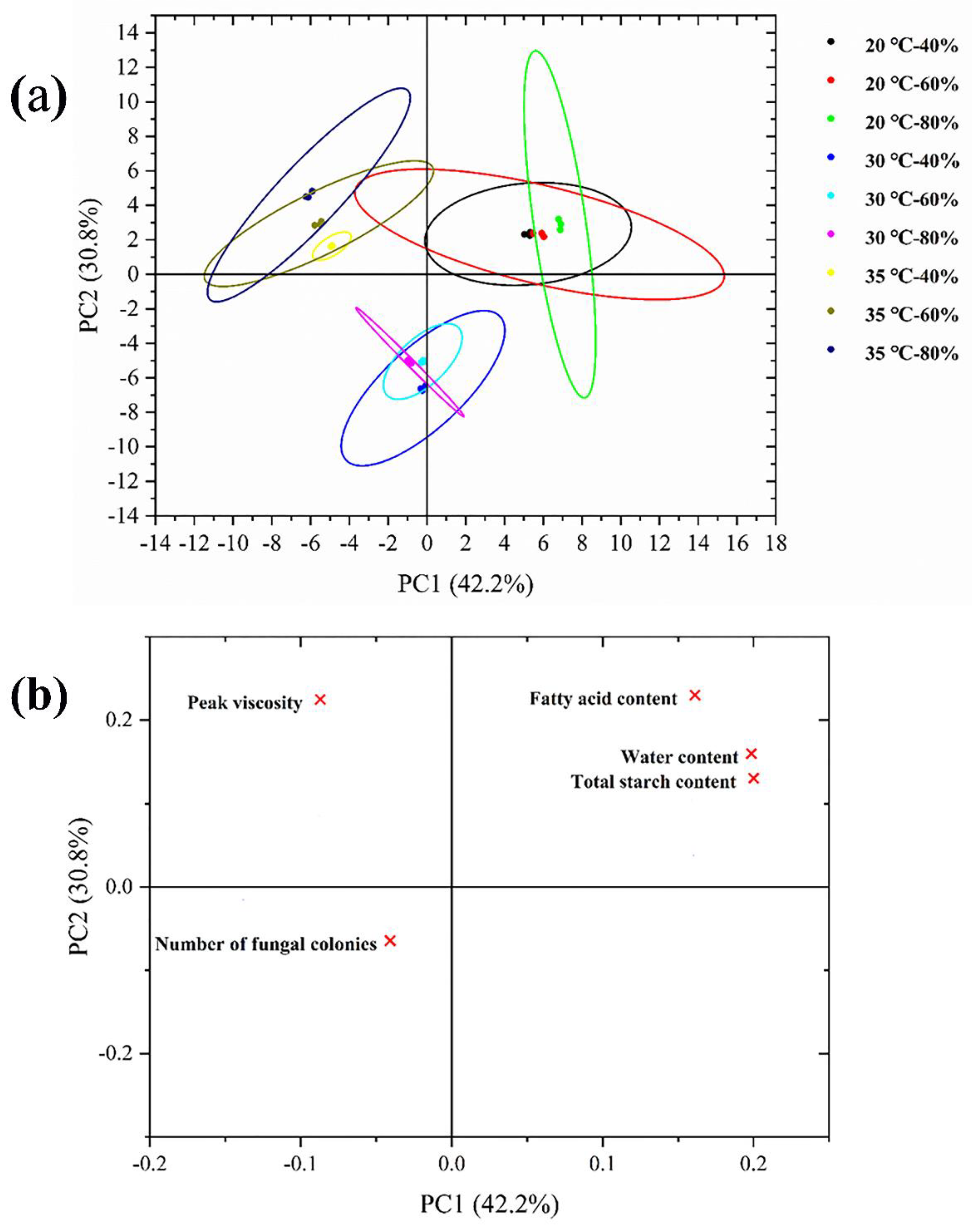
4.9. PCA Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perdon, A.A.; Tang, J.; Buescher, R.W.; Gbur, E.E. Starch Retrogradation and Texture of Cooked Milled Rice During Storage. J. Food Sci. 1999, 64, 828–832. [Google Scholar] [CrossRef]
- Van Nguyen, N.; Ferrero, A. Meeting the challenges of global rice production. Paddy Water Environ. 2006, 4, 1–9. [Google Scholar] [CrossRef]
- Peng, S.; Tang, Q.; Zou, Y. Current Status and Challenges of Rice Production in China. Plant Prod. Sci. 2015, 12, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; She, N.; Wang, M.; Zhang, B.; Qin, J.; Dong, J.; Fang, G.; Wang, S. Changes in Physicochemical Properties and Qualities of Red Brown Rice at Different Storage Temperatures. Foods 2021, 10, 2658. [Google Scholar] [CrossRef] [PubMed]
- Fleurat-Lessard, F. Integrated management of the risks of stored grain spoilage by seedborne fungi and contamination by storage mould mycotoxins—An update. J. Stored Prod. Res. 2017, 71, 22–40. [Google Scholar] [CrossRef]
- Atungulu, G.G.; Thote, S.; Wilson, S. Storage of hybrid rough rice—Consideration of microbial growth kinetics and prediction models. J. Stored Prod. Res. 2016, 69, 235–244. [Google Scholar] [CrossRef]
- Carvalho, M.O.; Fradinho, P.; Martins, M.J.; Magro, A.; Raymundo, A.; Sousa, I. Paddy rice stored under hermetic conditions: The effect of relative humidity, temperature and storage time in suppressing Sitophilus zeamais and impact on rice quality. J. Stored Prod. Res. 2019, 80, 21–27. [Google Scholar] [CrossRef]
- Chidanand, K.D.; Chidanand, D.V.; Srinivasan, K.; Shanmugasundaram, S.; Alice, R.P.; Sujeetha, J. Chemical Changes during rice storage at metal silo: Fatty acid profile changes. Asian J. Pharm. Clin. Res. 2016, 9, 121–125. [Google Scholar]
- Gobbi, E.; Falasconi, M.; Torelli, E.; Sberveglieri, G. Electronic nose predicts high and low fumonisin contamination in maize cultures. Food Res. Int. 2011, 44, 992–999. [Google Scholar] [CrossRef]
- Zhou, J. The biochemistry during grian mildewing. J. Grain Storage 2004, 1, 9–12. [Google Scholar]
- Park, C.-E.; Kim, Y.-S.; Park, K.-J.; Kim, B.-K. Changes in physicochemical characteristics of rice during storage at different temperatures. J. Stored Prod. Res. 2012, 48, 25–29. [Google Scholar] [CrossRef]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. Effect of storage temperature on rice thermal properties. Food Res. Int. 2010, 43, 709–715. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Wu, G.; Qi, X.; Dag, D.; Kong, F.; Zhang, H. Characteristics of pasting properties and morphology changes of rice starch and flour under different heating modes. Int. J. Biol. Macromol. 2020, 149, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. Effect of storage temperature on cooking behaviour of rice. Food Chem. 2007, 105, 491–497. [Google Scholar] [CrossRef]
- Chemists (AACC). Approved Methods of the American Association of Cereal Chemists; American Association of Cereal Chemists, Inc.: St. Paul, MN, USA, 2000. [Google Scholar]
- Choi, S.; Seo, H.-S.; Lee, K.R.; Lee, S.; Lee, J.; Lee, J. Effect of milling and long-term storage on volatiles of black rice (Oryza sativa L.) determined by headspace solid-phase microextraction with gas chromatography-mass spectrometry. Food Chem. 2019, 276, 572–582. [Google Scholar] [CrossRef]
- Yang, C.Z.; Shu, X.L.; Zhang, L.L.; Wang, X.Y.; Zhao, H.J.; Ma, A.C.X.; Wu, D.X. Starch Properties of Mutant Rice High in Resistant Starch. J. Agric. Food Chem. 2019, 54, 523–528. [Google Scholar] [CrossRef]
- Standard, I. Cereals and milled cereal products -Determination of the viscosity of flour—Method using an amylograph. Int. Stand. 1992, 7973, 1. [Google Scholar]
- GB 4789.15-2016. Food Microbiology Tests for Mold and Yeast Counts; The Standardization Administration of the People’s Republic of China: Beijing, China, 2010.
- Qin, J.; Su, Z.; Mao, Y.; Liu, C.; Qi, B.; Fang, G.; Wang, S. Carboxyl-functionalized hollow polymer microspheres for detection of trace metal elements in complex food matrixes by ICP-MS assisted with solid-phase extraction. Ecotoxicol. Environ. Saf. 2021, 208, 111729. [Google Scholar] [CrossRef]
- Zheng, H.; Du, X.; Guo, L.; Hu, J.; Xu, Y.; Zhao, H. Using NMR to study changes in the characteristic constituents of stored rice. J. Cereal Sci. 2017, 75, 179–185. [Google Scholar] [CrossRef]
- Bhat, F.M.; Riar, C.S. Effect of composition, granular morphology and crystalline structure on the pasting, textural, thermal and sensory characteristics of traditional rice cultivars. Food Chem. 2019, 280, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, T.; Geng, S.; Liang, F.; Wang, T. Effect of accumulated temperature on flavour and microbial diversity of japonica rice during storage. J. Stored Prod. Res. 2021, 92, 101779. [Google Scholar] [CrossRef]
- Luthra, S.; Kalonia, D.S.; Pikal, M.J. Effect of hydration on the secondary structure of lyophilized proteins as measured by fourier transform infrared (FTIR) spectroscopy. J. Pharm. Sci. 2007, 96, 2910–2921. [Google Scholar] [CrossRef] [PubMed]
- Putthang, R.; Sirisomboon, C.D. Shortwave Near-Infrared Spectroscopy for Rapid Detection of Aflatoxin B 1 Contamination in Polished Rice. J. Food Prot. 2019, 82, 796–803. [Google Scholar] [CrossRef]
- Yilmaz, F.; Yilmaz Tuncel, N.; Tuncel, N.B. Stabilization of immature rice grain using infrared radiation. Food Chem. 2018, 253, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Saikrishna, A.; Dutta, S.; Subramanian, V.; Moses, J.A.; Anandharamakrishnan, C. Ageing of rice: A review. J. Cereal Sci. 2018, 81, 161–170. [Google Scholar] [CrossRef]
- Yang, X.; Hao, J. Study on a correlation between the fatty acid value change and mould of paddy in different storage conditions. Grain Storage 2006, 35, 49–52. [Google Scholar]
- Thammapat, P.; Meeso, N.; Siriamornpun, S. Effects of the traditional method and an alternative parboiling process on the fatty acids, vitamin E, gamma-oryzanol and phenolic acids of glutinous rice. Food Chem. 2016, 194, 230–236. [Google Scholar] [CrossRef]
- Tao, K.; Li, C.; Yu, W.; Li, E. How amylose molecular fine structure of rice starch affects functional properties. Carbohydr. Polym. 2018, 204, 24–31. [Google Scholar] [CrossRef]
- Balindong, J.L.; Ward, R.M.; Liu, L.; Rose, T.; Pallas, L.A.; Ovenden, B.W.; Snell, P.J.; Waters, D. Rice grain protein composition influences instrumental measures of rice cooking and eating quality. J. Cereal Sci. 2018, 79, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Kraic, J.; Mihalik, D.; Klčová, L.; Gubišová, M.; Klempová, T.; Hudcovicová, M.; Ondreičková, K.; Mrkvová, M.; Havrlentová, M.; Gubiš, J.; et al. Progress in the genetic engineering of cereals to produce essential polyunsaturated fatty acids. J. Biotechnol. 2018, 284, 115–122. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, S.; Ramaswamy, H.S.; Hu, F.; Yu, Y. Effect of high pressure processing on rancidity of brown rice during storage. LWT 2018, 93, 405–411. [Google Scholar] [CrossRef]
- Tang, S.; Zhou, Y.; Yao, M.; Shao, B.; Cereal, J. Study on the quality changes of storage of germinated brown rice at different temperatures. Feed Ind. 2013, 3. [Google Scholar]
- Zhu, L.; Wu, G.; Cheng, L.; Zhang, H.; Wang, L.; Qian, H.; Qi, X. Effect of soaking and cooking on structure formation of cooked rice through thermal properties, dynamic viscoelasticity, and enzyme activity. Food Chem. 2019, 289, 616–624. [Google Scholar] [CrossRef]
- Yan, C.-J.; Tian, Z.-X.; Fang, Y.-W.; Yang, Y.-C.; Li, J.; Zeng, S.-Y.; Gu, S.-L.; Xu, C.-W.; Tang, S.-Z.; Gu, M.-H. Genetic analysis of starch paste viscosity parameters in glutinous rice (Oryza sativa L.). Theor. Appl. Genet. 2011, 122, 63–76. [Google Scholar] [CrossRef]
- Ziegler, V.; Ferreira, C.D.; Goebel, J.T.S.; El Halal, S.L.M.; Santetti, G.S.; Gutkoski, L.C.; Zavareze, E.D.R.; Elias, M.C. Changes in properties of starch isolated from whole rice grains with brown, black, and red pericarp after storage at different temperatures. Food Chem. 2017, 216, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Tu, Z.; Liu, C.; Liu, W.; Xu, X.; Ai, Y.; Liu, W.; Chen, J.; Wu, J. Effect of microwave irradiation on composition, structure and properties of rice (Oryza sativa L.) with different milling degrees. J. Cereal Sci. 2013, 58, 228–233. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, X.; Si, X.; Blanchard, C.; Strappe, P. The ageing mechanism of stored rice: A concept model from the past to the present. J. Stored Products Res. 2015, 64, 80–87. [Google Scholar] [CrossRef]
- Luo, X.; Li, Y.; Yang, D.; Xing, J.; Li, K.; Yang, M.; Wang, R.; Wang, L.; Zhang, Y.; Chen, Z. Effects of electron beam irradiation on storability of brown and milled rice. J. Stored Prod. Res. 2019, 81, 22–30. [Google Scholar] [CrossRef]
- Al-Zoreky, N.S.; Saleh, F.A. Limited survey on aflatoxin contamination in rice. Saudi J. Biol. Sci. 2019, 26, 225–231. [Google Scholar] [CrossRef] [PubMed]


| Storge Days | α-Helix | β-Sheet | β-Turn | Random Coli |
|---|---|---|---|---|
| 0 days | 54.61% | 12.23% | 14.53% | 18.63% |
| 1 days | 49.81% | 10.64% | 12.36% | 27.21% |
| 2 days | 54.12% | 13.58% | 14.67% | 17.63% |
| 3 days | 47.96% | 12.15% | 14.62% | 25.27% |
| 4 days | 55.32% | 13.17% | 15.63% | 15.88% |
| 5 days | 53.63% | 12.89% | 13.98% | 19.50% |
| 6 days | 48.56% | 15.26% | 13.54% | 22.64% |
| 7 days | 50.23% | 14.89% | 13.95% | 20.93% |
| 8 days | 53.21% | 11.78% | 12.10% | 22.91% |
| 9 days | 55.69% | 14.78% | 14.62% | 14.91% |
| 10 days | 54.30% | 13.58% | 15.21% | 16.91% |
| Storge Days | α-Helix | β-Sheet | β-Turn | Random Coli |
|---|---|---|---|---|
| 0 days | 53.16% | 18.32% | 11.54% | 16.98% |
| 1 days | 52.64% | 15.26% | 10.98% | 21.12% |
| 2 days | 54.65% | 17.62% | 13.17% | 14.56% |
| 3 days | 49.85% | 20.16% | 14.52% | 15.47% |
| 4 days | 52.17% | 16.13% | 12.85% | 18.85% |
| 5 days | 50.64% | 13.69% | 11.27% | 24.40% |
| 6 days | 51.67% | 11.25% | 13.42% | 23.66% |
| 7 days | 51.29% | 12.56% | 15.86% | 20.29% |
| 8 days | 54.39% | 13.18% | 15.26% | 17.17% |
| Storge Days | α-Helix | β-Sheet | β-Turn | Random Coli |
|---|---|---|---|---|
| 1 days | 49.25% | 13.14% | 16.58% | 21.03% |
| 2 days | 52.17% | 11.89% | 14.00% | 21.94% |
| 3 days | 52.86% | 13.65% | 15.77% | 17.72% |
| 4 days | 50.29% | 11.64% | 14.89% | 23.09% |
| 5 days | 53.02% | 12.15% | 15.77% | 19.06% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Lin, Y.; Gao, Y.; Tian, Y.; Zhang, J.; Fang, G. Nutritional Changes and Early Warning of Moldy Rice under Different Relative Humidity and Storage Temperature. Foods 2022, 11, 185. https://doi.org/10.3390/foods11020185
Du J, Lin Y, Gao Y, Tian Y, Zhang J, Fang G. Nutritional Changes and Early Warning of Moldy Rice under Different Relative Humidity and Storage Temperature. Foods. 2022; 11(2):185. https://doi.org/10.3390/foods11020185
Chicago/Turabian StyleDu, Jing, Yingxue Lin, Yuan Gao, Yanyan Tian, Jixiang Zhang, and Guozhen Fang. 2022. "Nutritional Changes and Early Warning of Moldy Rice under Different Relative Humidity and Storage Temperature" Foods 11, no. 2: 185. https://doi.org/10.3390/foods11020185
APA StyleDu, J., Lin, Y., Gao, Y., Tian, Y., Zhang, J., & Fang, G. (2022). Nutritional Changes and Early Warning of Moldy Rice under Different Relative Humidity and Storage Temperature. Foods, 11(2), 185. https://doi.org/10.3390/foods11020185