Effects of Repeated Heating on Fatty Acid Composition of Plant-Based Cooking Oils
Abstract
1. Introduction
2. Materials and Methods
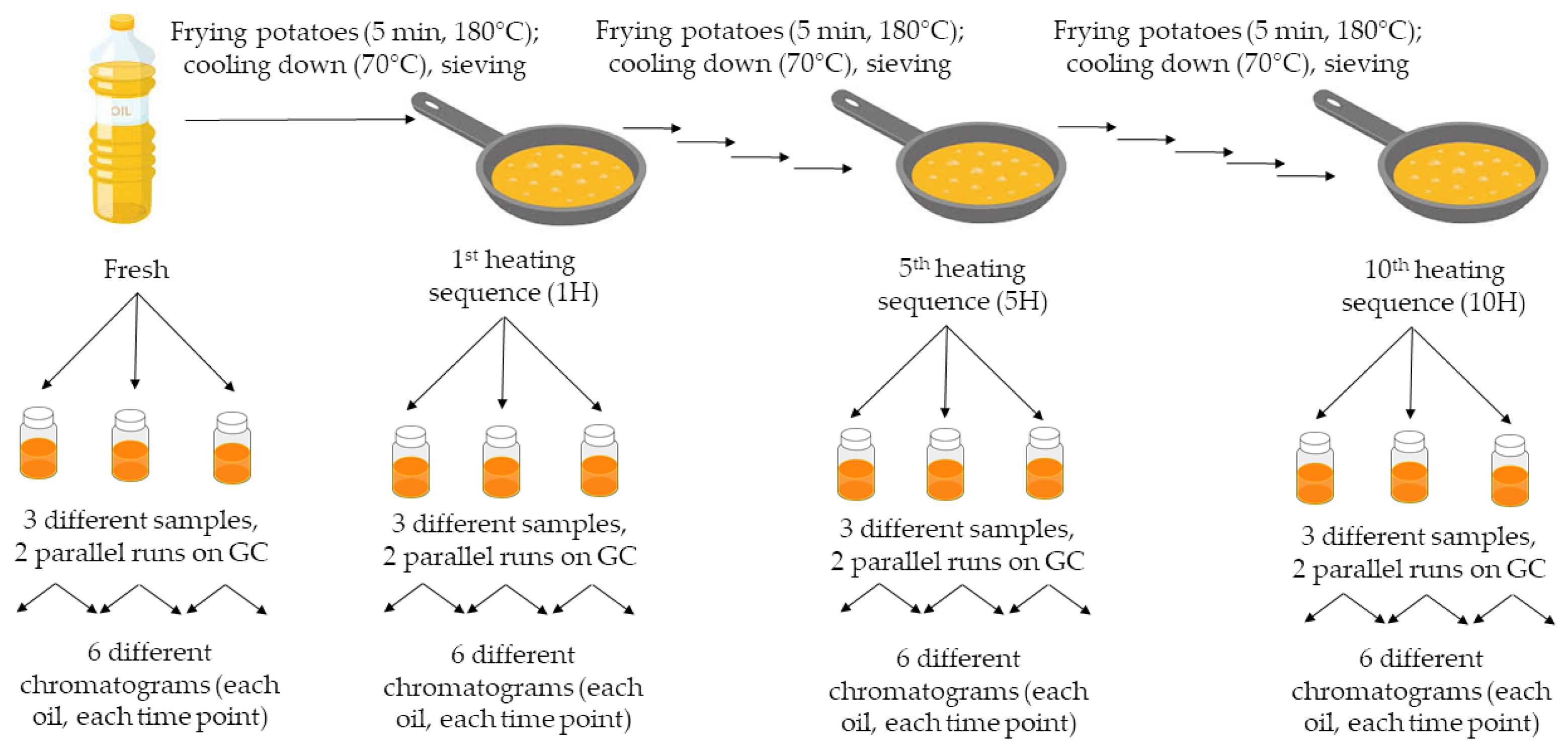
2.1. Frying Protocol
2.2. Reagents and Standards
2.3. Fatty Acid Analysis
2.4. Index Calculation
2.5. Statistical Analysis
3. Results
3.1. Fatty Acid Composition of Vegetable Oils
3.2. Changes in Fatty Acid Composition
3.2.1. Changes in LA Contents during the Heating Sequences
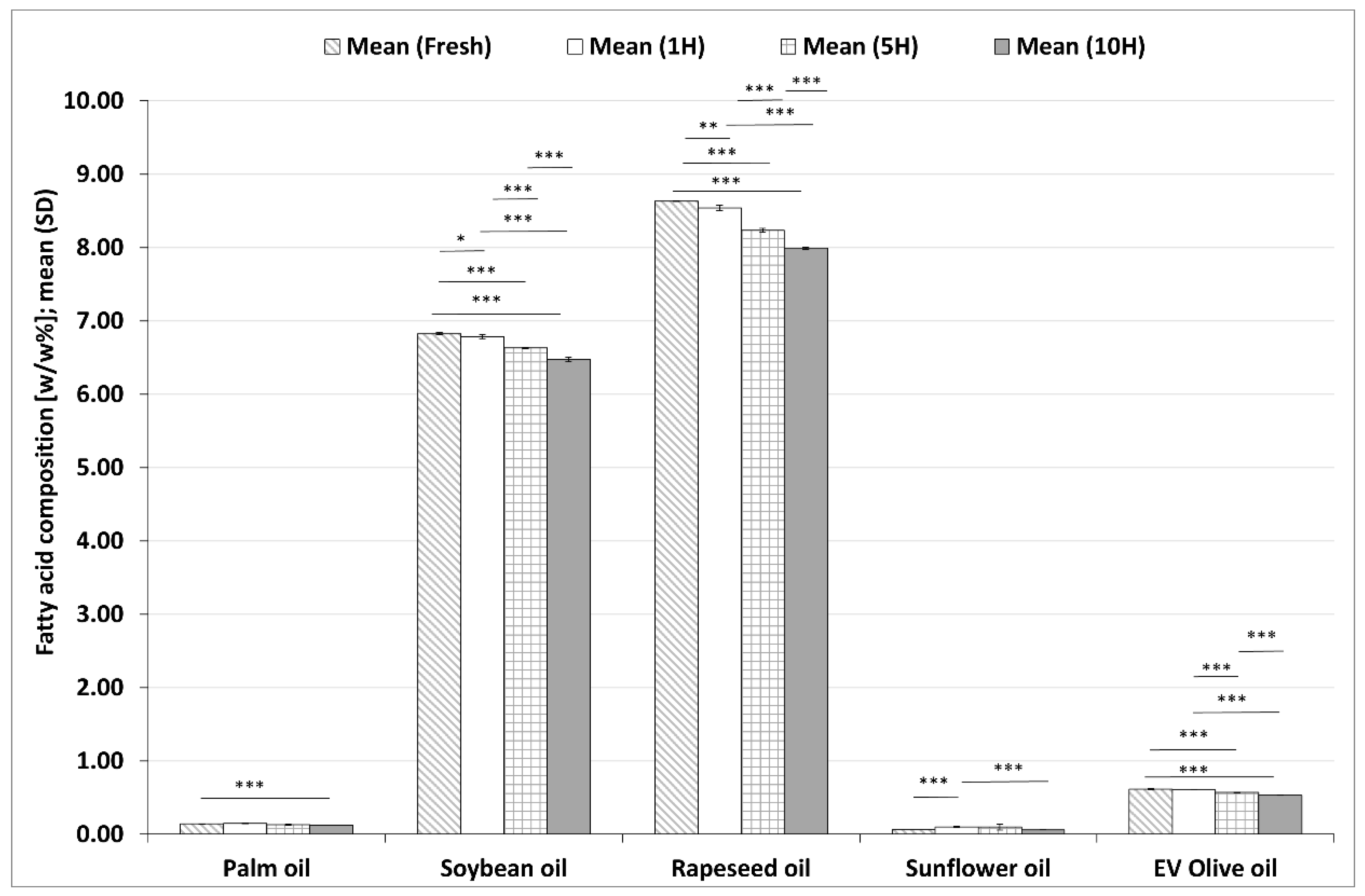
3.2.2. Changes in ALA Contents during the Heating Sequences
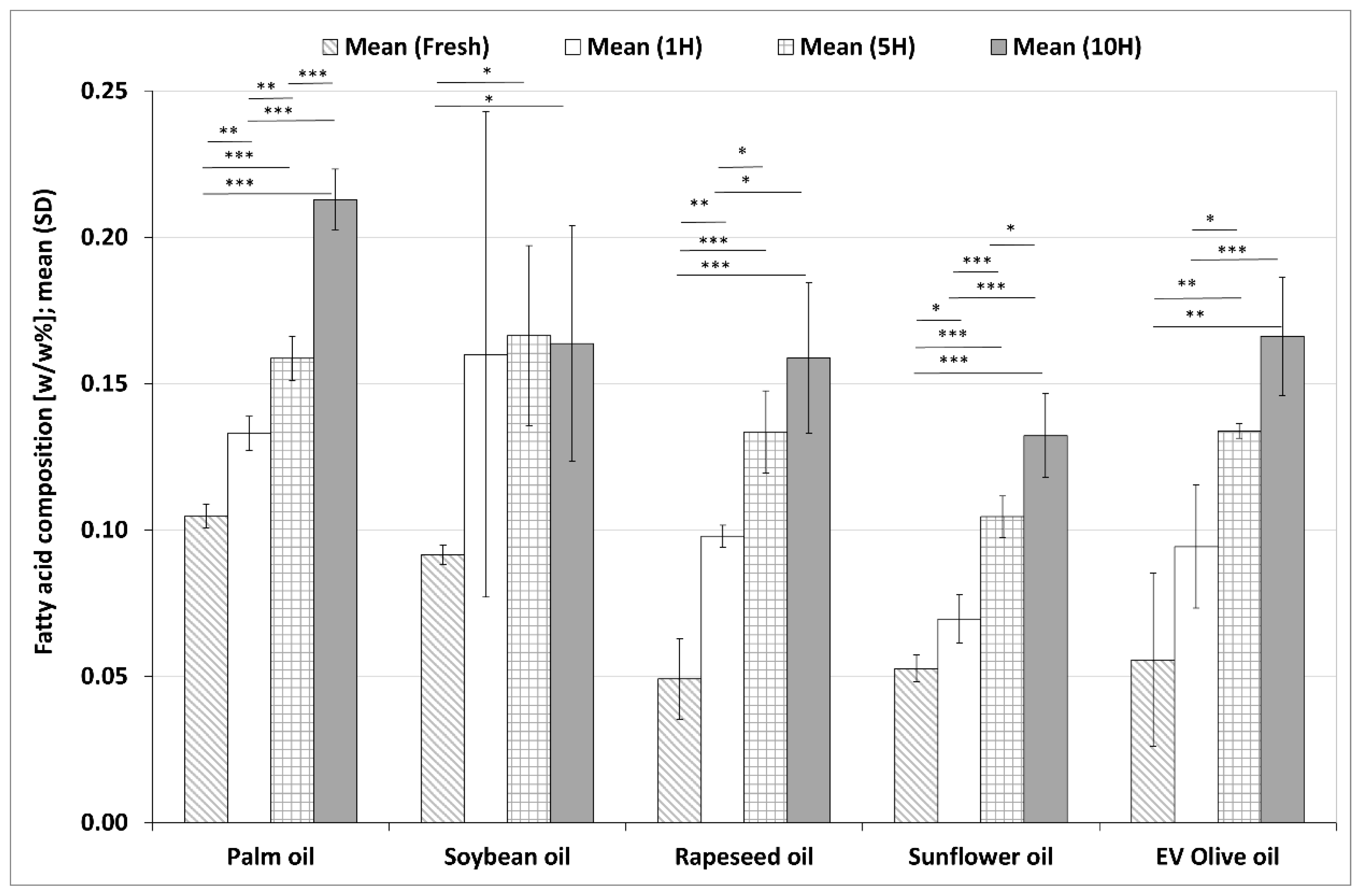
3.2.3. Changes in Total TFA Contents during the Heating Sequences
3.3. Changes in Nutritional Indices
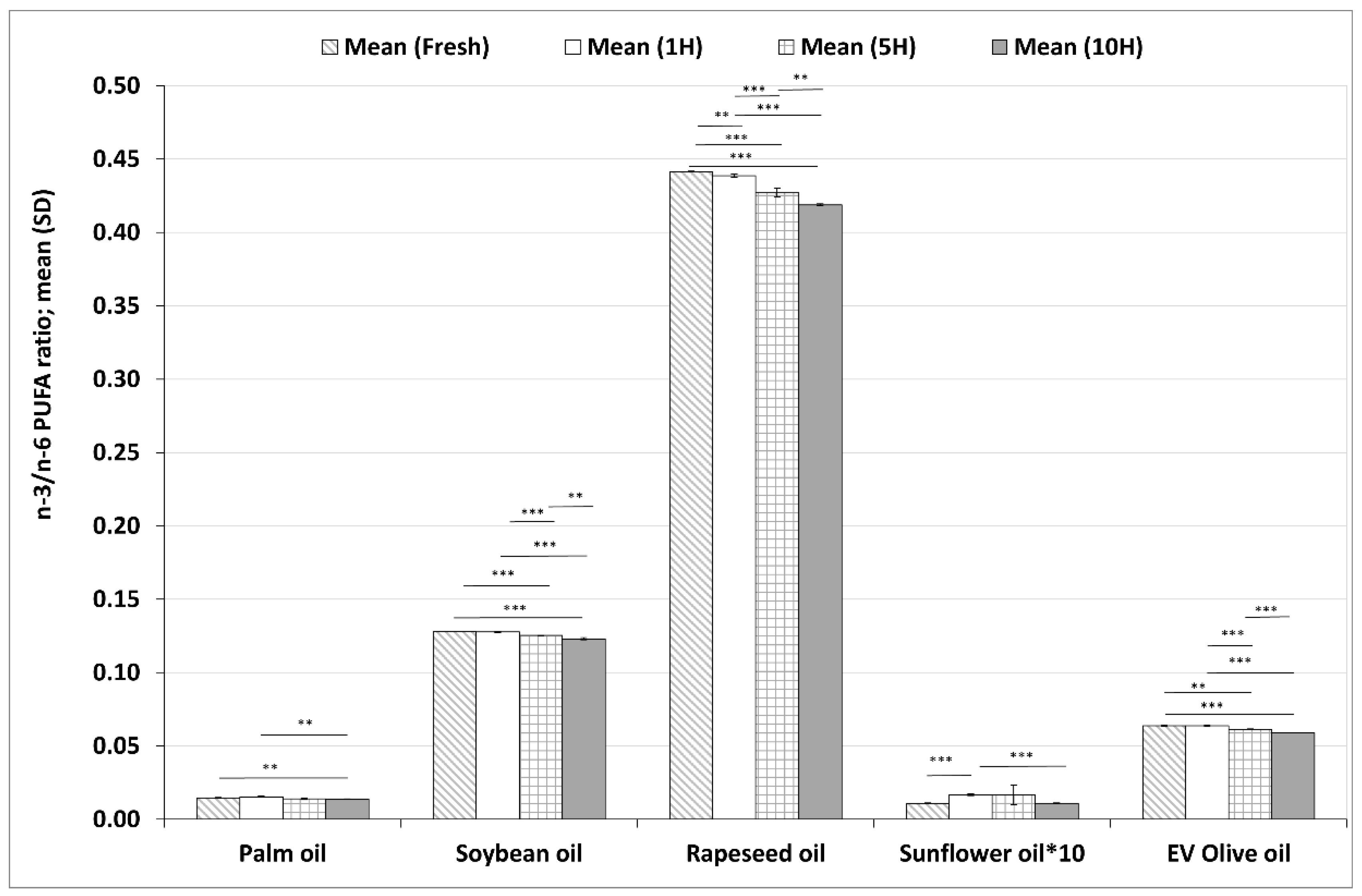
3.3.1. Changes in n–3/n–6 Polyunsaturated Fatty Acid Ratios during Heating Sequences
3.3.2. Changes in the Unsaturation Index (UI) during the Heating Sequences
3.3.3. Changes in the Index of Atherogenicity (IA) during Heating Sequences
3.3.4. Changes in the Index of Thrombogenicity (IT) during the Heating Sequences
3.3.5. Changes in the Hypocholesterolemic/Hypercholesterolemic Ratio (HH) during the Heating Sequences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lands, B. Consequences of essential fatty acids. Nutrients 2012, 4, 1338–1357. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids epa and dha: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 1991, 54, 438–463. [Google Scholar] [CrossRef]
- Innis, S.M. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008, 1237, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Oteng, A.B.; Kersten, S. Mechanisms of action of trans fatty acids. Adv. Nutr. 2020, 11, 697–708. [Google Scholar] [CrossRef]
- Kummerow, F.A. The negative effects of hydrogenated trans fats and what to do about them. Atherosclerosis 2009, 205, 458–465. [Google Scholar] [CrossRef]
- Raccuglia, G.; Privett, O.S. Influence of dietary trans,trans-linoleate on hematologic and hemostatic properties of rat blood. Lipids 1970, 5, 85–89. [Google Scholar] [CrossRef]
- Takatori, T.; Phillips, F.C.; Shimasaki, H.; Privett, O.S. Effects of dietary saturated and trans fatty acids on tissue lipid composition and serum lcat activity in the rat. Lipids 1976, 11, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Privett, O.S.; Phillips, F.; Shimasaki, H.; Nozawa, T.; Nickell, E.C. Studies of effects of trans fatty acids in the diet on lipid metabolism in essential fatty acid deficient rats. Am. J. Clin. Nutr. 1977, 30, 1009–1017. [Google Scholar] [CrossRef]
- Ascherio, A.; Katan, M.B.; Zock, P.L.; Stampfer, M.J.; Willett, W.C. Trans fatty acids and coronary heart disease. N. Engl. J. Med. 1999, 340, 1994–1998. [Google Scholar] [CrossRef]
- Mensink, R.P.; Katan, M.B. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N. Engl. J. Med. 1990, 323, 439–445. [Google Scholar] [CrossRef]
- Willett, W.C.; Stampfer, M.J.; Manson, J.E.; Colditz, G.A.; Speizer, F.E.; Rosner, B.A.; Sampson, L.A. Intake of trans fatty acids and risk of coronary heart disease among women. Lancet 1993, 341, 5. [Google Scholar] [CrossRef]
- Hudgins, L.C.; Hirsch, J.; Emken, E.A. Correlation of isomeric fatty acids in human adipose tissue with clinical risk factors for cardiovascular disease. Am. J. Clin. Nutr. 1991, 53, 474–482. [Google Scholar] [CrossRef]
- Gebauer, S.K.; Destaillats, F.; Dionisi, F.; Krauss, R.M.; Baer, D.J. Vaccenic acid and trans fatty acid isomers from partially hydrogenated oil both adversely affect ldl cholesterol: A double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Tavani, A.; Negri, E.; D’Avanzo, B.; La Vecchia, C. Margarine intake and risk of nonfatal acute myocardial infarction in italian women. Eur. J. Clin. Nutr. 1997, 51, 30–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pietinen, P.; Ascherio, A.; Korhonen, P.; Hartman, A.M.; Willett, W.C.; Albanes, D.; Virtamo, J. Intake of fatty acids and risk of coronary heart disease in a cohort of finnish men. The alpha-tocopherol, beta-carotene cancer prevention study. Am. J. Epidemiol. 1997, 145, 876–887. [Google Scholar] [CrossRef]
- Bendsen, N.T.; Haugaard, S.B.; Larsen, T.M.; Chabanova, E.; Stender, S.; Astrup, A. Effect of trans-fatty acid intake on insulin sensitivity and intramuscular lipids—A randomized trial in overweight postmenopausal women. Metabolism 2011, 60, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; de Oliveira Otto, M.C.; Lemaitre, R.N.; Fretts, A.M.; Hotamisligil, G.; Tsai, M.Y.; Siscovick, D.S.; Nettleton, J.A. Trans-palmitoleic acid, other dairy fat biomarkers, and incident diabetes: The multi-ethnic study of atherosclerosis (mesa). Am. J. Clin. Nutr. 2013, 97, 854–861. [Google Scholar] [CrossRef]
- Riserus, U.; Vessby, B.; Arner, P.; Zethelius, B. Supplementation with trans10cis12-conjugated linoleic acid induces hyperproinsulinaemia in obese men: Close association with impaired insulin sensitivity. Diabetologia 2004, 47, 1016–1019. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Katan, M.B.; Ascherio, A.; Stampfer, M.J.; Willett, W.C. Trans fatty acids and cardiovascular disease. N. Engl. J. Med. 2006, 354, 1601–1613. [Google Scholar] [CrossRef]
- de Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schunemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef]
- Stender, S.; Astrup, A.; Dyerberg, J. Ruminant and industrially produced trans fatty acids: Health aspects. Food Nutr. Res. 2008, 52, 1651. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Passi, S.J.; Misra, A.; Pant, K.K.; Anwar, K.; Pandey, R.M.; Kardam, V. Effect of heating/reheating of fats/oils, as used by asian indians, on trans fatty acid formation. Food Chem. 2016, 212, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Wu, G.; Zhang, H.; Jin, Q.; Wang, X. Deep-fried flavor: Characteristics, formation mechanisms, and influencing factors. Crit. Rev. Food Sci. Nutr. 2020, 60, 1496–1514. [Google Scholar] [CrossRef] [PubMed]
- Gere, A. A survey on operating conditions and quality of commercial frying fats in hungary. Z. Ernahr. 1985, 24, 120–132. [Google Scholar] [CrossRef]
- Dhaka, V.; Gulia, N.; Ahlawat, K.S.; Khatkar, B.S. Trans fats-sources, health risks and alternative approach—A review. J. Food Sci. Technol. 2011, 48, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Aro, A.; Willett, W.C. Health effects of trans-fatty acids: Experimental and observational evidence. Eur. J. Clin. Nutr. 2009, 63 (Suppl. S2), S5–S21. [Google Scholar] [CrossRef]
- Gadiraju, T.V.; Patel, Y.; Gaziano, J.M.; Djousse, L. Fried food consumption and cardiovascular health: A review of current evidence. Nutrients 2015, 7, 8424–8430. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Sukalingam, K.; Xu, B. Impact of consumption of repeatedly heated cooking oils on the incidence of various cancers—A critical review. Crit. Rev. Food Sci. Nutr. 2019, 59, 488–505. [Google Scholar] [CrossRef]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic aromatic hydrocarbons: From metabolism to lung cancer. Toxicol. Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef]
- Santos, C.S.P.; Garcia, L.M.; Cruz, R.; Cunha, S.C.; Fernandes, J.O.; Casal, S. Impact of potatoes deep-frying on common monounsaturated-rich vegetable oils: A comparative study. J. Food Sci. Technol. 2019, 56, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Chemistry of deep-fat frying oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef] [PubMed]
- Dobarganes, C.; Marquez-Ruiz, G. Possible adverse effects of frying with vegetable oils. Br. J. Nutr. 2015, 113 (Suppl. S2), S49–S57. [Google Scholar] [CrossRef] [PubMed]
- Gere, A. Decrease in essential fatty acid content of edible fats during the frying process. Z. Ernahr. 1982, 21, 191–201. [Google Scholar] [CrossRef]
- An, K.J.; Liu, Y.L.; Liu, H.L. Relationship between total polar components and polycyclic aromatic hydrocarbons in fried edible oil. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2017, 34, 1596–1605. [Google Scholar] [CrossRef]
- Multari, S.; Marsol-Vall, A.; Heponiemi, P.; Suomela, J.P.; Yang, B. Changes in the volatile profile, fatty acid composition and other markers of lipid oxidation of six different vegetable oils during short-term deep-frying. Food Res. Int. 2019, 122, 318–329. [Google Scholar] [CrossRef]
- Casal, S.; Malheiro, R.; Sendas, A.; Oliveira, B.P.; Pereira, J.A. Olive oil stability under deep-frying conditions. Food Chem. Toxicol. 2010, 48, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, T.L.; Southgate, D.A. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs ii. Fatty acid composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Chen, S.; Bobe, G.; Zimmerman, S.; Hammond, E.G.; Luhman, C.M.; Boylston, T.D.; Freeman, A.E.; Beitz, D.C. Physical and sensory properties of dairy products from cows with various milk fatty acid compositions. J. Agric. Food Chem. 2004, 52, 3422–3428. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Food Outlook. Available online: http://www.fao.org/3/cb4479en/cb4479en.pdf (accessed on 8 October 2021).
- United States Department of Agriculture. Oilseeds: World Markets and Trade. Available online: https://usda.library.cornell.edu/concern/publications/tx31qh68h?locale=en (accessed on 8 October 2021).
- The European Food Information Council. How to Choose Your Culinary Oil. Available online: https://www.eufic.org/en/whats-in-food/article/how-to-choose-your-culinary-oil (accessed on 30 May 2021).
- National Food Chain Safety Office. Útmutató a Vendéglátás és Étkeztetés jó Higiéniai Gyakorlatához. Available online: https://portal.nebih.gov.hu/-/vendeglatas-ghp (accessed on 8 October 2021).
- Szabó, É.; Marosvölgyi, T.; Szilágyi, G.; Kőrösi, L.; Schmidt, J.; Csepregi, K.; Márk, L.; Bóna, Á. Correlations between total antioxidant capacity, polyphenol and fatty acid content of native grape seed and pomace of four different grape varieties in hungary. Antioxidants 2021, 10, 1101. [Google Scholar] [CrossRef] [PubMed]
- Alireza, S.; Tan, C.P.; Hamed, M.; Che Man, Y.B. Effect of frying process on fatty acid composition and iodine value of selected vegetable oils and their blends. Int. Food Res. J. 2010, 17, 295–302. [Google Scholar]
- Cui, Y.; Hao, P.; Liu, B.; Meng, X. Effect of traditional chinese cooking methods on fatty acid profiles of vegetable oils. Food Chem. 2017, 233, 77–84. [Google Scholar] [CrossRef]
- Li, A.; Ha, Y.; Wang, F.; Li, W.; Li, Q. Determination of thermally induced trans-fatty acids in soybean oil by attenuated total reflectance fourier transform infrared spectroscopy and gas chromatography analysis. J. Agric. Food Chem. 2012, 60, 10709–10713. [Google Scholar] [CrossRef]
- Liu, W.H.; Inbaraj, S.B.; Chen, B.H. Analysis and formation of trans fatty acids in hydrogenated soybean oil during heating. Food Chem. 2007, 104, 10. [Google Scholar] [CrossRef]
- Naseri, M.; Abedi, E.; Mohammadzadeh, B.; Afsharnaderi, A. Effect of frying in different culinary fats on the fatty acid composition of silver carp. Food Sci. Nutr. 2013, 1, 292–297. [Google Scholar] [CrossRef]
- Song, J.; Park, J.; Jung, J.; Lee, C.; Gim, S.Y.; Ka, H.; Yi, B.; Kim, M.J.; Kim, C.I.; Lee, J. Analysis of trans fat in edible oils with cooking process. Toxicol. Res. 2015, 31, 307–312. [Google Scholar] [CrossRef]
- Troegeler-Meynadier, A.; Puaut, S.; Farizon, Y.; Enjalbert, F. Effects of the heating process of soybean oil and seeds on fatty acid biohydrogenation in vitro. J. Dairy Sci. 2014, 97, 5657–5667. [Google Scholar] [CrossRef] [PubMed]
- Zribi, A.; Jabeur, H.; Aladedunye, F.; Rebai, A.; Matthaus, B.; Bouaziz, M. Monitoring of quality and stability characteristics and fatty acid compositions of refined olive and seed oils during repeated pan- and deep-frying using gc, ft-nirs, and chemometrics. J. Agric. Food Chem. 2014, 62, 10357–10367. [Google Scholar] [CrossRef]
- Ansorena, D.; Guembe, A.; Mendizabal, T.; Astiasaran, I. Effect of fish and oil nature on frying process and nutritional product quality. J. Food Sci. 2010, 75, H62–H67. [Google Scholar] [CrossRef]
- Zotos, A.; Kotaras, A.; Mikras, E. Effect of baking of sardine (Sardina pilchardus) and frying of anchovy (Engraulis encrasicholus) in olive and sunflower oil on their quality. Food Sci. Technol. Int. 2013, 19, 11–23. [Google Scholar] [CrossRef]
- Tsuzuki, W. Cis-trans isomerization of carbon double bonds in monounsaturated triacylglycerols via generation of free radicals. Chem. Phys. Lipids 2010, 163, 741–745. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Nie, S.; Xie, M.; Chen, F.; Luo, P.G. Formation of trans fatty acids during the frying of chicken fillet in corn oil. Int. J. Food Sci. Nutr. 2014, 65, 306–310. [Google Scholar] [CrossRef]
- Ben Hammouda, I.; Triki, M.; Matthaus, B.; Bouaziz, M. A comparative study on formation of polar components, fatty acids and sterols during frying of refined olive pomace oil pure and its blend coconut oil. J. Agric. Food Chem. 2018, 66, 3514–3523. [Google Scholar] [CrossRef]
- Berasategi, I.; Barriuso, B.; Ansorena, D.; Astiasaran, I. Stability of avocado oil during heating: Comparative study to olive oil. Food Chem. 2012, 132, 439–446. [Google Scholar] [CrossRef]
- WHO. An Action Package to Eliminate Industrially-Produced Trans-Fatty Acids. Available online: https://www.who.int/docs/default-source/documents/replace-transfats/replace-action-package.pdf (accessed on 11 November 2021).
- Astrup, A.; Bertram, H.C.; Bonjour, J.P.; de Groot, L.C.; de Oliveira Otto, M.C.; Feeney, E.L.; Garg, M.L.; Givens, I.; Kok, F.J.; Krauss, R.M.; et al. Who draft guidelines on dietary saturated and trans fatty acids: Time for a new approach? BMJ 2019, 366, l4137. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific and Technical Assistance on Transfatty Acids. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/sp.efsa.2018.EN-1433 (accessed on 11 November 2021).
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 acc/aha guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation 2019, 140, e563–e595. [Google Scholar] [CrossRef]
- Askin, B.; Kaya, Y. Effect of deep frying process on the quality of the refined oleic/linoleic sunflower seed oil and olive oil. J Food Sci. Technol. 2020, 57, 4716–4725. [Google Scholar] [CrossRef] [PubMed]
- Sayyad, R. Effects of deep-fat frying process on the oil quality during french fries preparation. J. Food Sci. Technol. 2017, 54, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Dordevic, D.; Kushkevych, I.; Jancikova, S.; Zeljkovic, S.C.; Zdarsky, M.; Hodulova, L. Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations. Open Life Sci. 2020, 15, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Afonso, C.; Bandarra, N.M.; Gueifao, S.; Castanheira, I.; Carvalho, M.L.; Cardoso, C.; Nunes, M.L. The emerging farmed fish species meagre (Argyrosomus regius): How culinary treatment affects nutrients and contaminants concentration and associated benefit-risk balance. Food Chem. Toxicol. 2013, 60, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Castro-Gonzalez, M.I.; Carrillo-Dominguez, S. Impact of six cooking techniques on fatty acid composition of marlin (Makaira nigricans) and hake (Merluccius productus). Nutr. Hosp. 2015, 32, 1289–1299. [Google Scholar] [CrossRef]
- Mekonnen, M.F.; Desta, D.T.; Alemayehu, F.R.; Kelikay, G.N.; Daba, A.K. Evaluation of fatty acid-related nutritional quality indices in fried and raw nile tilapia, (Oreochromis niloticus), fish muscles. Food Sci. Nutr. 2020, 8, 4814–4821. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naeem, H.H.S.; Sallam, K.I.; Zaki, H. Effect of different cooking methods of rabbit meat on topographical changes, physicochemical characteristics, fatty acids profile, microbial quality and sensory attributes. Meat Sci. 2021, 181, 108612. [Google Scholar] [CrossRef] [PubMed]
- Alkaltham, M.S.; Ozcan, M.M.; Uslu, N.; Salamatullah, A.M.; Hayat, K. Characterization of oil uptake and fatty acid composition of pre-treated potato slices fried in sunflower and olive oils. J. Oleo Sci. 2020, 69, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Al-Khusaibi, M.; Gordon, M.H.; Lovegrove, J.A.; Niranjan, K. Provenance of the oil in par-fried french fries after finish frying. J. Food Sci. 2012, 77, E32–E36. [Google Scholar] [CrossRef]
- Al-Saghir, S.; Thurner, K.; Wagner, K.H.; Frisch, G.; Luf, W.; Razzazi-Fazeli, E.; Elmadfa, I. Effects of different cooking procedures on lipid quality and cholesterol oxidation of farmed salmon fish (Salmo salar). J. Agric. Food Chem. 2004, 52, 5290–5296. [Google Scholar] [CrossRef]
- Sadhu, T.; Banerjee, I.; Lahiri, S.K.; Chakrabarty, J. Enhancement of nutritional value of fried fish using an artificial intelligence approach. Environ. Sci. Pollut. Res. Int. 2021. [Google Scholar] [CrossRef]
- Akintola, S.L. Effects of smoking and sun-drying on proximate, fatty and amino acids compositions of southern pink shrimp (Penaeus notialis). J. Food Sci. Technol. 2015, 52, 2646–2656. [Google Scholar] [CrossRef]
- Wijendran, V.; Hayes, K.C. Dietary n–6 and n–3 fatty acid balance and cardiovascular health. Annu. Rev. Nutr. 2004, 24, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Alfaia, C.M.; Alves, S.P.; Lopes, A.F.; Fernandes, M.J.; Costa, A.S.; Fontes, C.M.; Castro, M.L.; Bessa, R.J.; Prates, J.A. Effect of cooking methods on fatty acids, conjugated isomers of linoleic acid and nutritional quality of beef intramuscular fat. Meat Sci. 2010, 84, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Su, X.Q.; Babb, J.R. The effect of cooking process on the total lipid and n–3 lc-pufa contents of australian bass strait scallops, pecten fumatus. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. S1), 407–411. [Google Scholar] [PubMed]
- Biandolino, F.; Parlapiano, I.; Denti, G.; Di Nardo, V.; Prato, E. Effect of different cooking methods on lipid content and fatty acid profiles of mytilus galloprovincialis. Foods 2021, 10, 416. [Google Scholar] [CrossRef] [PubMed]

| Palm Oil | Soybean Oil | Rapeseed Oil | Sunflower Oil | EV Olive Oil | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Saturated fatty acids | ||||||||||
| C8:0 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 |
| C10:0 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| C12:0 | 0.15 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| C14:0 | 0.91 | 0.01 | 0.08 | 0.00 | 0.05 | 0.00 | 0.08 | 0.00 | 0.02 | 0.01 |
| C16:0 | 42.14 | 0.10 | 10.42 | 0.05 | 4.34 | 0.01 | 6.29 | 0.01 | 14.53 | 0.07 |
| C18:0 | 4.60 | 0.02 | 4.15 | 0.03 | 1.64 | 0.00 | 3.35 | 0.00 | 2.47 | 0.01 |
| C20:0 | 0.40 | 0.00 | 0.37 | 0.00 | 0.56 | 0.00 | 0.25 | 0.00 | 0.43 | 0.01 |
| C22:0 | 0.07 | 0.00 | 0.42 | 0.00 | 0.30 | 0.00 | 0.75 | 0.01 | 0.12 | 0.01 |
| C24:0 | 0.08 | 0.00 | 0.15 | 0.01 | 0.15 | 0.00 | 0.27 | 0.00 | 0.07 | 0.01 |
| SAT | 48.56 | 0.09 | 15.78 | 0.08 | 7.18 | 0.01 | 11.10 | 0.02 | 17.83 | 0.08 |
| Monounsaturated fatty acids | ||||||||||
| C16:1n–7 | 0.16 | 0.00 | 0.10 | 0.00 | 0.20 | 0.00 | 0.12 | 0.00 | 1.49 | 0.01 |
| C18:1n–9 | 40.34 | 0.07 | 22.30 | 0.03 | 59.98 | 0.03 | 27.65 | 0.01 | 66.87 | 0.12 |
| C18:1n–7 | 0.70 | 0.00 | 1.37 | 0.00 | 3.10 | 0.01 | 0.71 | 0.00 | 3.20 | 0.00 |
| C20:1n–9 | 0.15 | 0.00 | 0.21 | 0.00 | 1.20 | 0.00 | 0.15 | 0.00 | 0.29 | 0.01 |
| C22:1n–9 | 0.00 | 0.00 | 0.01 | 0.00 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| MUFA | 41.35 | 0.08 | 23.99 | 0.03 | 64.58 | 0.03 | 28.63 | 0.01 | 71.85 | 0.10 |
| Trans fatty acids | ||||||||||
| t18:1n–9/7 | 0.08 | 0.00 | 0.04 | 0.00 | 0.03 | 0.01 | 0.03 | 0.00 | 0.05 | 0.02 |
| C18:2n–6tt | 0.01 | 0.00 | 0.01 | 0.00 | n.d. | - | 0.00 | 0.00 | n.d. | - |
| C20:1n–9t | 0.02 | 0.00 | 0.05 | 0.00 | 0.02 | 0.01 | 0.02 | 0.00 | 0.01 | 0.01 |
| TFA | 0.10 | 0.00 | 0.09 | 0.00 | 0.05 | 0.01 | 0.05 | 0.00 | 0.06 | 0.03 |
| Polyunsaturated fatty acids | ||||||||||
| C18:2n–6 | 9.84 | 0.01 | 53.23 | 0.09 | 19.49 | 0.01 | 60.12 | 0.02 | 9.64 | 0.02 |
| C18:3n–3 | 0.14 | 0.00 | 6.82 | 0.01 | 8.63 | 0.01 | 0.07 | 0.00 | 0.62 | 0.00 |
| C20:2n–6 | 0.00 | 0.00 | 0.07 | 0.00 | 0.07 | 0.00 | 0.04 | 0.01 | 0.00 | 0.00 |
| n–6 PUFA | 9.84 | 0.01 | 53.32 | 0.09 | 19.56 | 0.01 | 60.16 | 0.02 | 9.65 | 0.02 |
| n–3 PUFA | 0.14 | 0.00 | 6.82 | 0.01 | 8.63 | 0.01 | 0.07 | 0.00 | 0.62 | 0.00 |
| Fresh | 1H | 5H | 10H | |
|---|---|---|---|---|
| Unsaturation index [UI] | ||||
| Palm oil | 61.462 aA (0.104) | 61.405 BC (0.458) | 60.548 aBD (0.274) | 59.646 ACD (0.275) |
| Soybean oil | 151.093 AB (0.206) | 150.618 C (0.577) | 149.923 AD (0.210) | 149.100 BCD (0.229) |
| Rapeseed oil | 129.597 aA (0.022) | 129.123 BC (0.125) | 128.111 aBD (0.153) | 127.422 ACD (0.081) |
| Sunflower oil | 149.138 aAB (0.039) | 148.524 abC (0.336) | 147.91 AbD (0.092) | 147.112 BCD (0.120) |
| EV Olive oil | 92.988 AB (0.114) | 92.732 CD (0.057) | 92.290 ACE (0.036) | 91.643 BDE (0.117) |
| Index of atherogenicity [IA] | ||||
| Palm oil | 0.895 ab (0.004) | 0.898 cA (0.013) | 0.915 acB (0.008) | 0.937 bAB (0.011) |
| Soybean oil | 0.128 AB (0.001) | 0.129 a (0.002) | 0.131 AC (0.001) | 0.133 BaC (0.000) |
| Rapeseed oil | 0.049 ab (0.000) | 0.051 cA (0.000) | 0.052 acB (0.002) | 0.052 bAB (0.001) |
| Sunflower oil | 0.074 aAB (0.000) | 0.077 ab (0.002) | 0.078 A (0.001) | 0.079 Bb (0.000) |
| EV Olive oil | 0.178 a (0.001) | 0.179 b (0.001) | 0.180 c (0.001) | 0.184 abc (0.002) |
| Index of thrombogenicity [IT] | ||||
| Palm oil | 1.830 aA (0.007) | 1.832 bB (0.029) | 1.872 abC (0.014) | 1.917 ABC (0.018) |
| Soybean oil | 0.247 AB (0.002) | 0.249 ab (0.003) | 0.255 AaC (0.002) | 0.261 BbC (0.001) |
| Rapeseed oil | 0.088 aA (0.000) | 0.091 bB (0.000) | 0.094 abC (0.002) | 0.095 ABC (0.001) |
| Sunflower oil | 0.218 AB (0.000) | 0.224 a (0.004) | 0.227 AC (0.001) | 0.231 BaC (0.001) |
| EV Olive oil | 0.399 ab (0.003) | 0.402 A (0.001) | 0.405 ac (0.001) | 0.414 bAc (0.003) |
| The hypocholesterolemic/hypercholesterolemic ratio [HH] | ||||
| Palm oil | 1.056 aA (0.004) | 1.054 bB (0.017) | 1.033 abC (0.008) | 1.009 ABC (0.010) |
| Soybean oil | 5.629 AB (0.040) | 5.583 a (0.075) | 5.496 AC (0.036) | 5.406 BaC (0.022) |
| Rapeseed oil | 14.611 aA (0.034) | 14.223 bB (0.035) | 13.851 abC (0.353) | 13.794 ABC (0.081) |
| Sunflower oil | 9.048 AB (0.013) | 8.777 a (0.161) | 8.672 AC (0.046) | 8.524 BaC (0.020) |
| EV Olive oil | 4.531 ab (0.029) | 4.504 ac (0.017) | 4.477 d (0.012) | 4.384 bcd (0.037) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabo, Z.; Marosvölgyi, T.; Szabo, E.; Koczka, V.; Verzar, Z.; Figler, M.; Decsi, T. Effects of Repeated Heating on Fatty Acid Composition of Plant-Based Cooking Oils. Foods 2022, 11, 192. https://doi.org/10.3390/foods11020192
Szabo Z, Marosvölgyi T, Szabo E, Koczka V, Verzar Z, Figler M, Decsi T. Effects of Repeated Heating on Fatty Acid Composition of Plant-Based Cooking Oils. Foods. 2022; 11(2):192. https://doi.org/10.3390/foods11020192
Chicago/Turabian StyleSzabo, Zoltan, Tamas Marosvölgyi, Eva Szabo, Viktor Koczka, Zsofia Verzar, Maria Figler, and Tamas Decsi. 2022. "Effects of Repeated Heating on Fatty Acid Composition of Plant-Based Cooking Oils" Foods 11, no. 2: 192. https://doi.org/10.3390/foods11020192
APA StyleSzabo, Z., Marosvölgyi, T., Szabo, E., Koczka, V., Verzar, Z., Figler, M., & Decsi, T. (2022). Effects of Repeated Heating on Fatty Acid Composition of Plant-Based Cooking Oils. Foods, 11(2), 192. https://doi.org/10.3390/foods11020192








