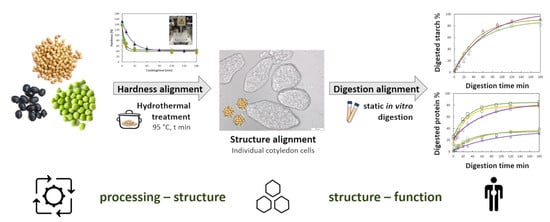
Utilizing Hydrothermal Processing to Align Structure and In Vitro Digestion Kinetics between Three Different Pulse Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Thermal Processing of Plant Material
2.2.2. Isolation and Characterization of ICC
2.3. Proximate Composition of Pulses
2.4. Structural Characterization
2.4.1. Microscopic Evaluation
2.4.2. Hardness Determination of Hydrothermally Treated Whole Pulse Seeds
2.4.3. Particle Size Distribution
2.5. Thermal Properties
2.6. Static In Vitro Digestion Protocol
2.7. Quantitative Evaluation of Starch and Protein Digestion Kinetics
2.7.1. Determination of Starch Digestion Products (%)
2.7.2. Determination of Protein Digestion Products (%)
2.8. Data Analysis: Kinetic Modeling and Statistical Analysis
3. Results
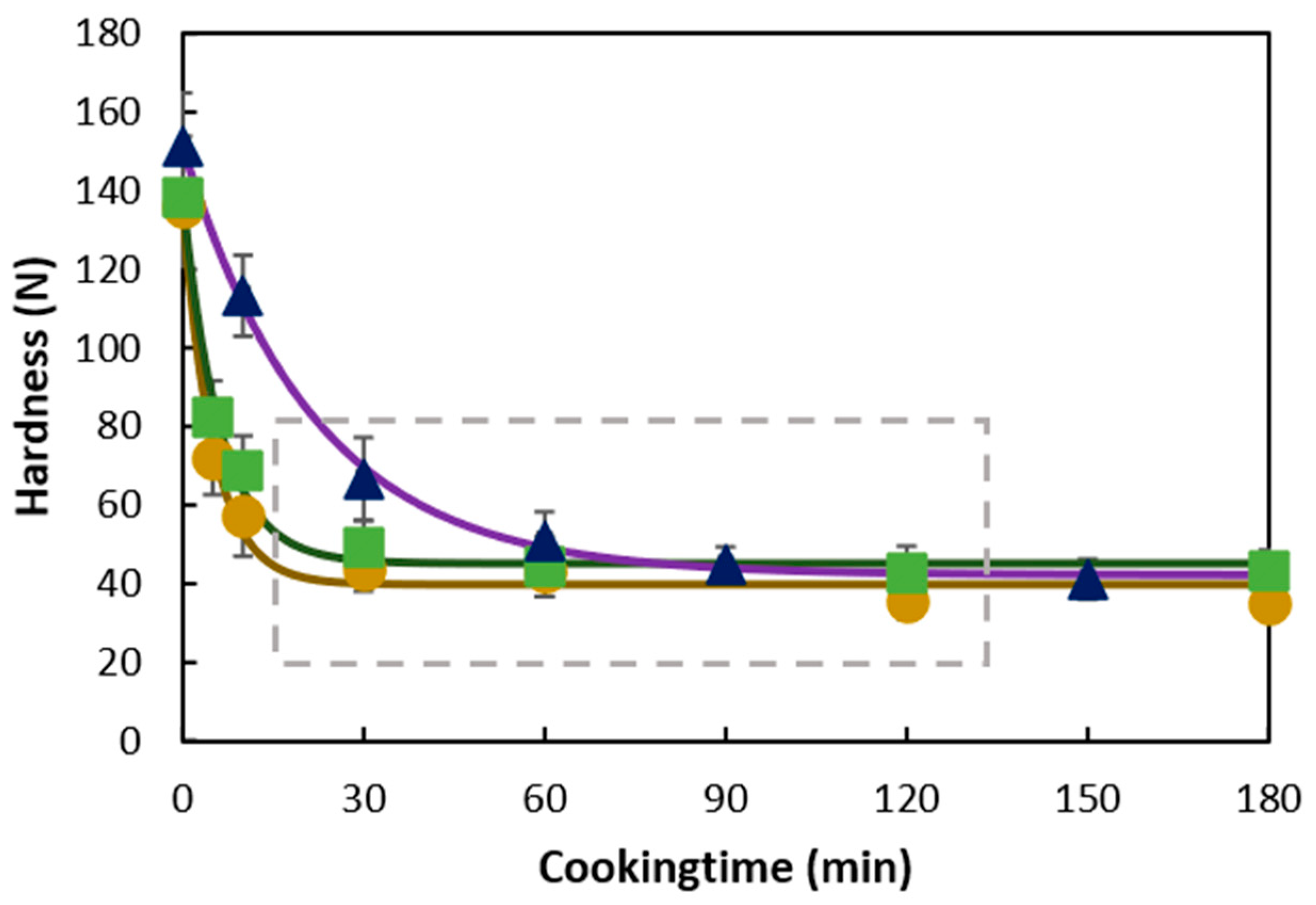
3.1. Hardness Evolution of Chickpea, Pea, or Black Bean during Hydrothermal Treatment
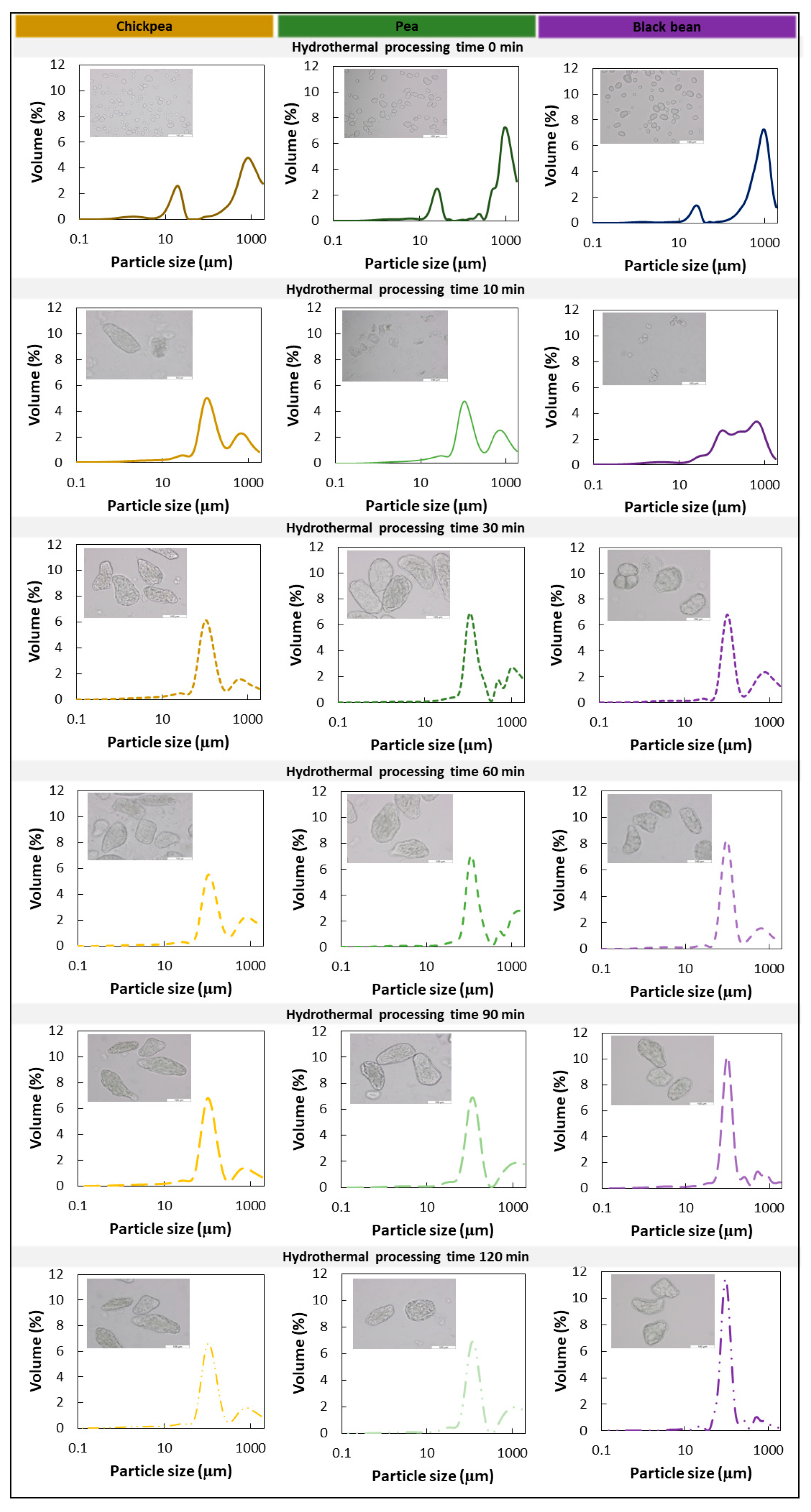
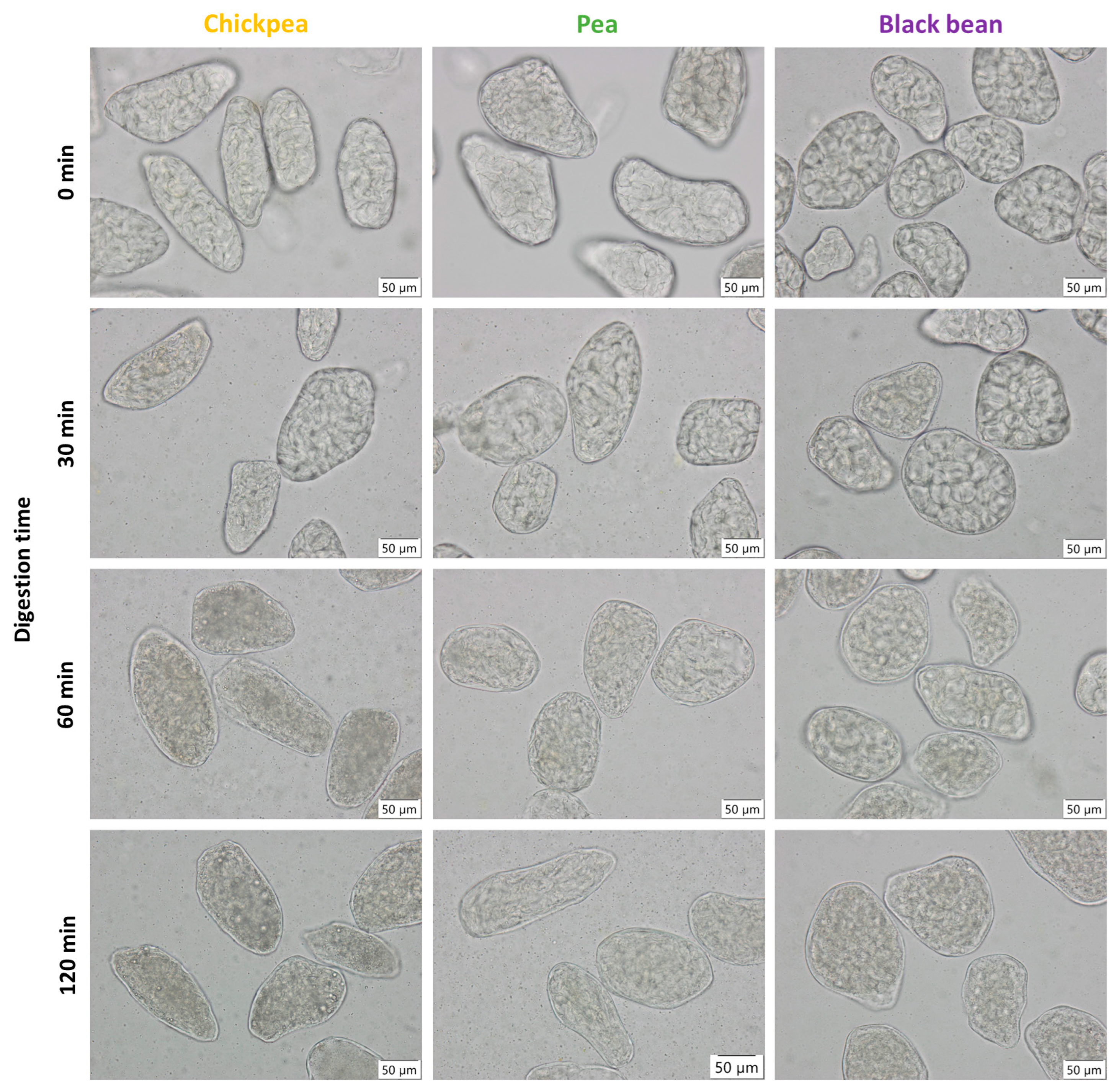
3.2. Microstructural Changes as a Result of Hydrothermal Treatment
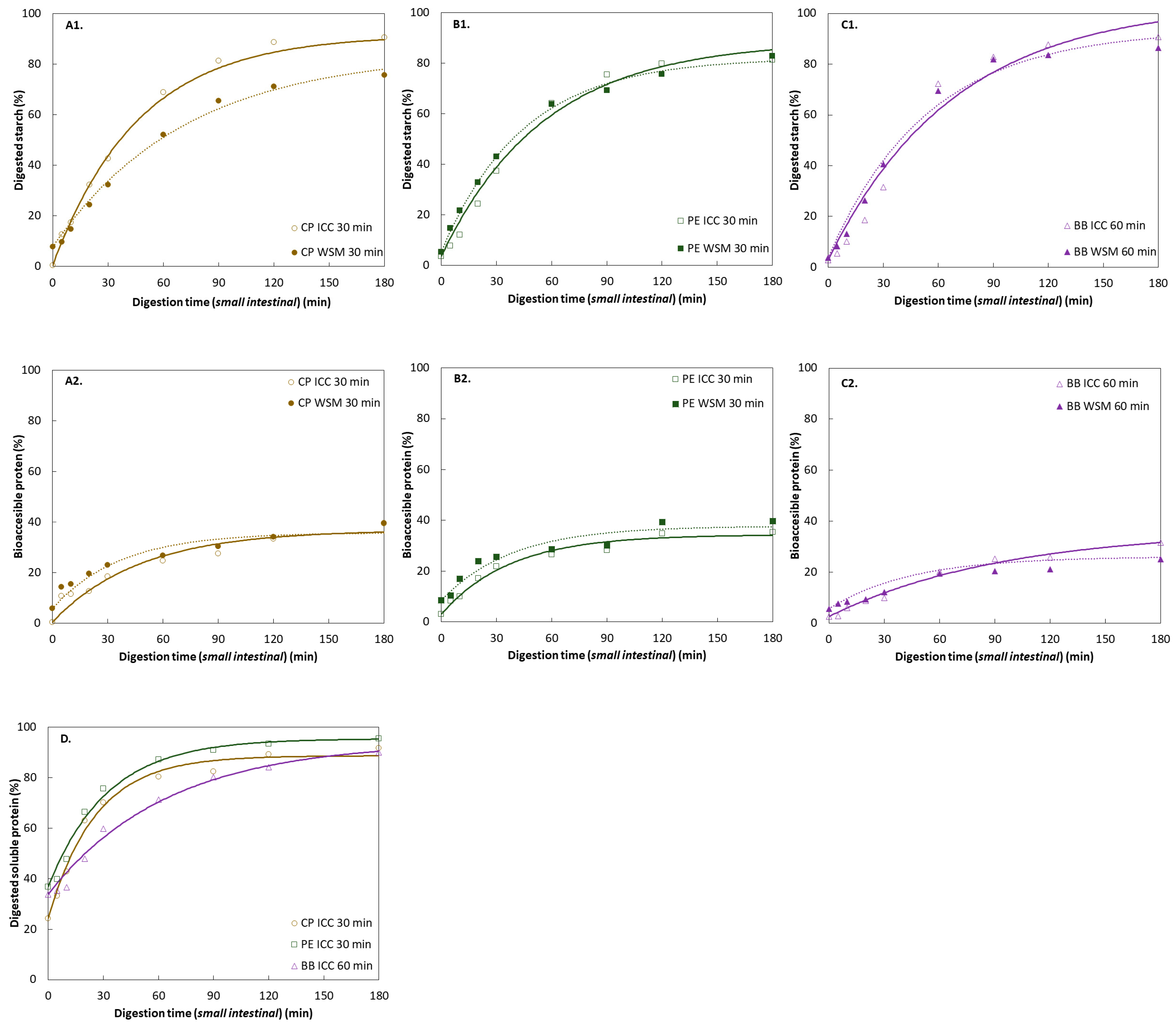
3.3. Macronutrient In Vitro Digestion Patterns of Hardness-Aligned Pulses
3.3.1. Proximate and Structural Characterization of Hardness-Aligned Pulses (ICC and WSM)
3.3.2. Starch Digestion of ICC Fraction of Hardness-Aligned Pulses
3.3.3. Protein Digestion of ICC Fraction of Hardness-Aligned Pulses
3.3.4. Effects of Increased Microstructural Heterogeneity on Starch and Protein Digestion of Hardness-Aligned Pulses
3.4. Hydrothermal Processing Duration and Hardness as a Tool to Align In Vitro Macronutrient Digestion: The Case of Chickpeas and Black Beans
3.4.1. Characterization of ICC Fraction of Hardness-Aligned Pulses after Different Hydrothermal Treatment Times
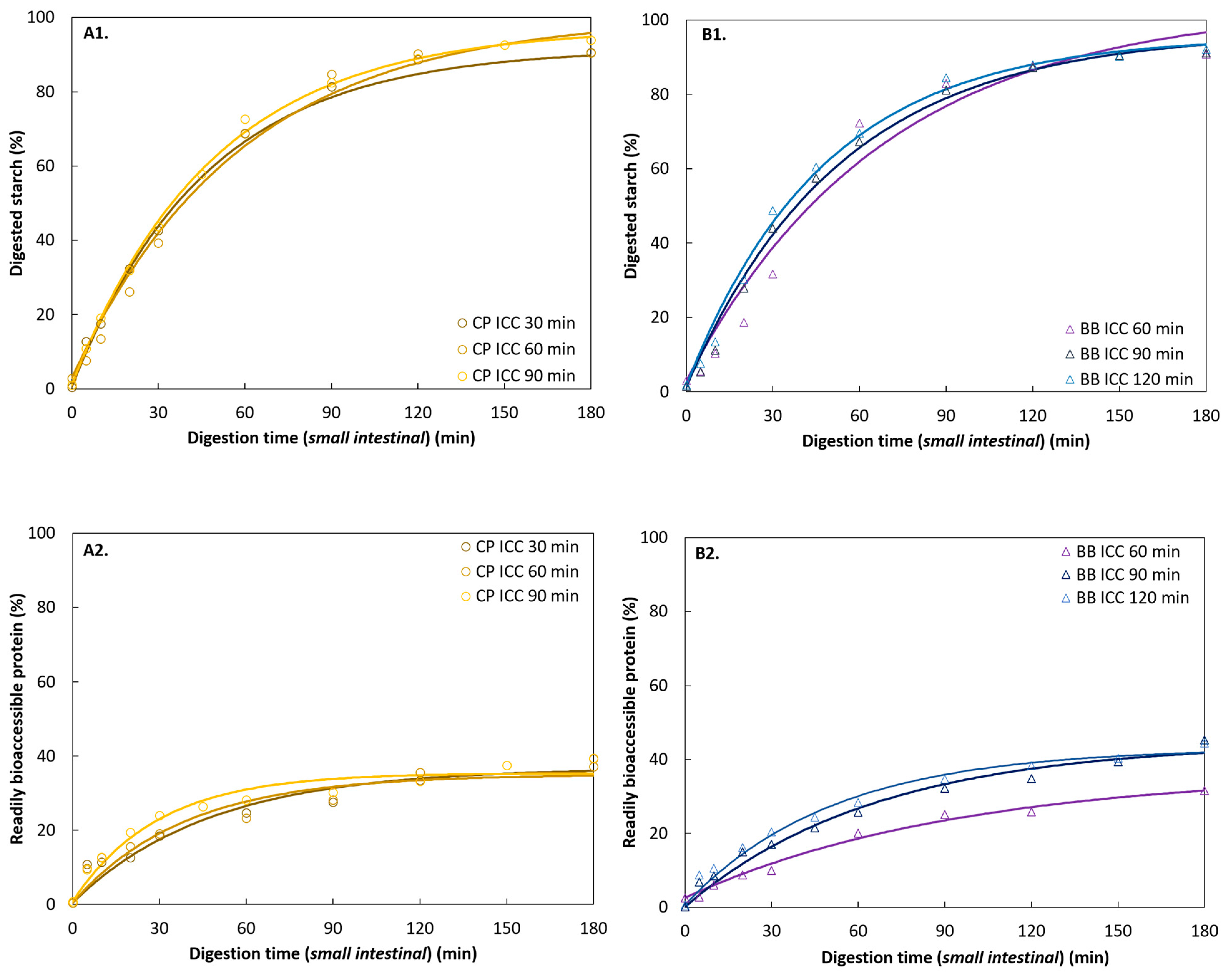
3.4.2. Starch Digestion of ICC from Different Hydrothermal Treatment Times
3.4.3. Protein Digestion of ICC from Different Hydrothermal Treatment Times
3.5. Correlation between Starch and Protein Digestion in Pulses
3.5.1. Effect of Pulse Type (Intrinsic Factors)
3.5.2. Effect of Different Hydrothermal Processing Times (Extrinsic Factors)
4. Discussion
4.1. Process-Induced Hardness Profile as a Material Property to Predict Microstructural Properties of Whole Pulses
4.2. Structure-Aligned Pulses: The Consequences for Their In Vitro Macronutrient Digestion
4.2.1. Comparative Study of Structure-Aligned Pulses: The Effect of Pulse Type
Starch Digestion
Protein Digestion
4.2.2. Comparative Study of Structure-Aligned Pulses: The Effect of Processing Time and Resulting Microstructure
Starch Digestion
Protein Digestion
4.3. Effect of Increased Microstructural Heterogeneity on the Digestive Response (ICC versus WSM)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Singh, N. Pulses: An overview. J. Food Sci. Technol. 2017, 54, 853–857. [Google Scholar] [CrossRef]
- Li, H.-T.; Chen, S.-Q.; Bui, A.T.; Xu, B.; Dhital, S. Natural ‘capsule’ in food plants: Cell wall porosity controls starch digestion and fermentation. Food Hydrocoll. 2021, 117, 106657. [Google Scholar] [CrossRef]
- Pallares, A.P.; Gwala, S.; Pälchen, K.; Duijsens, D.; Hendrickx, M.; Grauwet, T. Pulse seeds as promising and sustainable source of ingredients with naturally bioencapsulated nutrients: Literature review and outlook. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1524–1553. [Google Scholar] [CrossRef]
- Duijsens, D.; Gwala, S.; Pallares, A.P.; Pälchen, K.; Hendrickx, M.; Grauwet, T. How postharvest variables in the pulse value chain affect nutrient digestibility and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5067–5096. [Google Scholar] [CrossRef] [PubMed]
- Chigwedere, C.M.; Olaoye, T.F.; Kyomugasho, C.; Kermani, Z.J.; Pallares, A.P.; Van Loey, A.; Grauwet, T.; Hendrickx, M.E. Mechanistic insight into softening of Canadian wonder common beans (Phaseolus vulgaris) during cooking. Food Res. Int. 2018, 106, 522–531. [Google Scholar] [CrossRef]
- Tovar, J.; De Francisco, A.; Bjork, I.; Asp, N.G. Relationship Between Microstructure and Invitro Digestibility of Starch in Precooked Leguminous Seed Flours. Food Struct. 1991, 10, 19–26. Available online: http://digitalcommons.usu.edu/foodmicrostructurehttp://digitalcommons.usu.edu/foodmicrostructure/vol10/iss1/2 (accessed on 14 August 2021).
- Njoroge, D.M.; Kinyanjui, P.K.; Chigwedere, C.M.; Christiaens, S.; Makokha, A.O.; Sila, D.N.; Hendrickx, M.E. Mechanistic insight into common bean pectic polysaccharide changes during storage, soaking and thermal treatment in relation to the hard-to-cook defect. Food Res. Int. 2016, 81, 39–49. [Google Scholar] [CrossRef]
- Bernal-Lugo, I.; Parra, C.; Portilla, M.; Peña-Valdivia, C.B.; Moreno, E. Cotyledon thermal behavior and pectic solubility as related to cooking quality in common beans. Plant Foods Hum. Nutr. 1997, 50, 141–150. [Google Scholar] [CrossRef]
- Melito, C.; Tovar, J. Cell walls limit in vitro protein digestibility in processed legume seeds. Food Chem. 1995, 53, 305–307. [Google Scholar] [CrossRef]
- Tovar, J.; Bjorck, I.M.; Asp, N.G. Incomplete digestion of legume starches in rats: A study of precooked flours containing retrograded and physically inaccessible starch fractions. J. Nutr. 1992, 122, 1500–1507. [Google Scholar] [CrossRef]
- Singh, N. Functional and physicochemical properties of pulse starch. In Pulse Foods; Elsevier: Amsterdam, The Netherlands, 2021; pp. 87–112. [Google Scholar]
- Noah, L.; Guillon, F.; Bouchet, B.; Buléon, A.; Molis, C.; Gratas, M.; Champ, M. Digestion of Carbohydrate from White Beans (Phaseolus vulgaris L.) in Healthy Humans. J. Nutr. 1998, 128, 977–985. [Google Scholar] [CrossRef]
- Granfeldt, Y.; Björck, I.; Drews, A.; Tovar, J. An in vitro procedure based on chewing to predict metabolic response to starch in cereal and legume products. Am. J. Clin. Nutr. 1994, 59, 777S. [Google Scholar] [CrossRef]
- Dhital, S.; Bhattarai, R.R.; Gorham, J.; Gidley, M.J. Intactness of cell wall structure controls the in vitro digestion of starch in legumes. Food Funct. 2016, 7, 1367–1379. [Google Scholar] [CrossRef]
- Bhattarai, R.R.; Dhital, S.; Wu, P.; Chen, X.D.; Gidley, M.J. Digestion of isolated legume cells in a stomach-duodenum model: Three mechanisms limit starch and protein hydrolysis. Food Funct. 2017, 8, 2573–2582. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.H. The Role of Plant Cell Walls in Influenceing Starch Bioaccessibility; King’s College London: London, UK, 2014. [Google Scholar]
- Pallares, A.P.; Rousseau, S.; Chigwedere, C.M.; Kyomugasho, C.; Hendrickx, M.E.; Grauwet, T. Temperature-pressure-time combinations for the generation of common bean microstructures with different starch susceptibilities to hydrolysis. Food Res. Int. 2018, 106, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Rovalino-Córdova, A.M.; Fogliano, V.; Capuano, E. The effect of cell wall encapsulation on macronutrients digestion: A case study in kidney beans. Food Chem. 2019, 286, 557–566. [Google Scholar] [CrossRef]
- Bajka, B.H.; Pinto, A.M.; Ahn-Jarvis, J.; Ryden, P.; Perez-Moral, N.; van der Schoot, A.; Stocchi, C.; Bland, C.; Berry, S.E.; Ellis, P.R.; et al. The impact of replacing wheat flour with cellular legume powder on starch bioaccessibility, glycaemic response and bread roll quality: A double-blind randomised controlled trial in healthy participants. Food Hydrocoll. 2021, 114, 106565. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Manickavasagan, A.; Shobana, S.; Mohan, V. Glycemic index of pulses and pulse-based products: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1567–1588. [Google Scholar] [CrossRef]
- Pallares, A.P.; Miranda, B.A.; Truong, N.Q.A.; Kyomugasho, C.; Chigwedere, C.M.; Hendrickx, M.; Grauwet, T. Process-induced cell wall permeability modulates the in vitro starch digestion kinetics of common bean cotyledon cells. Food Funct. 2018, 9, 6544–6554. [Google Scholar] [CrossRef]
- Li, H.; Gidley, M.J.; Dhital, S. Wall porosity in isolated cells from food plants: Implications for nutritional functionality. Food Chem. 2019, 279, 416–425. [Google Scholar] [CrossRef]
- Rovalino-Córdova, A.M.; Fogliano, V.; Capuano, E. A closer look to cell structural barriers affecting starch digestibility in beans. Carbohydr. Polym. 2018, 181, 994–1002. [Google Scholar] [CrossRef]
- Gwala, S.; Pallares, A.P.; Pälchen, K.; Hendrickx, M.; Grauwet, T. In vitro starch and protein digestion kinetics of cooked Bambara groundnuts depend on processing intensity and hardness sorting. Food Res. Int. 2020, 137, 109512. [Google Scholar] [CrossRef]
- Khrisanapant, P.; Leong, S.; Kebede, B.; Oey, I. Effects of Hydrothermal Processing Duration on the Texture, Starch and Protein In Vitro Digestibility of Cowpeas, Chickpeas and Kidney Beans. Foods 2021, 10, 1415. [Google Scholar] [CrossRef]
- Li, P.; Dhital, S.; Fu, X.; Huang, Q.; Liu, R.; Zhang, B.; He, X. Starch digestion in intact pulse cotyledon cells depends on the extent of thermal treatment. Food Chem. 2020, 315, 126268. [Google Scholar] [CrossRef]
- Grundy, M.M.-L.; Edwards, C.H.; Mackie, A.R.; Gidley, M.J.; Butterworth, P.J.; Ellis, P.R. Re-evaluation of the mechanisms of dietary fibre and implications for macronutrient bioaccessibility, digestion and postprandial metabolism. Br. J. Nutr. 2016, 116, 816–833. [Google Scholar] [CrossRef] [PubMed]
- Rovalino-Córdova, A.M.; Montesdeoca, V.A.; Capuano, E. A mechanistic model to study the effect of the cell wall on starch digestion in intact cotyledon cells. Carbohydr. Polym. 2021, 253, 117351. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Hall, C.; Hillen, C.; Robinson, J.G. Composition, nutritional value, and health benefits of pulses. Cereal Chem. 2017, 94, 11–31. [Google Scholar] [CrossRef]
- Pälchen, K.; Michels, D.; Duijsens, D.; Gwala, S.; Pallares, A.P.; Hendrickx, M.; Van Loey, A.; Grauwet, T. In vitro protein and starch digestion kinetics of individual chickpea cells: From static to more complex in vitro digestion approaches. Food Funct. 2021, 12, 7787–7804. [Google Scholar] [CrossRef] [PubMed]
- Do, D.T.; Singh, J.; Oey, I.; Singh, H. Modulating effect of cotyledon cell microstructure on in vitro digestion of starch in legumes. Food Hydrocoll. 2019, 96, 112–122. [Google Scholar] [CrossRef]
- Edwards, C.H.; Ryden, P.; Pinto, A.M.; van der Schoot, A.; Stocchi, C.; Perez-Moral, N.; Butterworth, P.J.; Bajka, B.; Berry, S.E.; Hill, S.E.; et al. Chemical, physical and glycaemic characterisation of PulseON®: A novel legume cell-powder ingredient for use in the design of functional foods. J. Funct. Foods 2020, 68, 103918. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, B.; Dhital, S.; Huang, Q.; Fu, X. Structural features and starch digestion properties of intact pulse cotyledon cells modified by heat-moisture treatment. J. Funct. Foods 2019, 61, 103500. [Google Scholar] [CrossRef]
- Pallares, A.P.; Loosveldt, B.; Karimi, S.N.; Hendrickx, M.; Grauwet, T. Effect of process-induced common bean hardness on structural properties of in vivo generated boluses and consequences for in vitro starch digestion kinetics. Br. J. Nutr. 2019, 122, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Kyomugasho, C.; Kamau, P.G.; Aravindakshan, S.; Hendrickx, M.E. Evaluation of storage stability of low moisture whole common beans and their fractions through the use of state diagrams. Food Res. Int. 2021, 140, 109794. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.Y.; Kil, D.Y.; Kong, C.; Kim, B.G. Comparison of Oven-drying Methods for Determination of Moisture Content in Feed Ingredients. Asian-Aust. J. Anim. Sci. 2014, 27, 1615–1622. [Google Scholar] [CrossRef]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Janssen, F.; Wouters, A.G.B.; Pareyt, B.; Gerits, L.R.; Delcour, J.A.; Waelkens, E.; Derua, R. Wheat (Triticum aestivum L.) lipid species distribution in the different stages of straight dough bread making. Food Res. Int. 2018, 112, 299–311. [Google Scholar] [CrossRef]
- Infantes-Garcia, M.; Verkempinck, S.; Guevara-Zambrano, J.; Andreoletti, C.; Hendrickx, M.; Grauwet, T. Enzymatic and chemical conversions taking place during in vitro gastric lipid digestion: The effect of emulsion droplet size behavior. Food Chem. 2020, 326, 126895. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Gwala, S.; Wainana, I.; Pallares, A.P.; Kyomugasho, C.; Hendrickx, M.; Grauwet, T. Texture and interlinked post-process microstructures determine the in vitro starch digestibility of Bambara groundnuts with distinct hard-to-cook levels. Food Res. Int. 2019, 120, 1–11. [Google Scholar] [CrossRef]
- Verkempinck, S.; Salvia-Trujillo, L.; Moens, L.; Carrillo, C.; Van Loey, A.; Hendrickx, M.; Grauwet, T. Kinetic approach to study the relation between in vitro lipid digestion and carotenoid bioaccessibility in emulsions with different oil unsaturation degree. J. Funct. Foods 2018, 41, 135–147. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Zahir, M.; Fogliano, V.; Capuano, E. Food matrix and processing modulatein vitroprotein digestibility in soybeans. Food Funct. 2018, 9, 6326–6336. [Google Scholar] [CrossRef]
- Fujikawa, H.; Kai, A.; Morozumi, S. A new logistic model for Escherichia coli growth at constant and dynamic temperatures. Food Microbiol. 2004, 21, 501–509. [Google Scholar] [CrossRef]
- Costa, G.E.D.A.; Queiroz-Monici, K.D.S.; Reis, S.M.P.M.; de Oliveira, A.C. Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food Chem. 2006, 94, 327–330. [Google Scholar] [CrossRef]
- Bravo, L.; Siddhuraju, P.; Saura-Calixto, F. Effect of Various Processing Methods on the in Vitro Starch Digestibility and Resistant Starch Content of Indian Pulses. J. Agric. Food Chem. 1998, 46, 4667–4674. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, B.; Huang, Q.; Li, C.; Pletsch, E.A.; Fu, X. Variation in the rate and extent of starch digestion is not determined by the starch structural features of cooked whole pulses. Food Hydrocoll. 2018, 83, 340–347. [Google Scholar] [CrossRef]
- Bhattarai, R.R.; Dhital, S.; Gidley, M.J. Interactions among macronutrients in wheat flour determine their enzymic susceptibility. Food Hydrocoll. 2016, 61, 415–425. [Google Scholar] [CrossRef]
- Bassett, A.; Hooper, S.; Cichy, K. Genetic variability of cooking time in dry beans (Phaseolus vulgaris L.) related to seed coat thickness and the cotyledon cell wall. Food Res. Int. 2021, 141, 109886. [Google Scholar] [CrossRef]
- Tosh, S.M.; Yada, S. Dietary fibres in pulse seeds and fractions: Characterization, functional attributes, and applications. Food Res. Int. 2010, 43, 450–460. [Google Scholar] [CrossRef]
- Sadowska, J.; Jeliński, T.; Blaszczak, W.; Konopka, S.; Fornal, J.; Rybiński, W. The Effect of Seed Size and Microstructure on Their Mechanical Properties and Frictional Behavior. Int. J. Food Prop. 2013, 16, 814–825. [Google Scholar] [CrossRef][Green Version]
- Edwards, C.H.; Cochetel, N.; Setterfield, L.; Perez-Moral, N.; Warren, F.J. A single-enzyme system for starch digestibility screening and its relevance to understanding and predicting the glycaemic index of food products. Food Funct. 2019, 10, 4751–4760. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, B.; Dhital, S. Starch digestion in intact pulse cells depends on the processing induced permeability of cell walls. Carbohydr. Polym. 2019, 225, 115204. [Google Scholar] [CrossRef] [PubMed]
- Brummer, Y.; Kaviani, M.; Tosh, S.M. Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Res. Int. 2015, 67, 117–125. [Google Scholar] [CrossRef]
- Zahir, M.; Fogliano, V.; Capuano, E. Effect of soybean processing on cell wall porosity and protein digestibility. Food Funct. 2020, 11, 285–296. [Google Scholar] [CrossRef]
- Dhital, S.; Gidley, M.J.; Warren, F.J. Inhibition of α-amylase activity by cellulose: Kinetic analysis and nutritional implications. Carbohydr. Polym. 2015, 123, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Murillo, C.E.; Veyna-Torres, J.I.; Cavazos-Tamez, L.M.; de la Rosa-Millán, J.; Serna-Saldívar, S.O. Physicochemical characteristics, ATR-FTIR molecular interactions and in vitro starch and protein digestion of thermally-treated whole pulse flours. Food Res. Int. 2018, 105, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse proteins: Secondary structure, functionality and applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, M.; Grant, G.; Cappelloni, M.; Pusztai, A. Perspectives into Factors Limiting in Vivo Digestion of Legume Proteins: Antinutritional Compounds or Storage Proteins? J. Agric. Food Chem. 2000, 48, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Giusti, F.; Caprioli, G.; Ricciutelli, M.; Vittori, S.; Sagratini, G. Determination of fourteen polyphenols in pulses by high performance liquid chromatography-diode array detection (HPLC-DAD) and correlation study with antioxidant activity and colour. Food Chem. 2017, 221, 689–697. [Google Scholar] [CrossRef]
- Velickovic, T.D.C.; Stanic-Vucinic, D.J. The Role of Dietary Phenolic Compounds in Protein Digestion and Processing Technologies to Improve Their Antinutritive Properties. Compr. Rev. Food Sci. Food Saf. 2018, 17, 82–103. [Google Scholar] [CrossRef] [PubMed]
- Gilani, G.S.; Cockell, K.A.; Sepehr, E. Effects of Antinutritional Factors on Protein Digestibility and Amino Acid Availability in Foods. J. AOAC Int. 2005, 88, 967–987. [Google Scholar] [CrossRef]
- Gilani, G.S.; Xiao, C.W.; Cockell, K.A. Impact of Antinutritional Factors in Food Proteins on the Digestibility of Protein and the Bioavailability of Amino Acids and on Protein Quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef]
- Drulyte, D.; Orlien, V. The Effect of Processing on Digestion of Legume Proteins. Foods 2019, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: A Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 2012, 43, 911–921. [Google Scholar] [CrossRef]
- Torres, J.; Rutherfurd, S.M.; Muñoz, L.S.; Peters, M.; Montoya, C.A. The impact of heating and soaking on the in vitro enzymatic hydrolysis of protein varies in different species of tropical legumes. Food Chem. 2016, 194, 377–382. [Google Scholar] [CrossRef]
- Zhong, L.; Fang, Z.; Wahlqvist, M.L.; Wu, G.; Hodgson, J.M.; Johnson, S.K. Seed coats of pulses as a food ingredient: Characterization, processing, and applications. Trends Food Sci. Technol. 2018, 80, 35–42. [Google Scholar] [CrossRef]
- Capuano, E.; Pellegrini, N. An integrated look at the effect of structure on nutrient bioavailability in plant foods. J. Sci. Food Agric. 2019, 99, 493–498. [Google Scholar] [CrossRef]
- Edwards, C.; Maillot, M.; Parker, R.; Warren, F.J. A comparison of the kinetics of in vitro starch digestion in smooth and wrinkled peas by porcine pancreatic alpha-amylase. Food Chem. 2018, 244, 386–393. [Google Scholar] [CrossRef]
- Slaughter, S.L.; Ellis, P.R.; Jackson, E.C.; Butterworth, P.J. The effect of guar galactomannan and water availability during hydrothermal processing on the hydrolysis of starch catalysed by pancreatic α-amylase. Biochim. Biophys. Acta (BBA) Gen. Subj. 2002, 1571, 55–63. [Google Scholar] [CrossRef]



| Sample | Total Starch (g/100 g) | Total Protein (N*5.4) (g/100 g) | Total Non- starch Lipids (g/100 g) | Ash (g/100 g) | Fiber-Rich Residue (g/100 g Calculated) | Moisture (g/100 g) | Starch–Protein Ratio | Starch–Fiber-Rich Residue Ratio | Seed Coat–Cotyledon Ratio (w/w) |
|---|---|---|---|---|---|---|---|---|---|
| CP raw | 39.73 ± 1.61 a | 19.39 ± 0.12 a | 7.29 ± 0.28 a | 2.17 ± 0.40 a | 27.53 a | 3.89 ± 0.17 a | 2.05 | 1.44 | 0.14 |
| PE raw | 40.90 ± 1.42 a | 19.73 ± 1.10 a | 2.79 ± 0.10 b | 1.87 ± 0.28 a | 27.29 a | 7.43 ± 0.28 b | 2.07 | 1.50 | 0.15 |
| BB raw | 33.34 ± 1.72 c | 20.64 ± 0.53 a | 2.45 ± 0.39 b | 3.70 ± 0.28 b | 32.63 a | 7.23 ± 0.03 b | 1.62 | 1.02 | 0.11 |
| Sample | Total Starch (g/100 g dm) | Total Protein (N*5.4) (g/100 g dm) | Starch–Protein Ratio | Moisture (g/100 g) | |||||
| CP WSM 30 min | 41.24 ± 5.92 b | 19.65 ± 0.21 a | 2.10 | 64.42 ± 0.75 a | |||||
| PE WSM 30 min | 45.48 ± 3.19 a | 17.60 ± 0.56 b | 2.58 | 62.57 ± 4.52 a | |||||
| BB WSM 60 min | 38.61 ± 1.20 b | 18.67 ± 0.27 a,b | 2.07 | 62.66 ± 0.19 a | |||||
| Sample | Total Starch (g/100 g dm) | Total Protein (N*5.4) (g/100 g dm) | Starch–Protein Ratio | Moisture (g/100 g) | |||||
| PE ICC 30 min | 61.21 ± 1.22 a | 17.74 ± 0.06 c | 3.45 | 74.45 ± 0.11 a | |||||
| CP ICC 30 min | 54.96 ± 0.64 b | 16.94 ± 0.02 e | 3.24 | 74.24 ± 0.25 a | |||||
| CP ICC 60 min | 55.33 ± 2.65 b | 16.57 ± 0.02 e | 3.34 | 81.17 ± 1.14 a,b | |||||
| CP ICC 90 min | 54.56 ± 2.65 b | 17.33 ± 0.11 c,e | 3.15 | 81.31 ± 0.55 a,b | |||||
| BB ICC 60 min | 47.64 ± 2.95 c | 19.98 ± 0.13 b | 2.38 | 73.19 ± 0.02 b | |||||
| BB ICC 90 min | 51.31 ± 1.68 b,c | 21.06 ± 0.23 b | 2.44 | 82.28 ± 1.75 a | |||||
| BB ICC 120 min | 50.44 ± 0.01 b,c | 22.10 ± 0.01 a | 2.28 | 82.10 ± 0.01 a | |||||
| Sample | In Vitro Starch Digestion Kinetics (Small Intestinal) | |||
|---|---|---|---|---|
| k (min−1) | Starchf (%) | Initial Reaction Rate (% × min−1) | R2 adjusted | |
| CP ICC 30 min | 0.018 ± 0.001 a | 91.67 ± 1.63 a | 1.96 ± 0.10 a | 0.99 |
| PE ICC 30 min | 0.015 ± 0.002 a | 88.75 ± 4.68 a,b | 1.52 ± 0.22 b | 0.99 |
| BB ICC 60 min | 0.014 ± 0.004 a | 104.00 ± 11.02 a,b | 1.48 ± 0.39 b,d | 0.99 |
| CP WSM 30 min | 0.023 ± 0.002 a | 84.37 ± 3.89 a,b | 1.06 ± 0.13 c | 0.99 |
| PE WSM 30 min | 0.019 ± 0.001 a | 82.09 ± 1.46 b | 1.72 ± 0.09 b,d | 0.99 |
| BB WSM 60 min | 0.018 ± 0.003 a | 93.61 ± 5.06 a,b | 1.66 ± 0.25 b | 0.99 |
| Sample | In Vitro Protein Digestion Kinetics (Small Intestinal) | |||||||
|---|---|---|---|---|---|---|---|---|
| Readily Bioaccessible Protein | Digested Soluble Protein | |||||||
| k (min−1) | Proteinf (%) | Initial Reaction Rate (% × min−1) | R2adjusted | k (min−1) | Proteinf (%) | Initial Reaction Rate (% × min−1) | R2adjusted | |
| CP ICC 30 min | 0.021 ± 0.005 a,b | 36.84 ± 3.46 a,b | 0.77 ± 0.21 a | 0.98 | 0.039 ± 0.004 a | 88.73 ± 1.83 a | 2.51 ± 0.24 a | 0.99 |
| PE ICC 30 min | 0.027 ± 0.004 a | 34.35 ± 1.44 a | 0.84 ± 0.12 a | 0.99 | 0.031 ± 0.004 a | 95.50 ± 2.20 a | 1.84 ± 0.22 b | 0.99 |
| BB ICC 60 min | 0.015 ± 0.002 b | 36.68 ± 3.11 a,b | 0.36 ± 0.07 b | 0.99 | 0.016 ± 0.002 b | 94.22 ± 4.03 a | 0.94 ± 0.15 c | 0.99 |
| CP WSM 30 min | 0.027 ± 0.006 a,b | 35.83 ± 2.30 a | 0.82 ± 0.19 a | 0.99 | n.d | n.d | n.d | n.d |
| PE WSM 30 min | 0.026 ± 0.006 a,b | 37.78 ± 2.42 a | 0.78 ± 0.19 a | 0.99 | n.d | n.d | n.d | n.d |
| BB WSM 60 min | 0.022 ± 0.001 a,b | 26.10 ± 1.69 b | 0.46 ± 0.04 b | 0.99 | n.d | n.d | n.d | n.d |
| Sample | In Vitro Starch Digestion Kinetics (Small Intestinal) | |||
|---|---|---|---|---|
| k (min−1) | Starchf (%) | Initial Reaction Rate (% × min−1) | R2adjusted | |
| CP ICC 30 min | 0.021 ± 0.001 a | 91.67 ± 1.63 a | 1.96 ± 0.10 a | 0.99 |
| CP ICC 60 min | 0.017 ± 0.002 a | 100.30 ± 5.35 b | 1.67 ± 0.24 b | 0.99 |
| CP ICC 90 min | 0.020 ± 0.001 a | 97.17 ± 1.20 b | 1.97 ± 0.07 a | 0.99 |
| BB ICC 60 min | 0.015 ± 0.003 a | 104.00 ± 11.02 a,b | 1.48 ± 0.39 b | 0.99 |
| BB ICC 90 min | 0.019 ± 0.001 a | 96.68 ± 2.94 b | 1.78 ± 0.15 b | 0.99 |
| BB ICC 120 min | 0.021 ± 0.001 a | 95.58 ± 2.54 b | 1.97 ± 0.15 a | 0.99 |
| Sample | In Vitro Readily Bioaccessible Protein Digestion Kinetics (Small Intestinal) | |||
|---|---|---|---|---|
| k (min−1) | Proteinf (%) | Initial Reaction Rate (% × min−1) | R2adj | |
| CP ICC 30 min | 0.021 ± 0.005 a,b | 36.84 ± 3.46 a | 0.77 ± 0.21 a | 0.98 |
| CP ICC 60 min | 0.026 ± 0.006 a | 35.05 ± 2.82 a | 0.90 ± 0.22 a | 0.98 |
| CP ICC 90 min | 0.034 ± 0.005 a | 35.43 ± 1.50 a | 1.18 ± 0.18 b | 0.99 |
| BB ICC 60 min | 0.011 ± 0.002 b | 36.68 ± 3.11 a | 0.36 ± 0.07 c | 0.99 |
| BB ICC 90 min | 0.015 ± 0.002 a,b | 44.70 ± 2.71 a | 0.68 ± 0.10 a | 0.99 |
| BB ICC 120 min | 0.020 ± 0.002 a,b | 43.13 ± 1.81 a | 0.84 ± 0.10 a | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pälchen, K.; Van den Wouwer, B.; Duijsens, D.; Hendrickx, M.E.; Van Loey, A.; Grauwet, T. Utilizing Hydrothermal Processing to Align Structure and In Vitro Digestion Kinetics between Three Different Pulse Types. Foods 2022, 11, 206. https://doi.org/10.3390/foods11020206
Pälchen K, Van den Wouwer B, Duijsens D, Hendrickx ME, Van Loey A, Grauwet T. Utilizing Hydrothermal Processing to Align Structure and In Vitro Digestion Kinetics between Three Different Pulse Types. Foods. 2022; 11(2):206. https://doi.org/10.3390/foods11020206
Chicago/Turabian StylePälchen, Katharina, Ben Van den Wouwer, Dorine Duijsens, Marc E. Hendrickx, Ann Van Loey, and Tara Grauwet. 2022. "Utilizing Hydrothermal Processing to Align Structure and In Vitro Digestion Kinetics between Three Different Pulse Types" Foods 11, no. 2: 206. https://doi.org/10.3390/foods11020206
APA StylePälchen, K., Van den Wouwer, B., Duijsens, D., Hendrickx, M. E., Van Loey, A., & Grauwet, T. (2022). Utilizing Hydrothermal Processing to Align Structure and In Vitro Digestion Kinetics between Three Different Pulse Types. Foods, 11(2), 206. https://doi.org/10.3390/foods11020206