Effect of Conformation of Sugar Beet Pectin on the Interfacial and Emulsifying Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pectin Solutions
2.3. Zeta Potential and Hydrodynamic Diameter Measurement
2.4. Pendant Drop Method
2.5. Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D)
2.5.1. Preparation of Self-Assembled Monolayers (SAMs)
2.5.2. QCM-D Measurements
2.6. Emulsion Preparation and Characterization
2.7. Statistical Analysis
3. Results and Discussion
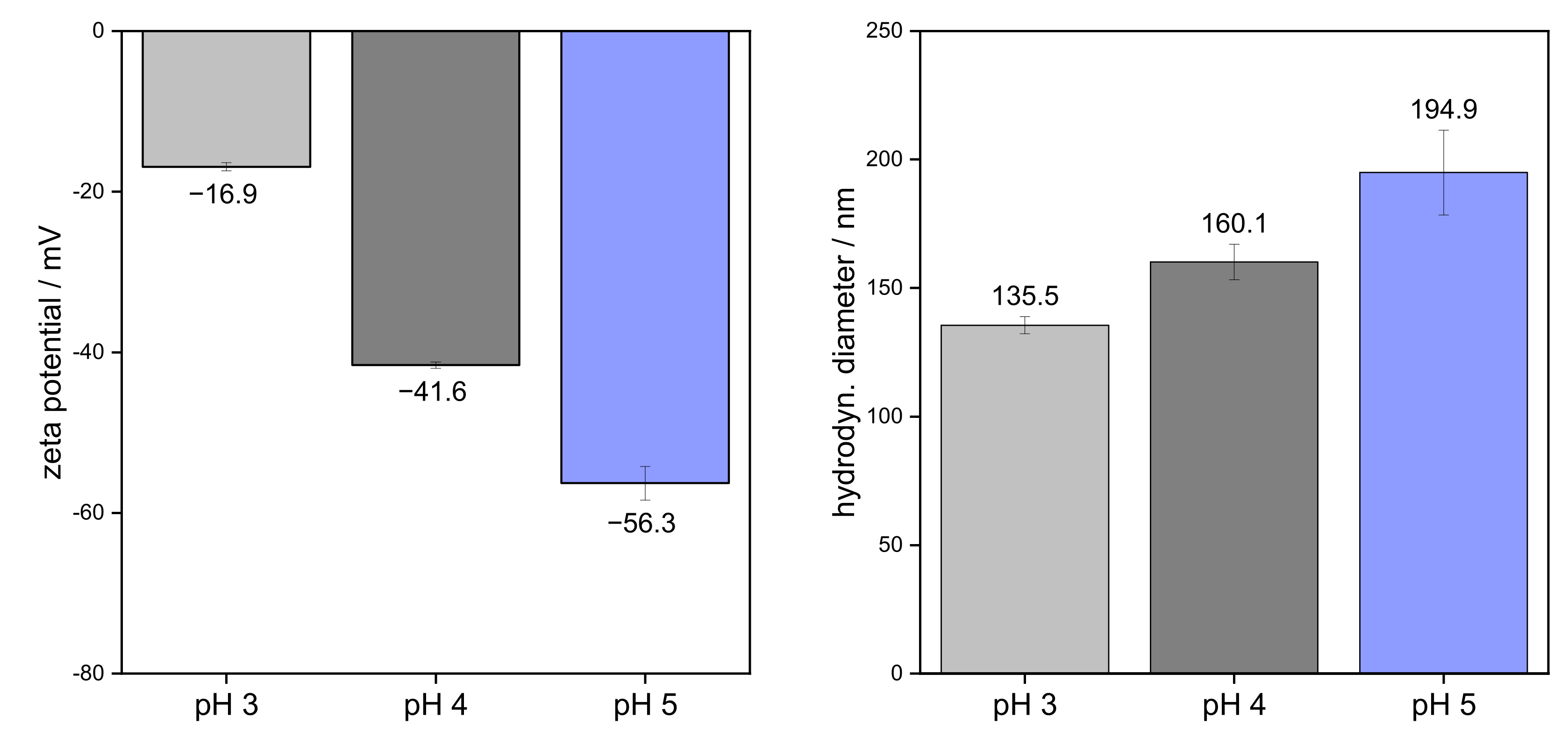
3.1. Colloidal Properties of Pectins in Aqueous Solution
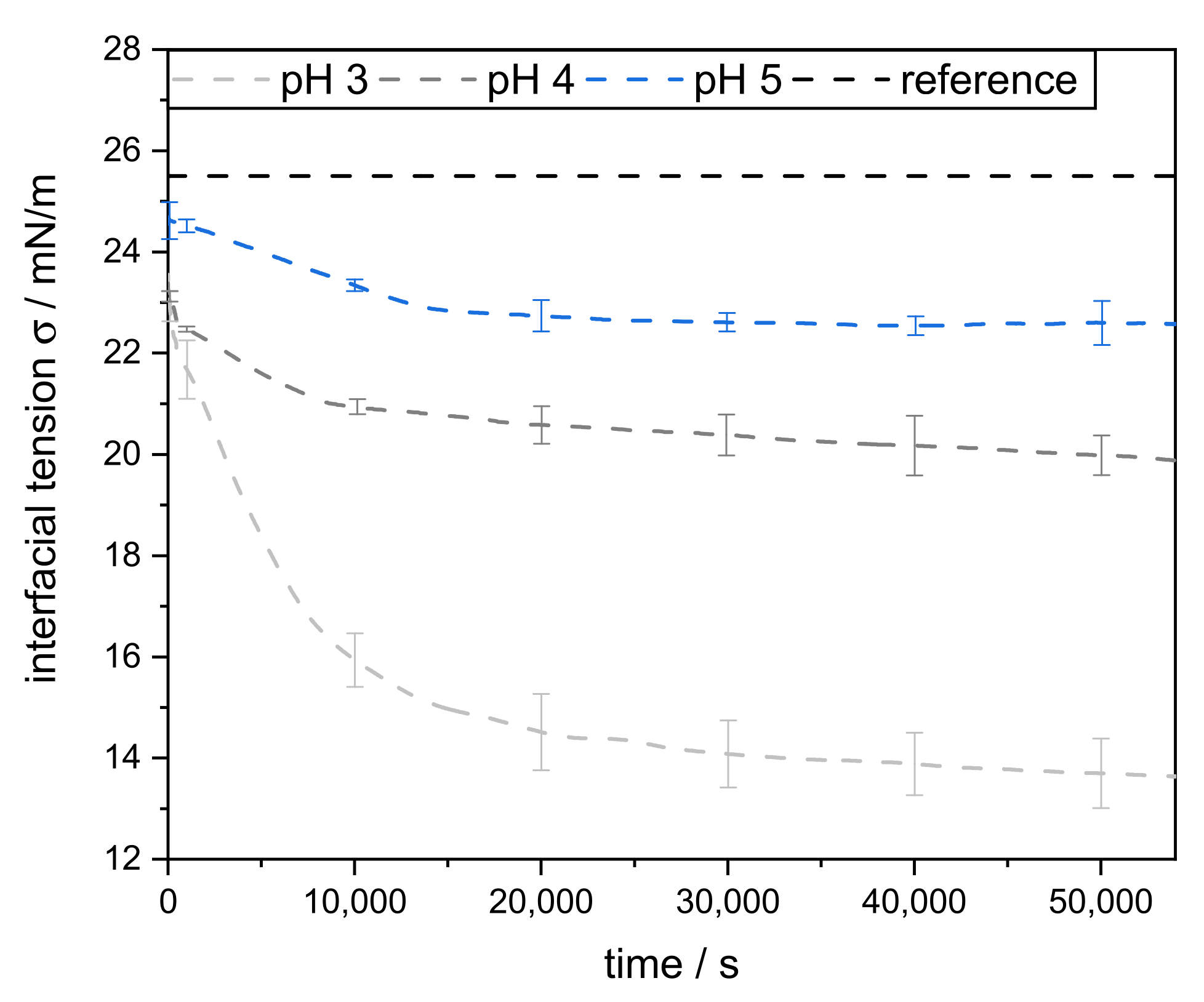
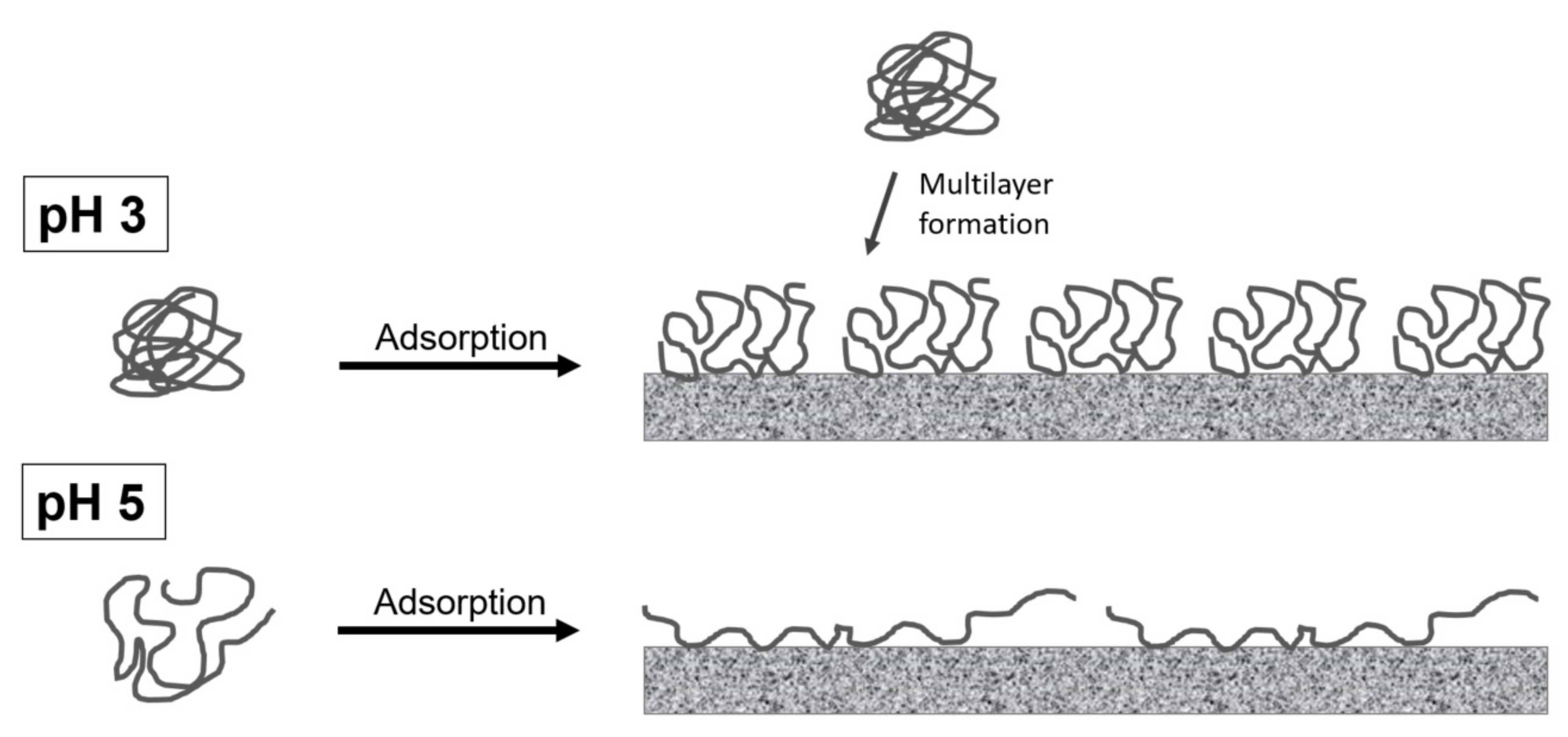
3.2. Interfacial Properties of Pectins
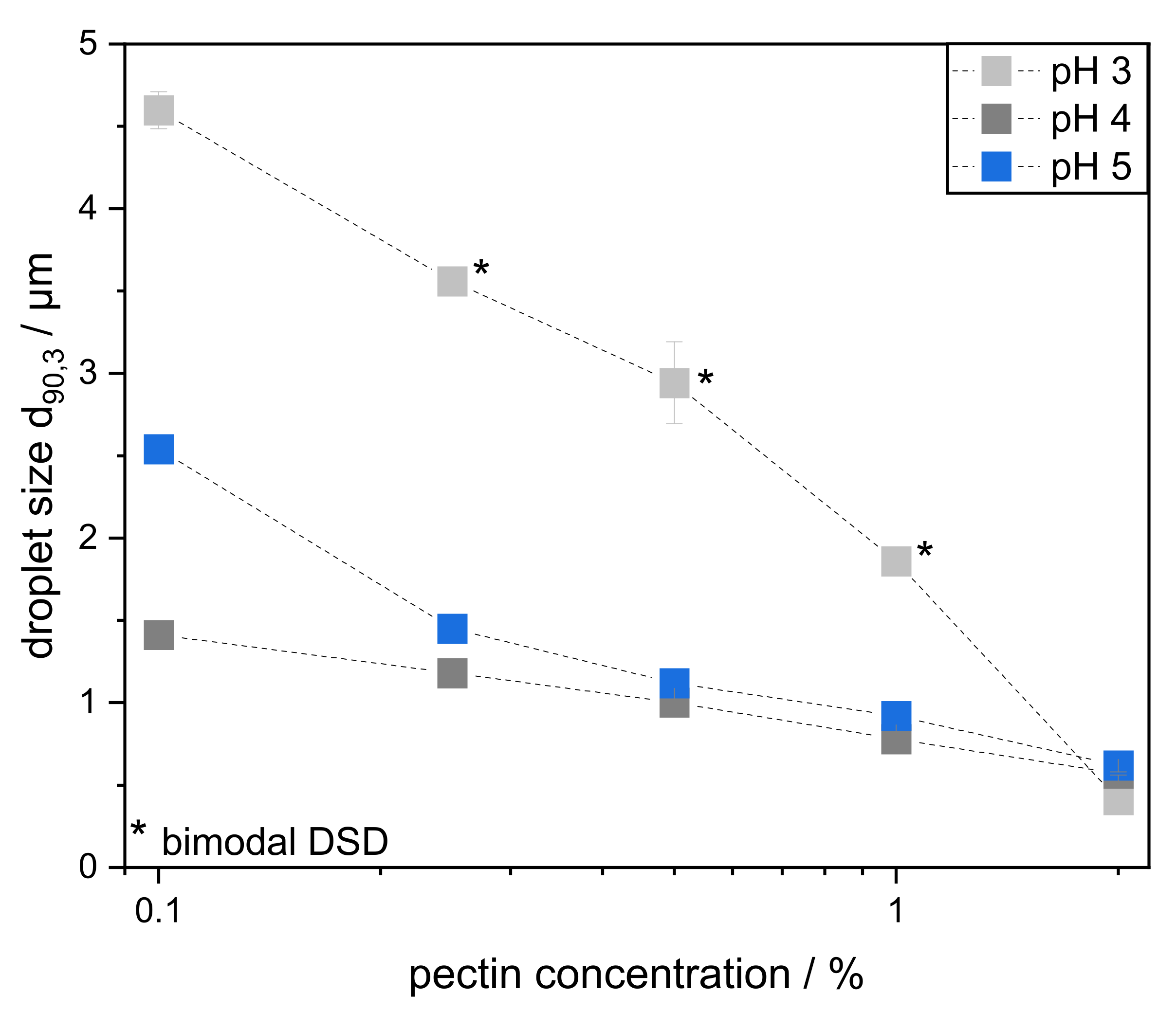
3.3. Characterization of Emulsions Prepared with Varying Pectin Concentration
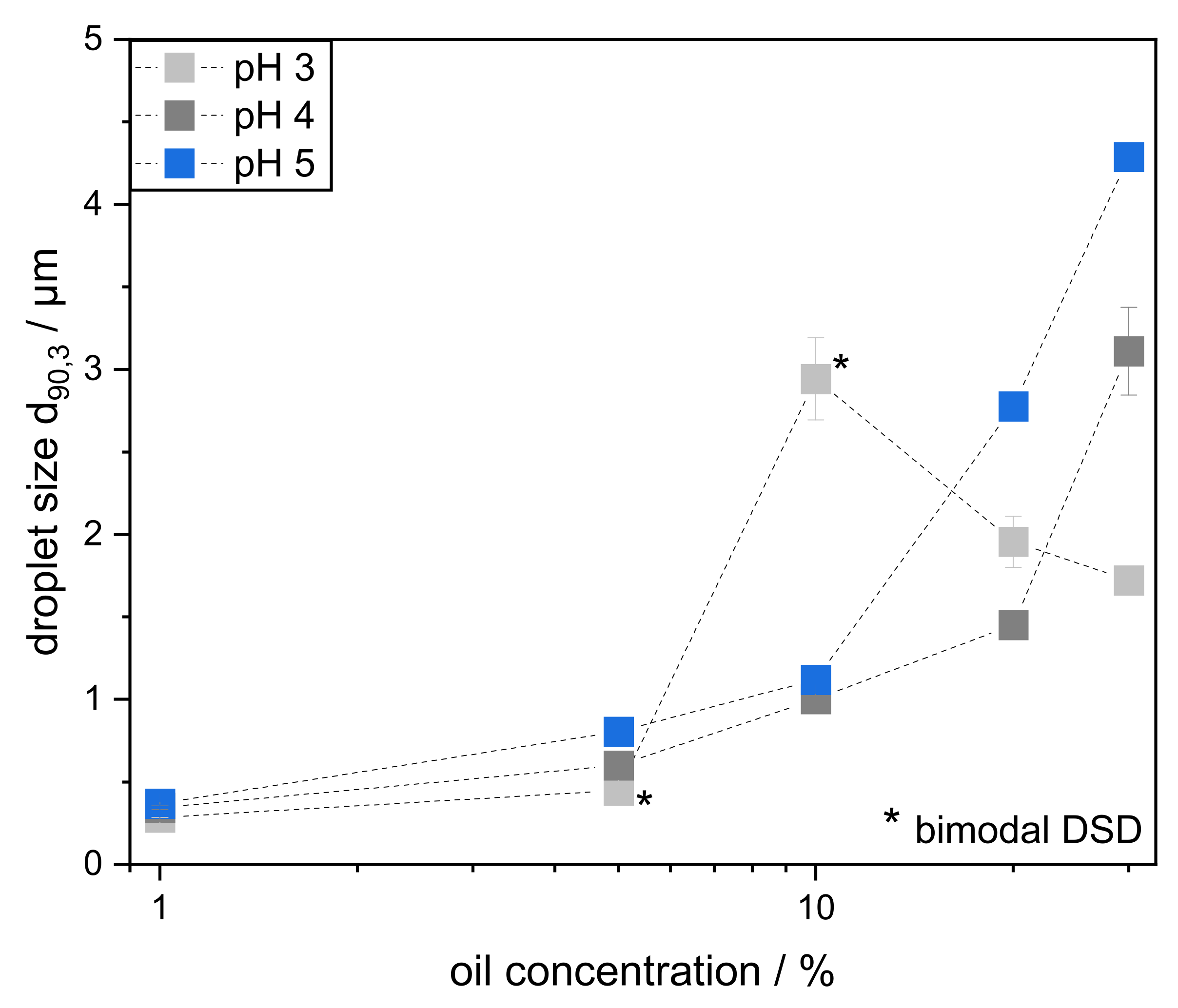
3.4. Effect of Varying Oil Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, K.C.; Dhurandhar, N.; You, X.; Miyamoto, A. Cultivar/Location and Processing Methods Affect Yield and Quality of Sunflower Pectin. J. Food Sci. 1994, 59, 602–605. [Google Scholar] [CrossRef]
- Schmidt, U.S.; Schmidt, K.; Kurz, T.; Endreß, H.-U.; Schuchmann, H.P. Pectins of different origin and their performance in forming and stabilizing oil-in-water-emulsions. Food Hydrocoll. 2015, 46, 59–66. [Google Scholar] [CrossRef]
- Yapo, B.M.; Robert, C.; Etienne, I.; Wathelet, B.; Paquot, M. Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chem. 2007, 100, 1356–1364. [Google Scholar] [CrossRef]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef] [PubMed]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.A.; Sayers, C.; Viebke, C.; Senan, C.; Mazoyer, J.; Boulenguer, P. Elucidation of the emulsification properties of sugar beet pectin. J. Agric. Food Chem. 2005, 53, 3592–3597. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.; Thibault, J.; Mercier, C.; Heitz, F.; Pouillaude, F. Extraction and Charcterization of Pectins from Sugar Beet Pulp. J. Food Sci. 1985, 50, 1499–1500. [Google Scholar] [CrossRef]
- Williamson, G.; Faulds, C.B.; Matthew, J.A.; Archer, D.B.; Morris, V.J.; Brownsey, G.J.; Ridout, M.J. Gelation of sugarbeet and citrus pectins using enzymes extracted from orange peel. Carbohydr. Polym. 1990, 13, 387–397. [Google Scholar] [CrossRef]
- Funami, T.; Nakauma, M.; Ishihara, S.; Tanaka, R.; Inoue, T.; Phillips, G.O. Structural modifications of sugar beet pectin and the relationship of structure to functionality. Food Hydrocoll. 2011, 25, 221–229. [Google Scholar] [CrossRef]
- Funami, T.; Nakauma, M.; Noda, S.; Ishihara, S.; Al-Assaf, S.; Phillips, G.O. Enhancement of the performance of sugar beet pectin as an emulsifier. Foods Food Ingred. J. Japan 2008, 213, 347–355. [Google Scholar]
- Nakauma, M.; Funami, T.; Noda, S.; Ishihara, S.; Al-Assaf, S.; Nishinari, K.; Phillips, G.O. Comparison of sugar beet pectin, soybean soluble polysaccharide, and gum arabic as food emulsifiers. 1. Effect of concentration, pH, and salts on the emulsifying properties. Food Hydrocoll. 2008, 22, 1254–1267. [Google Scholar] [CrossRef]
- Müller-Maatsch, J.; Bencivenni, M.; Caligiani, A.; Tedeschi, T.; Bruggeman, G.; Bosch, M.; Sforza, S. Pectin content and composition from different food waste streams. Food Chem. 2016, 201, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Rombouts, F.M.; Thibault, J.F. Sugar Beet Pectins: Chemical Structure and Gelation through Oxidative Coupling. In American Chemical Society Symposium; Fishman, M.L., Jen, J.J., Eds.; ACS Symposium Series 310, Chemistry and Function of Pectins; American Chemical Society: Washington, DC, USA, 1986; Volume 310, pp. 49–60. [Google Scholar] [CrossRef] [Green Version]
- Keenan, M.H.J.; Belton, P.S.; Matthew, J.A.; Howson, S.J. A 13C-n.m.r. study of sugar-beet pectin. Carbohydr. Res. 1985, 138, 168–170. [Google Scholar] [CrossRef]
- Siew, C.K.; Williams, P.A. Role of protein and ferulic acid in the emulsification properties of sugar beet pectin. J. Agric. Food Chem. 2008, 56, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Dickinson, E.; Mazoyer, J.; Langendorff, V. Emulsion stabilizing properties of depolymerized pectin. Food Hydrocoll. 2002, 16, 249–256. [Google Scholar] [CrossRef]
- Bindereif, B.; Eichhöfer, H.; Bunzel, M.; Karbstein, H.P.; Wefers, D.; Van der Schaaf, U.S. Arabinan side-chains strongly affect the emulsifying properties of acid-extracted sugar beet pectins. Food Hydrocoll. 2021, 7, 106968. [Google Scholar] [CrossRef]
- Chen, H.-M.; Fu, X.; Luo, Z.-G. Effect of molecular structure on emulsifying properties of sugar beet pulp pectin. Food Hydrocoll. 2016, 54, 99–106. [Google Scholar] [CrossRef]
- Endreß, H.-U.; Rentschler, C. Chances and limit for the use of pectin as emulsifier—Part 1. Eur. Food Drink Rev. 1999, 11, 49–53. [Google Scholar]
- Alba, K.; Bingham, R.J.; Kontogiorgos, V. Mesoscopic structure of pectin in solution. Biopolymers 2017, 107, e23016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellani, O.; Al-Assaf, S.; Axelos, M.; Phillips, G.O.; Anton, M. Hydrocolloids with emulsifying capacity. Part 2—Adsorption properties at the n-hexadecane–Water Interface. Food Hydrocoll. 2010, 24, 121–130. [Google Scholar] [CrossRef]
- Ngouémazong, E.D.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M. The Emulsifying and Emulsion-Stabilizing Properties of Pectin: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 705–718. [Google Scholar] [CrossRef]
- Schmidt, U.S.; Schütz, L.; Schuchmann, H.P. Interfacial and emulsifying properties of citrus pectin: Interaction of pH, ionic strength and degree of esterification. Food Hydrocoll. 2017, 62, 288–298. [Google Scholar] [CrossRef]
- Zhao, S.; Ren, W.; Gao, W.; Tian, G.; Zhao, C.; Bao, Y.; Zheng, J. Effect of mesoscopic structure of citrus pectin on its emulsifying properties: Compactness is more important than size. J. Colloid Interf. Sci. 2020, 570, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Alba, K.; Bingham, R.J.; Gunning, P.A.; Wilde, P.J.; Kontogiorgos, V. Pectin Conformation in Solution. J. Phys. Chem. B 2018, 122, 7286–7294. [Google Scholar] [CrossRef]
- Bhattacharyya, L.; Rohrer, J.S. Applications of Ion Chromatography for Pharmaceutical and Biological Products; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Zhao, S.; Gao, W.; Tian, G.; Zhao, C.; DiMarco-Crook, C.; Fan, B.; Zheng, J. Citrus Oil Emulsions Stabilized by Citrus Pectin: The Influence Mechanism of Citrus Variety and Acid Treatment. J. Agric. Food Chem. 2018, 66, 12978–12988. [Google Scholar] [CrossRef]
- Dopierala, K.; Javadi, A.; Krägel, J.; Schano, K.-H.; Kalogianni, E.P.; Leser, M.E.; Miller, R. Dynamic interfacial tensions of dietary oils. Colloids Surf. Physicochem. Eng. Asp. 2011, 382, 261–265. [Google Scholar] [CrossRef]
- Berry, J.D.; Neeson, M.J.; Dagastine, R.R.; Chan, D.Y.C.; Tabor, R.F. Measurement of surface and interfacial tension using pendant drop tensiometry. J. Colloid Interf. Sci. 2015, 454, 226–237. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef] [PubMed]
- Maccarini, M.; Himmelhaus, M.; Stoycheva, S.; Grunze, M. Characterisation and stability of hydrophobic surfaces in water. Appl. Surf. Sci. 2005, 252, 1941–1946. [Google Scholar] [CrossRef]
- Teo, A.; Dimartino, S.; Lee, S.J.; Goh, K.K.T.; Wen, J.; Oey, I.; Kwak, H.-S. Interfacial structures of whey protein isolate (WPI) and lactoferrin on hydrophobic surfaces in a model system monitored by quartz crystal microbalance with dissipation (QCM-D) and their formation on nanoemulsions. Food Hydrocoll. 2016, 56, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.X.; Kim, J.-T. Application of Kevin—Voigt Model in Quantifying Whey Protein Adsorption on Polyethersulfone Using QCM-D. JALA J. Assoc. Lab. Autom. 2009, 14, 213–220. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Doost, A.S.; Su, J.; Van der Meeren, P. Electrostatic interaction between whey proteins and low methoxy pectin studied by quartz crystal microbalance with dissipation monitoring. Food Hydrocoll. 2021, 113, 106489. [Google Scholar] [CrossRef]
- Zeeb, B.; Salminen, H.; Fischer, L.; Weiss, J. Impact of Heat and Laccase on the pH and Freeze-Thaw Stability of Oil-in-Water Emulsions Stabilized by Adsorbed Biopolymer Nanoparticles. Food Biophys. 2014, 9, 125–137. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Yao, X.; Zhang, Y.; Jiang, F. Complexation of bovine serum albumin and sugar beet pectin: Structural transitions and phase diagram. Langmuir 2012, 28, 10164–10176. [Google Scholar] [CrossRef] [PubMed]
- Siew, C.K.; Williams, P.A.; Cui, S.W.; Wang, Q. Characterization of the surface-active components of sugar beet pectin and the hydrodynamic thickness of the adsorbed pectin layer. J. Agric. Food Chem. 2008, 56, 8111–8120. [Google Scholar] [CrossRef]
- Guzey, D.; McClements, D.J. Formation, stability and properties of multilayer emulsions for application in the food industry. Adv. Colloid Interf. Sci. 2006, 128–130, 227–248. [Google Scholar] [CrossRef] [PubMed]
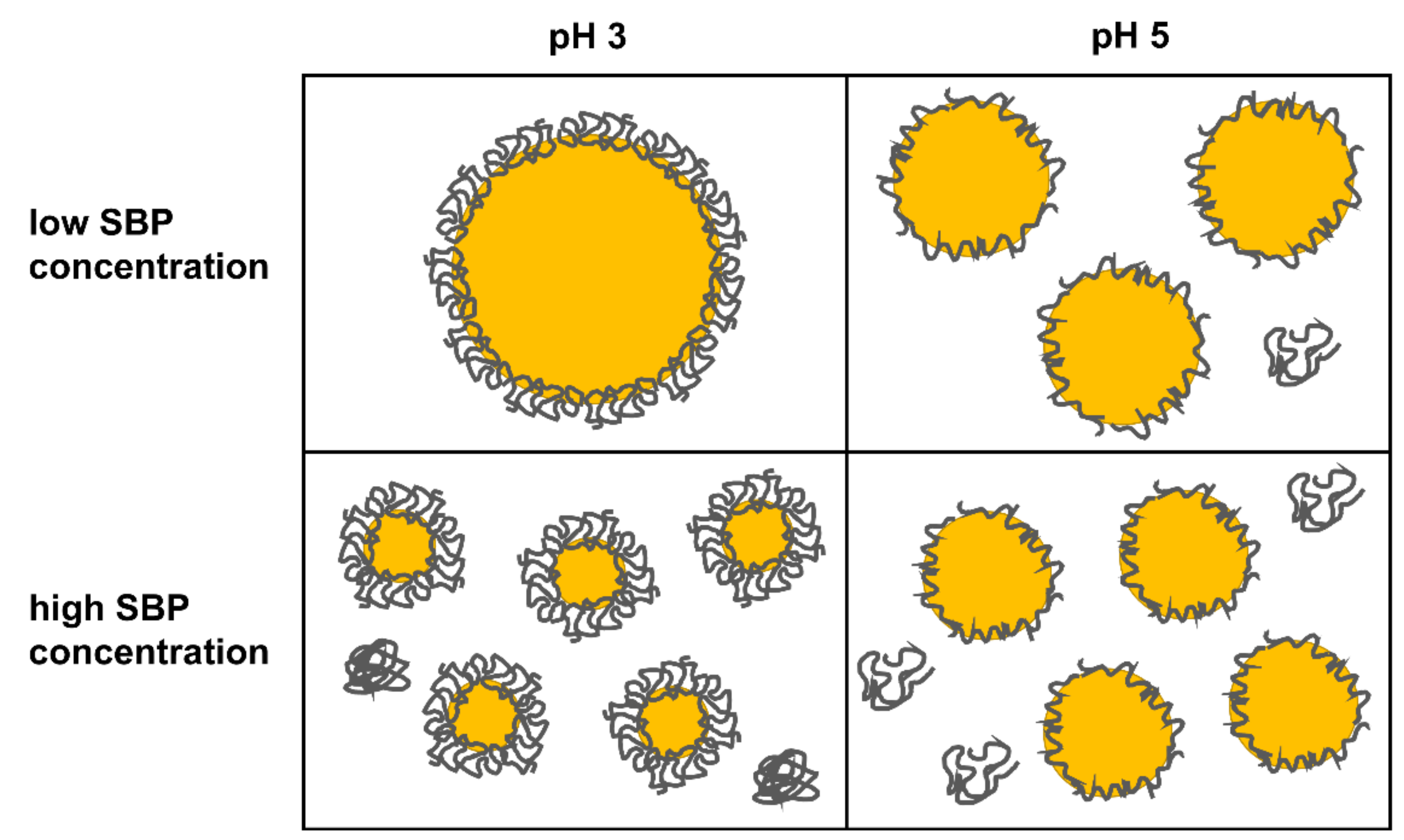
| pH 3 | pH 4 | pH 5 | ||||
|---|---|---|---|---|---|---|
| 0.1 wt% | 1.0 wt% | 0.1 wt% | 1.0 wt% | 0.1 wt% | 1.0 wt% | |
| Density (kg/m3) | 1001 | 1003 | 1001 | 1003 | 1001 | 1003 |
| Viscosity (mPa·s) | 1.2 | 8.0 | 1.2 | 8.5 | 1.3 | 8.8 |
| Adsorbed mass (ng/cm2) | 524 ± 91 | 1462 ± 105 | 257 ± 23 | 388 ± 69 | 147 ± 39 | 442 ± 148 |
| Layer thickness (nm) | 9.3 ± 0.7 | 11.6 ± 0.9 | 2.1 ± 0.2 | 3.1 ± 0.6 | 1.2 ± 0.3 | 3.7 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bindereif, B.; Karbstein, H.P.; Zahn, K.; van der Schaaf, U.S. Effect of Conformation of Sugar Beet Pectin on the Interfacial and Emulsifying Properties. Foods 2022, 11, 214. https://doi.org/10.3390/foods11020214
Bindereif B, Karbstein HP, Zahn K, van der Schaaf US. Effect of Conformation of Sugar Beet Pectin on the Interfacial and Emulsifying Properties. Foods. 2022; 11(2):214. https://doi.org/10.3390/foods11020214
Chicago/Turabian StyleBindereif, Benjamin, Heike Petra Karbstein, Katharina Zahn, and Ulrike Sabine van der Schaaf. 2022. "Effect of Conformation of Sugar Beet Pectin on the Interfacial and Emulsifying Properties" Foods 11, no. 2: 214. https://doi.org/10.3390/foods11020214
APA StyleBindereif, B., Karbstein, H. P., Zahn, K., & van der Schaaf, U. S. (2022). Effect of Conformation of Sugar Beet Pectin on the Interfacial and Emulsifying Properties. Foods, 11(2), 214. https://doi.org/10.3390/foods11020214







