Nondestructive Prediction of Isoflavones and Oligosaccharides in Intact Soybean Seed Using Fourier Transform Near-Infrared (FT-NIR) and Fourier Transform Infrared (FT-IR) Spectroscopic Techniques
Abstract
1. Introduction
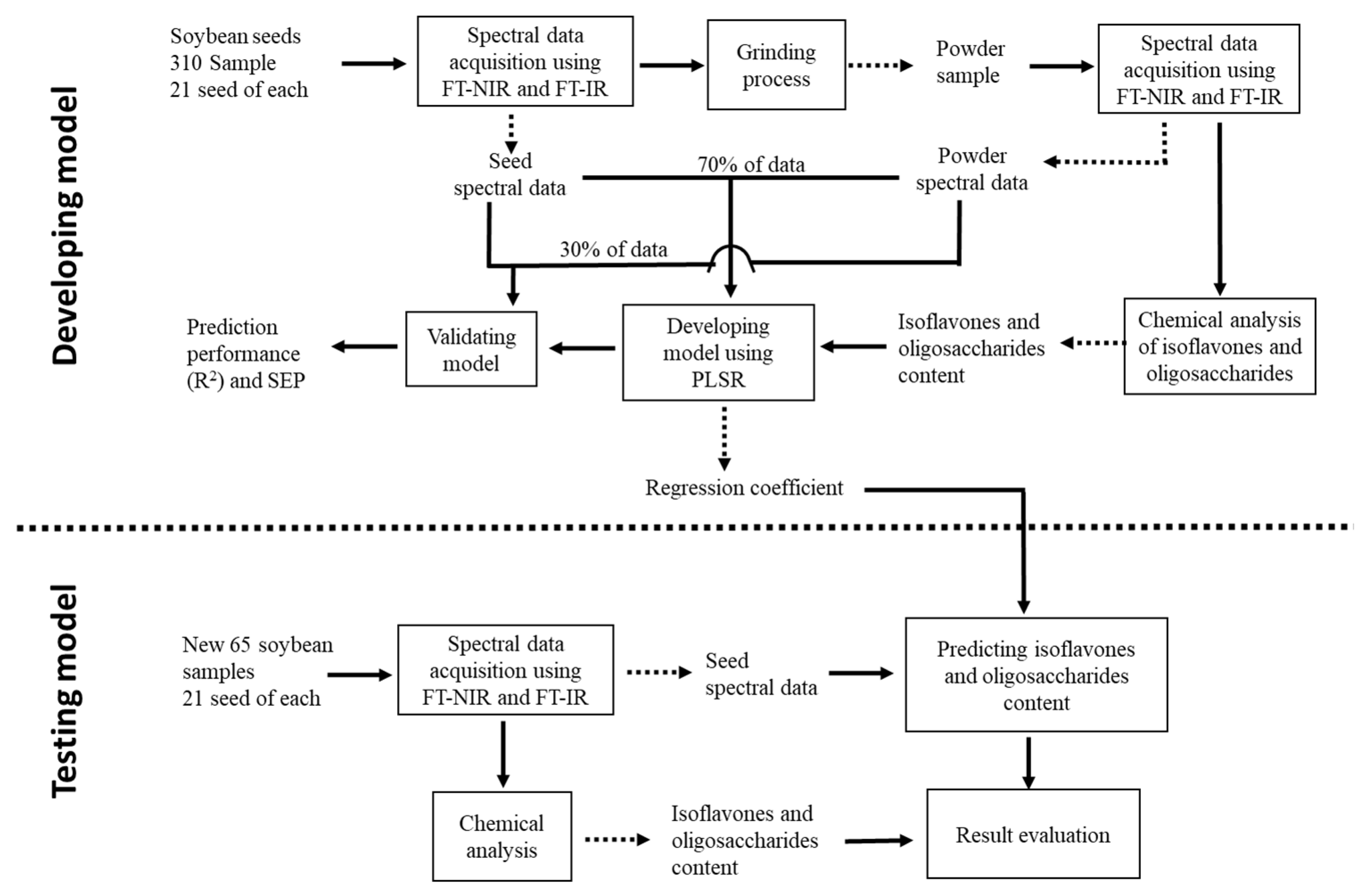
2. Materials and Methods
2.1. Sample Preparation
2.2. Spectral Data Acquisition
2.2.1. FT-NIR Spectroscopy
2.2.2. FT-IR Spectroscopy
2.3. Reference Value Investigation
2.3.1. Chemicals
2.3.2. Isoflavones Determination
2.3.3. Oligosaccharides Determination
2.4. Spectral Data Preprocessing and Multivariate Analysis
2.5. Model Testing
3. Results and Discussion
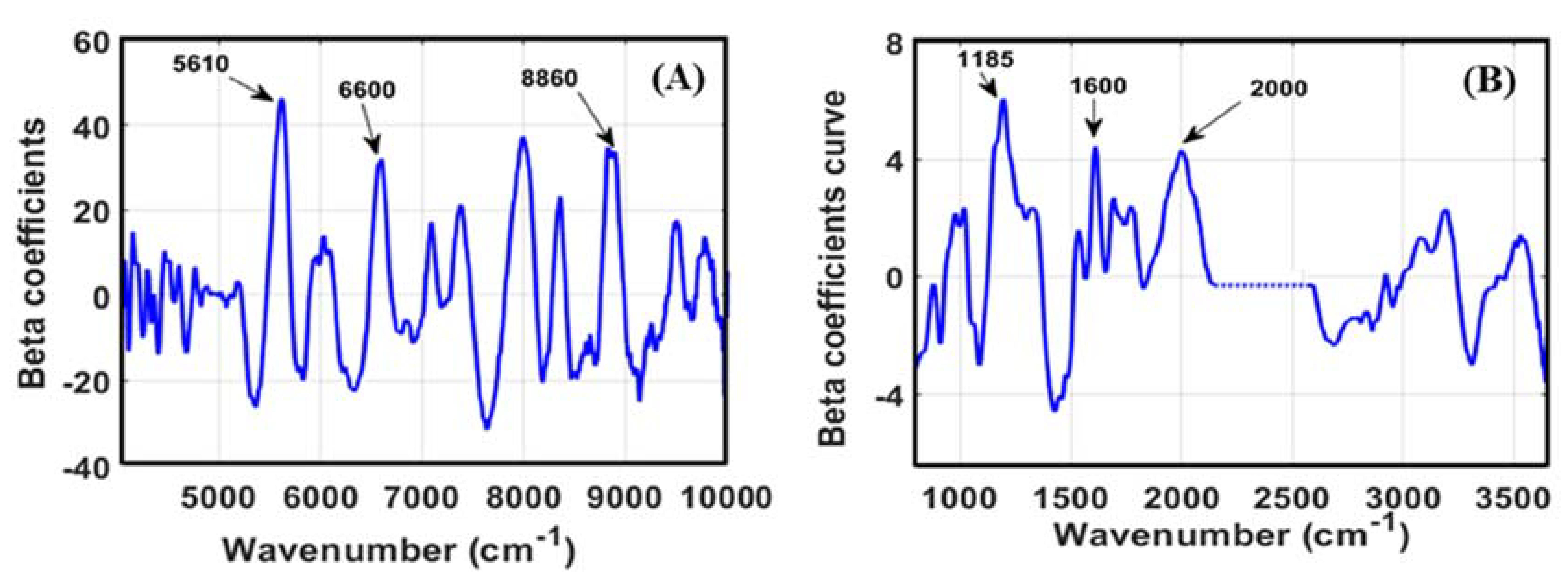
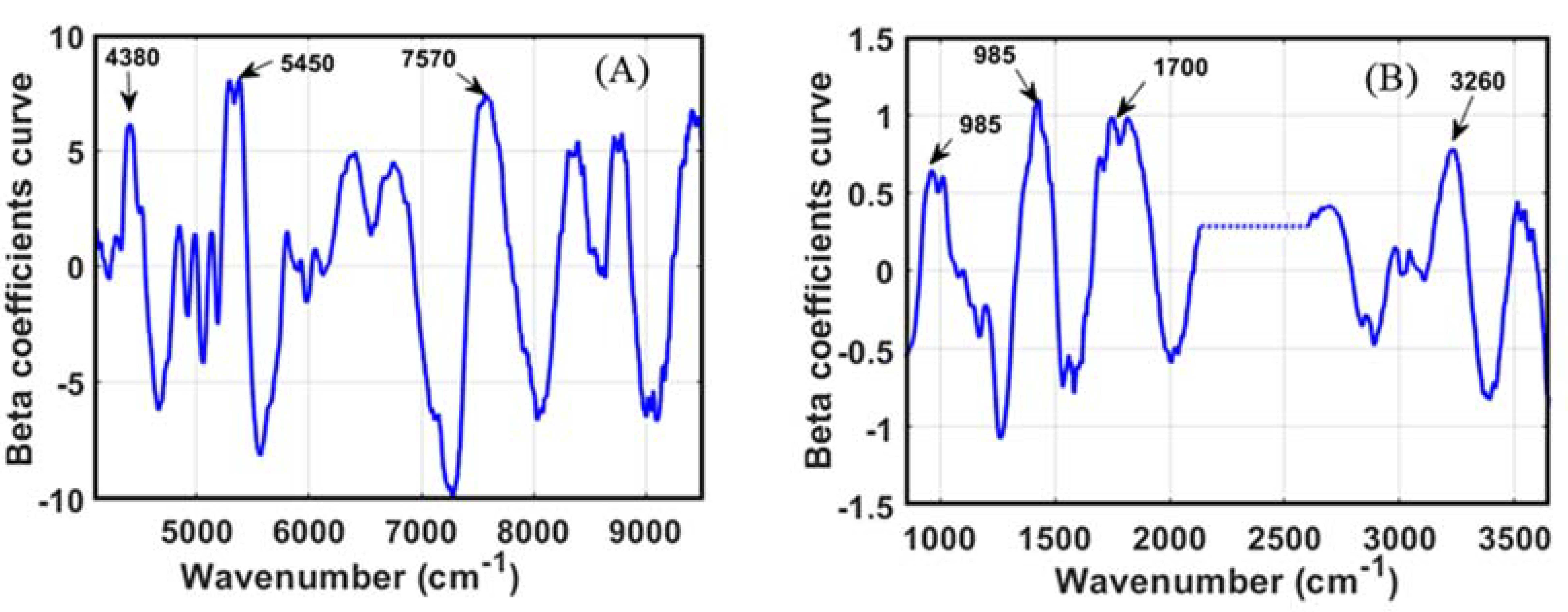
3.1. Spectral Data Interpretation
3.2. Reference Values Analysis
3.3. Prediction Model of Soybean Chemical Components
3.3.1. Isoflavones Model
3.3.2. Oligosaccharides Model
3.4. Testing Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Farid, M.; Kodama, K.; Arato, T.; Okazaki, T.; Oda, T.; Ikeda, H.; Sengoku, S. Comparative Study of Functional Food Regulations in Japan and Globally. Glob. J. Health Sci. 2019, 11, 132. [Google Scholar] [CrossRef]
- Roger Boerma, H.; Specht, J.E. Soybeans: Improvement, Production, and Uses; Shibles, R.M., Harper, J.E., Wilson, R.F., Shoemaker, R.C., Eds.; Agronomy Monographs; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 2004; ISBN 9780891182665.
- Liu, K. Soybeans: Chemistry, Technology, and Utilization; Springer: Cham, Switzerland, 1999; ISBN 0834212994. [Google Scholar]
- Karr-Lilienthal, L.; Kadzere, C.; Grieshop, C.; Fahey, G. Chemical and nutritional properties of soybean carbohydrates as related to nonruminants: A review. Livest. Prod. Sci. 2005, 97, 1–12. [Google Scholar] [CrossRef]
- Borejszo, Z.; Khan, K. Reduction of Flatulence-Causing Sugars by High Temperature Extrusion of Pinto Bean High Starch Fractions. J. Food Sci. 1992, 57, 771–777. [Google Scholar] [CrossRef]
- Bakker-Zierikzee, A.M.; Alles, M.S.; Knol, J.; Kok, F.J.; Tolboom, J.J.M.; Bindels, J.G. Effects of infant formula containing a mixture of galacto- and fructo-oligosaccharides or viable Bifidobacterium animalison the intestinal microflora during the first 4 months of life. Br. J. Nutr. 2005, 94, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Kogiso, M. Soy isoflavones and immunity. J. Med. Investig. 2008, 55, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P.S.; Selvaraj, V.; Yellayi, S. Genistein, Estrogen Receptors, and the Acquired Immune Response. J. Nutr. 2006, 136, 704–708. [Google Scholar] [CrossRef]
- Zuniga, K.; Clinton, S.K.; Erdman, J.W. The Interactions of Dietary Tomato Powder and Soy Germ on Prostate Carcinogenesis in the TRAMP Model. Cancer Prev. Res. 2013, 6, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Bhathena, S.J. Role of Dietary Soy Protein in Obesity. Int. J. Med. Sci. 2007, 4, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.; Cederroth, C.R.; Bourgoin, L.; Foti, M.; Nef, S. Prevention of Diabetes in db/db Mice by Dietary Soy Is Independent of Isoflavone Levels. Endocrinol. 2012, 153, 5200–5211. [Google Scholar] [CrossRef]
- Wei, P.; Liu, M.; Chen, Y.; Chen, D.-C. Systematic review of soy isoflavone supplements on osteoporosis in women. Asian Pac. J. Trop. Med. 2012, 5, 243–248. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.-T.; Yang, G.; Li, H.; Cai, Q.; Xiang, Y.-B.; Ji, B.-T.; Franke, A.A.; Zheng, W.; Shu, X.-O. Urinary isoflavonoids and risk of coronary heart disease. Int. J. Epidemiol. 2012, 41, 1367–1375. [Google Scholar] [CrossRef]
- Kim, G.-H.; Hwang, Y.-S.; Ahn, K.-G.; Kim, G.-P.; Kim, M.-J.; Hong, S.-B.; Moon, J.-K.; Choung, M.-G. Determination of Soluble Carbohydrates in Soybean Seeds Using High Performance Liquid Chromatography with Evaporative Light Scattering Detection. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1062–1067. [Google Scholar] [CrossRef]
- Aguiar, C.L.; Haddad, R.; Eberlin, M.N.; Carrão-Panizzi, M.C.; Tsai, S.M.; Park, Y.K. Thermal behavior of malonylglucoside isoflavones in soybean flour analyzed by RPHPLC/DAD and eletrospray ionization mass spectrometry. LWT Food Sci. Technol. 2012, 48, 114–119. [Google Scholar] [CrossRef]
- Bustamante-Rangel, M.; Delgado-Zamarreño, M.; Pérez-Martín, L.; Carabias-Martínez, R. QuEChERS method for the extraction of isoflavones from soy-based foods before determination by capillary electrophoresis-electrospray ionization-mass spectrometry. Microchem. J. 2013, 108, 203–209. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Xiao, C.; Liu, L.; Hao, M.; Wang, J.; Liu, X. Simultaneous Determination of 15 Phenolic Constituents of Chinese Black Rice Wine by HPLC-MS/MS with SPE. J. Food Sci. 2014, 79, C1100–C1105. [Google Scholar] [CrossRef]
- Hao, J.; Zhu, H.; Zhang, Z.; Yang, S.; Li, H. Identification of anthocyanins in black rice (Oryza sativa L.) by UPLC/Q-TOF-MS and their in vitro and in vivo antioxidant activities. J. Cereal Sci. 2015, 64, 92–99. [Google Scholar] [CrossRef]
- Cho, C.; Jung, Y.S.; Nam, T.G.; Rha, C.; Ko, M.; Jang, D.; Kim, H.; Kim, D. pH-adjusted solvent extraction and reversed-phase HPLC quantification of isoflavones from soybean (Glycine max (L.) Merr.). J. Food Sci. 2020, 85, 673–681. [Google Scholar] [CrossRef]
- Yasmin, J.; Ahmed, M.R.; Lohumi, S.; Wakholi, C.; Kim, M.S.; Cho, B.-K. Classification Method for Viability Screening of Naturally Aged Watermelon Seeds Using FT-NIR Spectroscopy. Sensors 2019, 19, 1190. [Google Scholar] [CrossRef] [PubMed]
- Mureşan, A.E.; Muste, S.; Vlaic, R.A.; Bobiş, O.; Mureşan, C.; Socaciu, C.; Mureşan, V. HPLC Determination and FT-MIR Prediction of Sugars from Juices of Different Apple Cultivars during Fruits Development. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 222–228. [Google Scholar] [CrossRef]
- You, Z.-H.; Liu, Z.-H.; Gong, C.-Y.; Yang, X.-L.; Cheng, F. Applying Attenuated Total Reflection-Mid-Infrared (ATR-MIR) Spectroscopy to Detect Hairtail Surimi in Mixed Surimi and Their Surimi Products. Guang Pu Xue Yu Guang Pu Fen Xi 2015, 35, 2930–2939. [Google Scholar]
- Determination of quality parameters in maize grain by NIR reflectance spectroscopy. Tarım Bilim. Derg. 2012, 18, 31–42. [CrossRef]
- Masithoh, R.E.; Amanah, H.Z.; Yoon, W.-S.; Joshi, R.; Cho, B.-K. Determination of protein and glucose of tuber and root flours using NIR and MIR spectroscopy. Infrared Phys. Technol. 2021, 113, 103577. [Google Scholar] [CrossRef]
- Brás, L.P.; Bernardino, S.A.; Lopes, J.; Menezes, J.C. Multiblock PLS as an approach to compare and combine NIR and MIR spectra in calibrations of soybean flour. Chemom. Intell. Lab. Syst. 2005, 75, 91–99. [Google Scholar] [CrossRef]
- Ferreira, D.; Galão, O.; Pallone, J.; Poppi, R. Comparison and application of near-infrared (NIR) and mid-infrared (MIR) spectroscopy for determination of quality parameters in soybean samples. Food Control. 2014, 35, 227–232. [Google Scholar] [CrossRef]
- Zhang, G.; Li, P.; Zhang, W.; Zhao, J. Analysis of multiple soybean phytonutrients by near-infrared reflectance spectroscopy. Anal. Bioanal. Chem. 2017, 409, 3515–3525. [Google Scholar] [CrossRef]
- Kim, Y.; Ahn, H.; Lee, E.; Kim, H. Development of Prediction Model by NIRS for Anthocyanin Contents in Black Colored Soybean. Korean J. Crop Sci. 2008, 53, 15–20. [Google Scholar]
- Kovalenko, I.V.; Rippke, G.R.; Hurburgh, C.R. Determination of Amino Acid Composition of Soybeans (Glycine max) by Near-Infrared Spectroscopy. J. Agric. Food Chem. 2006, 54, 3485–3491. [Google Scholar] [CrossRef]
- Lee, H.-S.; Kim, J.-B.; Lee, Y.-Y.; Lee, S.-Y.; Gwag, J.-G.; Baek, H.-J.; Kim, C.-K.; Yoon, M.-S. Estimating the Important Components in Three Different Sample Types of Soybean by Near Infrared Reflectance Spectroscopy. Korean J. Crop. Sci. 2011, 56, 88–93. [Google Scholar] [CrossRef]
- Amanah, H.Z.; Joshi, R.; Masithoh, R.E.; Choung, M.-G.; Kim, K.-H.; Kim, G.; Cho, B.-K. Nondestructive measurement of anthocyanin in intact soybean seed using Fourier Transform Near-Infrared (FT-NIR) and Fourier Transform Infrared (FT-IR) spectroscopy. Infrared Phys. Technol. 2020, 111, 103477. [Google Scholar] [CrossRef]
- Choi, Y.-M.; Yoon, H.; Lee, S.; Ko, H.-C.; Shin, M.-J.; Lee, M.C.; Hur, O.S.; Ro, N.Y.; Desta, K.T. Isoflavones, anthocyanins, phenolic content, and antioxidant activities of black soybeans (Glycine max (L.) Merrill) as affected by seed weight. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Gorla, G.; Mestres, M.; Boqué, R.; Riu, J.; Spanu, D.; Giussani, B. ATR-MIR spectroscopy to predict commercial milk major components: A comparison between a handheld and a benchtop instrument. Chemom. Intell. Lab. Syst. 2020, 200, 103995. [Google Scholar] [CrossRef]
- Romani, A.; Vignolini, P.; Galardi, C.; Aroldi, C.; Vazzana, A.C.; Heimler, D. Polyphenolic Content in Different Plant Parts of Soy Cultivars Grown under Natural Conditions. J. Agric. Food Chem. 2003, 51, 5301–5306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Osborne, B.G.; Fearn, T.; Hindle, P.H. Practical NIR spectroscopy with applications in food and beverage analysis. Longman Sci. Tech. 1993, 4, 12. [Google Scholar]
- Candolfi, A.; De Maesschalck, R.; Jouan-Rimbaud, D.; Hailey, P.; Massart, D. The influence of data pre-processing in the pattern recognition of excipients near-infrared spectra. J. Pharm. Biomed. Anal. 1999, 21, 115–132. [Google Scholar] [CrossRef]
- Varmuza, K.; Filzmoser, P. Introduction to Multivariate Statistical Analysis in Chemometrics; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Masithoh, R.E.; Lohumi, S.; Yoon, W.-S.; Amanah, H.Z.; Cho, B.-K. Development of multi-product calibration models of various root and tuber powders by fourier transform near infra-red (FT-NIR) spectroscopy for the quantification of polysaccharide contents. Heliyon 2020, 6, e05099. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.S.; Poppi, R.J.; Pallone, J.A.L. Evaluation of dietary fiber of Brazilian soybean (Glycine max) using near-infrared spectroscopy and chemometrics. J. Cereal Sci. 2015, 64, 43–47. [Google Scholar] [CrossRef]
- Lohumi, S.; Lee, S.; Cho, B.-K. Optimal variable selection for Fourier transform infrared spectroscopic analysis of starch-adulterated garlic powder. Sensors Actuators B Chem. 2015, 216, 622–628. [Google Scholar] [CrossRef]
- Berhow, M.A.; Singh, M.; Bowman, M.J.; Price, N.P.J.; Vaughn, S.F.; Liu, S.X. Quantitative NIR determination of isoflavone and saponin content of ground soybeans. Food Chem. 2020, 317, 126373. [Google Scholar] [CrossRef]
- Hollung, K.; Øverland, M.; Hrustić, M.; Sekulić, P.; Miladinović, J.; Martens, H.; Narum, B.; Sahlstrøm, S.; Sørensen, M.; Storebakken, T.; et al. Evaluation of Nonstarch Polysaccharides and Oligosaccharide Content of Different Soybean Varieties (Glycine max) by Near-Infrared Spectroscopy and Proteomics. J. Agric. Food Chem. 2005, 53, 9112–9121. [Google Scholar] [CrossRef]
- Sato, T.; Equchi, K.; Hatano, T.; Nishiba, Y. Use of Near-infrared Reflectance Spectroscopy for the Estimation of the Isoflavone Contents of Soybean Seeds. Plant Prod. Sci. 2008, 11, 481–486. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, H.; Yang, H.; Nie, L.; Zang, H. Rapid determination of major bioactive isoflavonoid compounds during the extraction process of kudzu (Pueraria lobata) by near-infrared transmission spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 1403–1408. [Google Scholar] [CrossRef]
- Aenugu, H.P.R.; Sathis Kumar, D.; Srisudharson, N.P.; Ghosh, S.S.; Banji, D. Near infra red spectroscopy–An overview. Int. J. Chem Tech Res. 2011, 3, 825–836. [Google Scholar]
- Coates, J. Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Chen, J.; Ren, X.; Zhang, Q.; Diao, X.; Shen, Q. Determination of protein, total carbohydrates and crude fat contents of foxtail millet using effective wavelengths in NIR spectroscopy. J. Cereal Sci. 2013, 58, 241–247. [Google Scholar] [CrossRef]
- Choung, M.-G. Determination of Sucrose Content in Soybean Using Near-infrared Reflectance Spectroscopy. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 478–484. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Justino, C.I.L.; Duarte, K.; Freitas, A.C. Analysis of Marine Samples in Search of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2014; Volume 65, ISBN 9780444633590. [Google Scholar]
- Schoonjans, V.; Questier, F.; Guo, Q.; Van der Heyden, Y.; Massart, D. Assessing molecular similarity/diversity of chemical structures by FT-IR spectroscopy. J. Pharm. Biomed. Anal. 2001, 24, 613–627. [Google Scholar] [CrossRef]
- Williams, P.; Norris, K. Near-Infrared Technology in the Agricultural and Food Industries, 2nd ed.; Amer Assn of Cereal Chemists: Washington, DC, USA, 2001; ISBN 1891127241. [Google Scholar]

| Components | Number of Samples | Mean ± SD | Max | Min |
|---|---|---|---|---|
| Isoflavones (mg/g) | ||||
| Daidzin | 289 | 0.129 ± 0.057 | 0.317 | 0.018 |
| Genistin | 289 | 0.136 ± 0.043 | 0.250 | 0.039 |
| Glycitin | 265 | 0.042 ± 0.018 | 0.112 | 0.012 |
| 6-O-Malonyl daidzin | 310 | 0.665 ± 0.271 | 1.403 | 0.110 |
| 6-O-Malonyl genistin | 310 | 1.080 ± 0.375 | 2.028 | 0.200 |
| 6-O-Malonyl glycitin | 280 | 0.147 ± 0.061 | 0.376 | 0.005 |
| Acetyl daidzin | 270 | 0.065 ± 0.021 | 0.123 | 0.020 |
| Total isoflavones | 310 | 2.320 ± 0.77 | 4.339 | 0.728 |
| Oligosaccharides (%) | ||||
| Sucrose | 310 | 6.001 ± 1.210 | 8.257 | 2.889 |
| Stachyose | 310 | 2.885 ± 0.517 | 4.067 | 1.781 |
| Raffinose | 310 | 1.124 ± 0.133 | 1.456 | 0.806 |
| Total oligosaccharides | 310 | 11.131 ± 1.645 | 14.643 | 6.879 |
| Components (Preprocessing Method) | FT-NIR | FT-IR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2c | SEC | R2p | SEP | LVs | R2c | SEC | R2p | SEP | LVs | |
| Seed | ||||||||||
| Daidzin (MN/MN) | 0.74 | 0.03 | 0.72 | 0.03 | 25 | 0.72 | 0.03 | 0.70 | 0.03 | 25 |
| Genistin (RD/RD) | 0.73 | 0.02 | 0.70 | 0.03 | 25 | 0.70 | 0.02 | 0.67 | 0.03 | 25 |
| Glycitin (MN/MN) | 0.78 | 0.01 | 0.76 | 0.01 | 25 | 0.73 | 0.01 | 0.72 | 0.01 | 25 |
| 6-O-Malonyl daidzin (MN/MN) | 0.77 | 0.14 | 0.75 | 0.12 | 25 | 0.72 | 0.15 | 0.70 | 0.20 | 25 |
| 6-O-Malonyl genistin (MN/MN) | 0.79 | 0.17 | 0.77 | 0.18 | 25 | 0.71 | 0.19 | 0.70 | 0.29 | 25 |
| 6-O-Malonyl glycitin (MN/MN) | 0.75 | 0.03 | 0.71 | 0.03 | 25 | 0.70 | 0.03 | 0.70 | 0.03 | 25 |
| Acetyl daidzin (SNV/SNV) | 0.76 | 0.01 | 0.73 | 0.01 | 23 | 0.71 | 0.02 | 0.68 | 0.02 | 22 |
| Total isoflavones (MN/MN) | 0.80 | 0.32 | 0.80 | 0.30 | 25 | 0.74 | 0.29 | 0.73 | 0.30 | 25 |
| Powder | ||||||||||
| Daidzin (RD/MN) | 0.77 | 0.03 | 0.75 | 0.03 | 22 | 0.79 | 0.02 | 0.78 | 0.02 | 22 |
| Genistin (RD/MN) | 0.81 | 0.02 | 0.76 | 0.03 | 23 | 0.84 | 0.02 | 0.73 | 0.02 | 22 |
| Glycitin (MN/MN) | 0.76 | 0.01 | 0.74 | 0.01 | 24 | 0.74 | 0.01 | 0.70 | 0.01 | 22 |
| 6-O-Malonyl daidzin (MN/MN) | 0.83 | 0.11 | 0.73 | 0.13 | 22 | 0.83 | 0.11 | 0.74 | 0.15 | 22 |
| 6-O-Malonyl genistin (MN/MN) | 0.83 | 0.16 | 0.77 | 0.17 | 22 | 0.88 | 0.14 | 0.77 | 0.19 | 23 |
| 6-O-Malonyl glycitin (MN/MN) | 0.73 | 0.03 | 0.72 | 0.03 | 24 | 0.78 | 0.03 | 0.74 | 0.03 | 24 |
| Acetyl daidzin (SNV/SNV) | 0.77 | 0.01 | 0.75 | 0.01 | 24 | 0.77 | 0.01 | 0.76 | 0.01 | 24 |
| Total isoflavones (MN/MN) | 0.92 | 0.21 | 0.84 | 0.33 | 25 | 0.92 | 0.21 | 0.84 | 0.33 | 25 |
| Components (Preprocessing Method) | FT-NIR | FT-IR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2c | SEC | R2p | SEP | LVs | R2c | SEC | R2p | SEP | LVs | |
| Seed | ||||||||||
| Sucrose (RD/MN) | 0.72 | 0.71 | 0.70 | 0.75 | 19 | 0.72 | 0.67 | 0.71 | 0.68 | 19 |
| Stachyose (RD/SNV) | 0.70 | 0.28 | 0.66 | 0.29 | 19 | 0.67 | 0.30 | 0.66 | 0.33 | 19 |
| Raffinose (SNV/SNV) | 0.72 | 0.06 | 0.70 | 0.07 | 20 | 0.68 | 0.07 | 0.66 | 0.08 | 20 |
| Total soluuble Carb (SNV/MN) | 0.72 | 0.80 | 0.70 | 0.82 | 18 | 0.70 | 0.88 | 0.70 | 0.95 | 18 |
| Powder | ||||||||||
| Sucrose (RD/MN) | 0.83 | 0.55 | 0.75 | 0.67 | 19 | 0.73 | 0.62 | 0.74 | 0.64 | 19 |
| Stachyose (RD/SNV) | 0.77 | 0.24 | 0.70 | 0.28 | 19 | 0.66 | 0.29 | 0.67 | 0.30 | 19 |
| Raffinose (SNV/SNV) | 0.77 | 0.06 | 0.72 | 0.06 | 20 | 0.73 | 0.07 | 0.72 | 0.07 | 20 |
| Total soluuble Carb (SNV/MN) | 0.78 | 0.74 | 0.75 | 0.80 | 18 | 0.73 | 0.87 | 0.72 | 0.84 | 18 |
| Components (Unit) | Number of Varieties | Mean ± SD | Max | Min |
|---|---|---|---|---|
| Total isoflvones (mg/g) | 60 | 2.799 ± 0.736 | 4.176 | 0.946 |
| Total oligosaccharides (%) | 65 | 11.317 ± 0.938 | 13.419 | 9.135 |
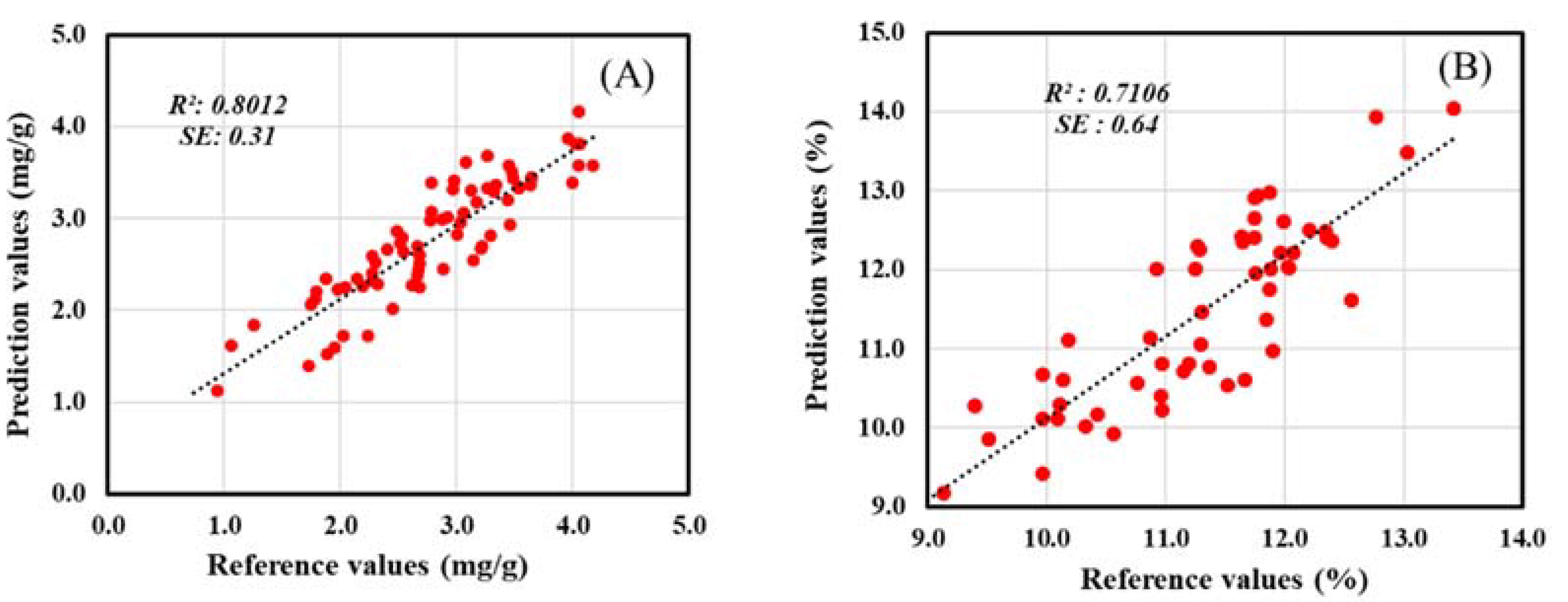
| Components | N Seeds | N Varieties | R2 | SE |
|---|---|---|---|---|
| FT-NIR | ||||
| Total Isoflavones | 1260 | 60 | 0.80 | 0.31 |
| Total oligosaccharides | 1365 | 65 | 0.71 | 0.64 |
| FT-IR | ||||
| Total Isoflavones | 1260 | 60 | 0.71 | 0.35 |
| Total oligosaccharides | 1365 | 65 | 0.68 | 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amanah, H.Z.; Tunny, S.S.; Masithoh, R.E.; Choung, M.-G.; Kim, K.-H.; Kim, M.S.; Baek, I.; Lee, W.-H.; Cho, B.-K. Nondestructive Prediction of Isoflavones and Oligosaccharides in Intact Soybean Seed Using Fourier Transform Near-Infrared (FT-NIR) and Fourier Transform Infrared (FT-IR) Spectroscopic Techniques. Foods 2022, 11, 232. https://doi.org/10.3390/foods11020232
Amanah HZ, Tunny SS, Masithoh RE, Choung M-G, Kim K-H, Kim MS, Baek I, Lee W-H, Cho B-K. Nondestructive Prediction of Isoflavones and Oligosaccharides in Intact Soybean Seed Using Fourier Transform Near-Infrared (FT-NIR) and Fourier Transform Infrared (FT-IR) Spectroscopic Techniques. Foods. 2022; 11(2):232. https://doi.org/10.3390/foods11020232
Chicago/Turabian StyleAmanah, Hanim Z., Salma Sultana Tunny, Rudiati Evi Masithoh, Myoung-Gun Choung, Kyung-Hwan Kim, Moon S. Kim, Insuck Baek, Wang-Hee Lee, and Byoung-Kwan Cho. 2022. "Nondestructive Prediction of Isoflavones and Oligosaccharides in Intact Soybean Seed Using Fourier Transform Near-Infrared (FT-NIR) and Fourier Transform Infrared (FT-IR) Spectroscopic Techniques" Foods 11, no. 2: 232. https://doi.org/10.3390/foods11020232
APA StyleAmanah, H. Z., Tunny, S. S., Masithoh, R. E., Choung, M.-G., Kim, K.-H., Kim, M. S., Baek, I., Lee, W.-H., & Cho, B.-K. (2022). Nondestructive Prediction of Isoflavones and Oligosaccharides in Intact Soybean Seed Using Fourier Transform Near-Infrared (FT-NIR) and Fourier Transform Infrared (FT-IR) Spectroscopic Techniques. Foods, 11(2), 232. https://doi.org/10.3390/foods11020232










