Active Packaging Films Made by Complex Coacervation of Tragacanth Gum and Gelatin Loaded with Curcumin; Characterization and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Soluble Fraction of TG
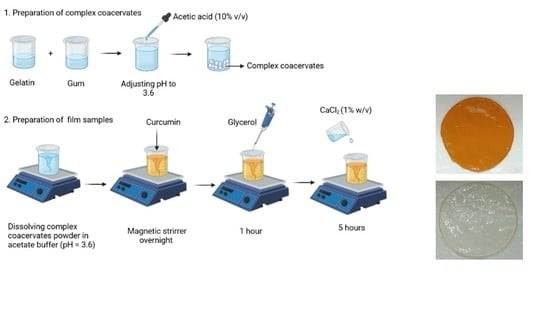
2.3. Preparation of Complex Coacervates
2.4. Film Formation Process
2.5. Characterization of Films
2.5.1. The Thickness and Mechanical Properties
2.5.2. Water Vapor Permeability (WVP)
2.5.3. Moisture Content (MC) and Water Solubility
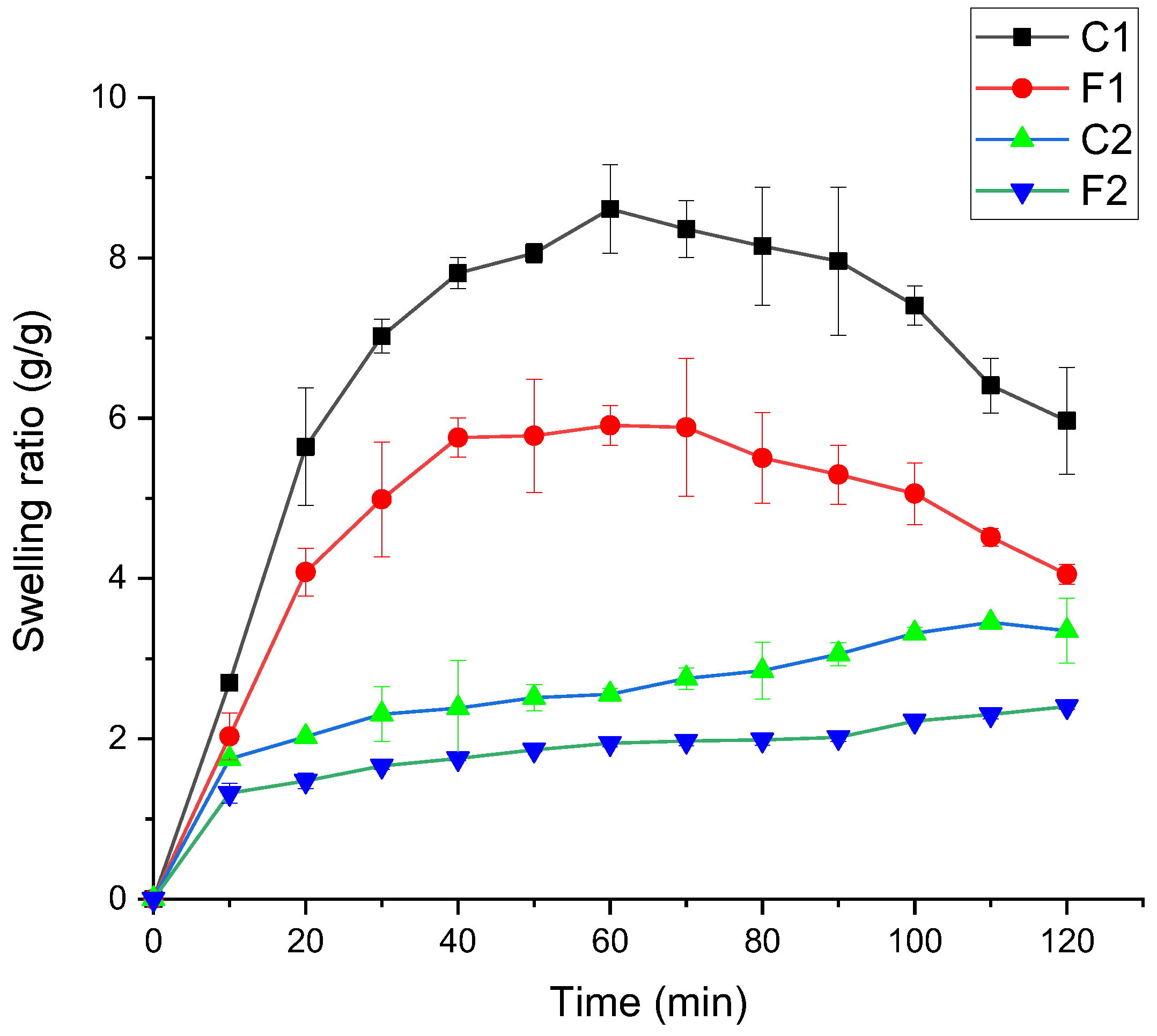
2.5.4. The Swelling Test
2.5.5. Curcumin Release from Films
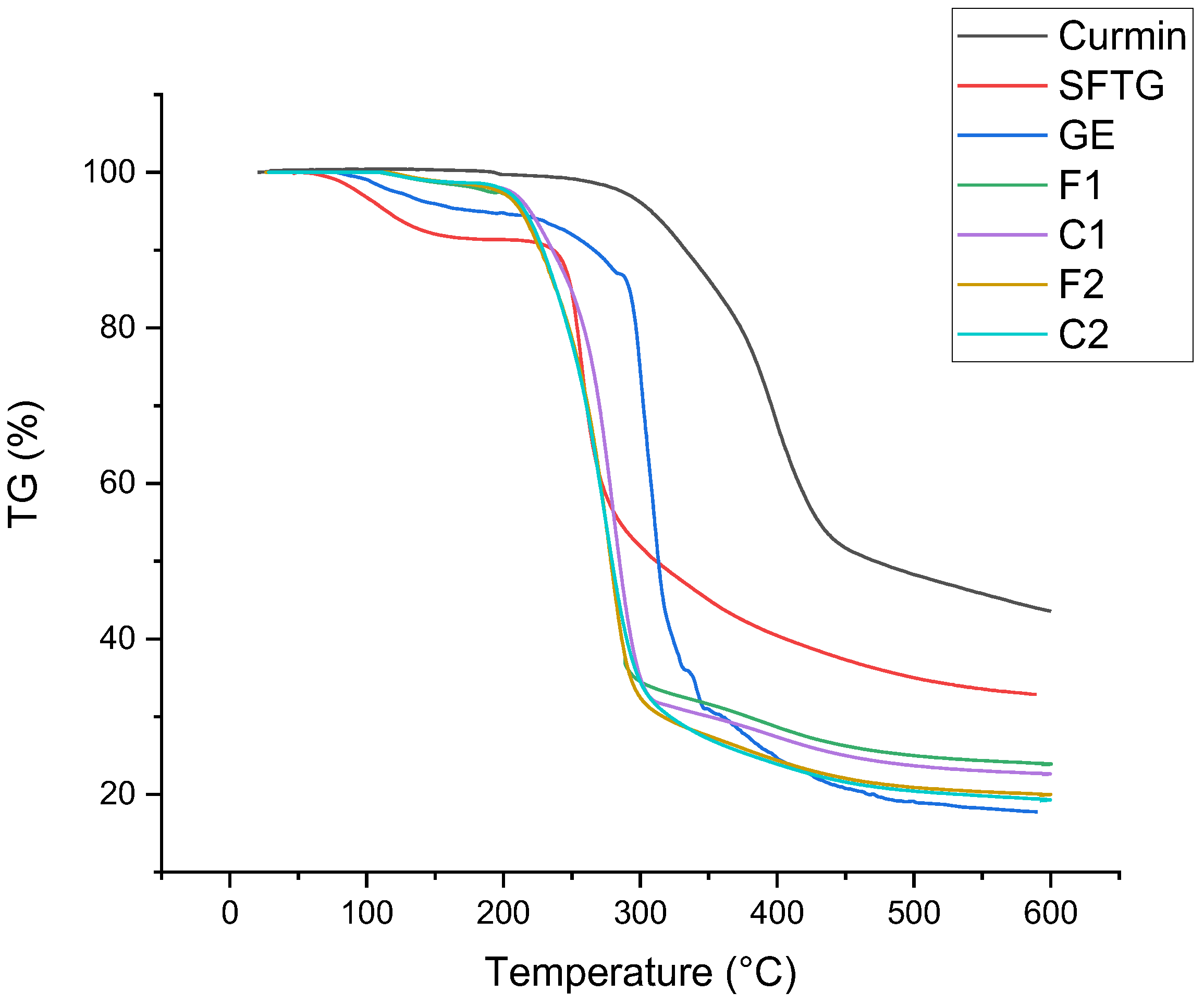
2.5.6. Thermal Analysis
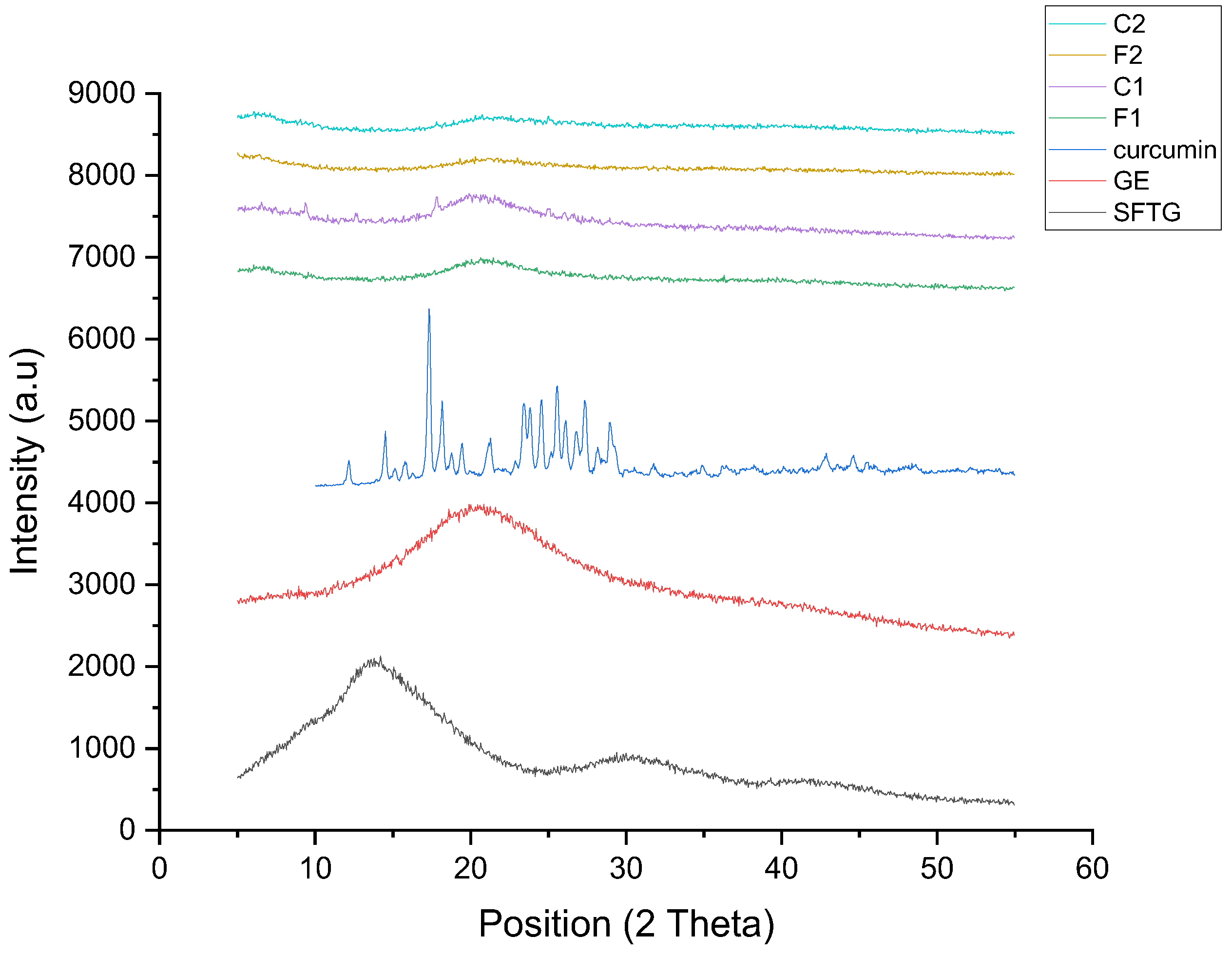
2.5.7. X-ray Diffraction (XRD)
2.5.8. Fourier Transform Infrared (FTIR) Spectroscopy
2.6. Antioxidant Activity of Films
2.7. Antibacterial Activity of Films
2.8. Statistical Analysis
3. Results and Discussion
3.1. Yield of Complex Coacervates
3.2. Physicochemical and Mechanical Properties of the Produced Active Films
3.2.1. Thickness and Mechanical Properties
3.2.2. Water Vapor Permeability
3.2.3. Moisture Content and Water Solubility
3.2.4. The Swelling Results of the Films
3.2.5. Thermal Properties
3.2.6. XRD Results
3.2.7. Chemical and Functional Groups of the Films
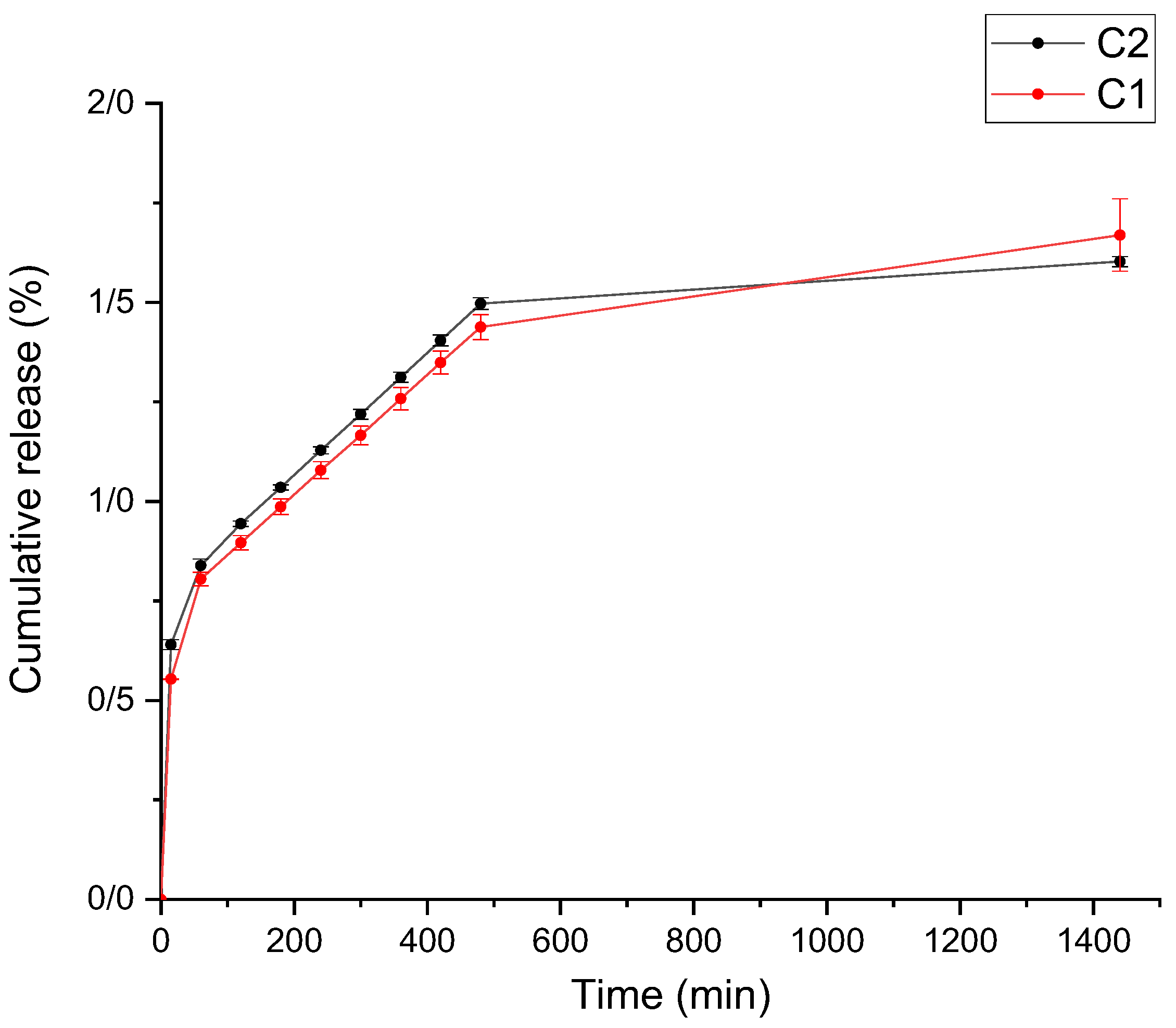
3.3. Release Results of Curcumin from Active Films
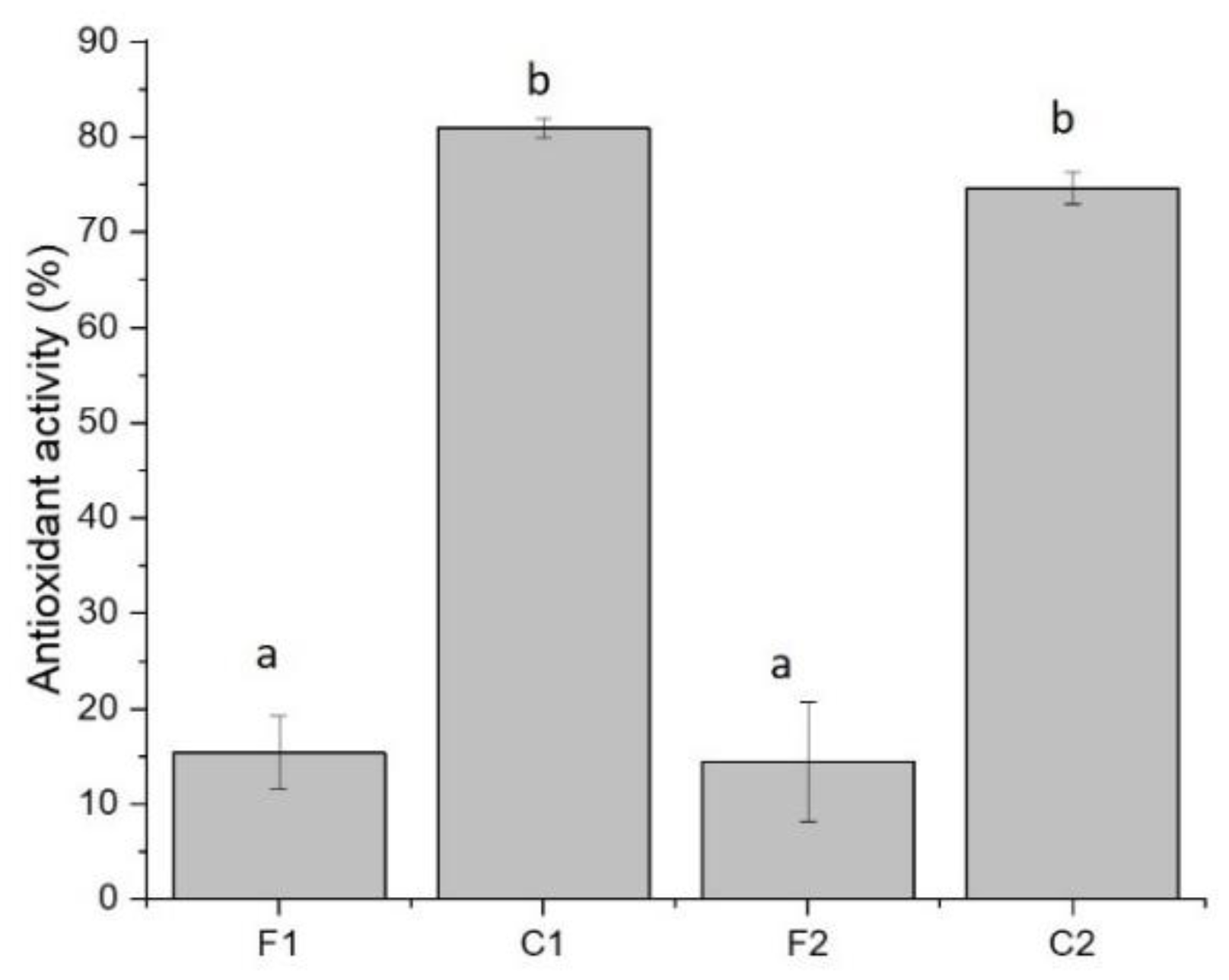
3.4. Antioxidant Activity of Curcumin-Loaded Films
3.5. Antibacterial Activity of Curcumin-Loaded Films
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Sani, M.A.; Azizi-Lalabadi, M.; Tavassoli, M.; Mohammadi, K.; McClements, D.J. Recent advances in the development of smart and active biodegradable packaging materials. Nanomaterials 2021, 11, 1331. [Google Scholar] [CrossRef] [PubMed]
- Ivonkovic, A.; Zeljko, K.; Talic, S.; Lasic, M. Biodegradable packaging in the food industry. J. Food Saf. Food Qual. 2017, 68, 26–38. [Google Scholar]
- Nesterenko, A.; Alric, I.; Silvestre, F.; Durrieu, V. Vegetable proteins in microencapsulation: A review of recent interventions and their effectiveness. Ind. Crops Prod. 2013, 42, 469–479. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Ikram, S. Chitosan and gelatin based biodegradable packaging films with UV-light protection. J. Photochem. Photobiol. B Biol. 2016, 163, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Mohammadifar, M.A.; Musavi, S.M.; Kiumarsi, A.; Williams, P.A. Solution properties of targacanthin (water-soluble part of gum tragacanth exudate from Astragalus gossypinus). Int. J. Biol. Macromol. 2006, 38, 31–39. [Google Scholar] [PubMed]
- Karimi, N.; Mohammadifar, M.A. Role of water soluble and water swellable fractions of gum tragacanth on stability and characteristic of model oil in water emulsion. Food Hydrocoll. 2014, 37, 124–133. [Google Scholar] [CrossRef]
- Khodaei, D.; Oltrogge, K.; Hamidi-Esfahani, Z. Preparation and characterization of blended edible films manufactured using gelatin, tragacanth gum and, Persian gum. LWT 2020, 117, 108617. [Google Scholar] [CrossRef]
- Gentile, L. Protein–polysaccharide interactions and aggregates in food formulations. Curr. Opin. Colloid Interface Sci. 2020, 48, 18–27. [Google Scholar]
- Wei, Z.; Huang, Q. Assembly of protein–polysaccharide complexes for delivery of bioactive ingredients: A perspective paper. J. Agric. Food Chem. 2019, 67, 1344–1352. [Google Scholar] [CrossRef]
- Schmitt, C.; Turgeon, S.L. Protein/polysaccharide complexes and coacervates in food systems. Adv. Colloid Interface Sci. 2011, 167, 63–70. [Google Scholar] [CrossRef]
- Weinbreck, F.; De Vries, R.; Schrooyen, P.; De Kruif, C. Complex coacervation of whey proteins and gum arabic. Biomacromolecules 2003, 4, 293–303. [Google Scholar] [CrossRef]
- Eghbal, N.; Yarmand, M.S.; Mousavi, M.; Degraeve, P.; Oulahal, N.; Gharsallaoui, A. Complex coacervation for the development of composite edible films based on LM pectin and sodium caseinate. Carbohydr. Polym. 2016, 151, 947–956. [Google Scholar] [CrossRef]
- Tavares, L.; Souza, H.K.; Gonçalves, M.P.; Rocha, C.M. Physicochemical and microstructural properties of composite edible film obtained by complex coacervation between chitosan and whey protein isolate. Food Hydrocoll. 2021, 113, 106471. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly bioavailable forms of curcumin and promising avenues for curcumin-based research and application: A review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [Green Version]
- Sharifi, S.; Fathi, N.; Memar, M.Y.; Hosseiniyan Khatibi, S.M.; Khalilov, R.; Negahdari, R.; Zununi Vahed, S.; Maleki Dizaj, S. Anti-microbial activity of curcumin nanoformulations: New trends and future perspectives. Phytother. Res. 2020, 34, 1926–1946. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Rezaei, A.; Nasirpour, A.; Tavanai, H. Fractionation and some physicochemical properties of almond gum (Amygdalus communis L.) exudates. Food Hydrocoll. 2016, 60, 461–469. [Google Scholar] [CrossRef]
- Devi, N.; Maji, T.K. Genipin crosslinked microcapsules of gelatin A and κ-carrageenan polyelectrolyte complex for encapsulation of Neem (Azadirachta Indica A. Juss.) seed oil. Polym. Bull. 2010, 65, 347–362. [Google Scholar] [CrossRef]
- ASTM D882-02; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 1995.
- ASTM. Standard test method for water vapor transmission of materials. In Annual Books of ASTM Standards. Designation E 96-01, Philadelphia: ASTM, American Society for Testing Materials; ASTM: West Conshohocken, PA, USA, 2001. [Google Scholar]
- Haghighi, H.; Biard, S.; Bigi, F.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Comprehensive characterization of active chitosan-gelatin blend films enriched with different essential oils. Food Hydrocoll. 2019, 95, 33–42. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Drozłowska, E. Polysaccharide/gelatin blend films as carriers of ascorbyl palmitate—A comparative study. Food Chem. 2020, 333, 127465. [Google Scholar] [CrossRef] [PubMed]
- Kochkina, N.E.; Lukin, N.D. Structure and properties of biodegradable maize starch/chitosan composite films as affected by PVA additions. Int. J. Biol. Macromol. 2020, 157, 377–384. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to a 16th list of substances for food contact materials. EFSA J. 2007, 5, 555. [Google Scholar] [CrossRef]
- Amani, F.; Sami, M.; Rezaei, A. Characterization and antibacterial activity of encapsulated rosemary essential oil within amylose nanostructures as a natural antimicrobial in food applications. Starch-Stärke 2021, 73, 2100021. [Google Scholar] [CrossRef]
- Rezaei, A.; Nasirpour, A. Evaluation of release kinetics and mechanisms of curcumin and curcumin-β-cyclodextrin inclusion complex incorporated in electrospun almond gum/PVA nanofibers in simulated saliva and simulated gastrointestinal conditions. BioNanoScience 2019, 9, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Hanani, Z.N.; Yee, F.C.; Nor-Khaizura, M. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Rukmanikrishnan, B.; Ramalingam, S.; Lee, J. Quaternary ammonium silane-reinforced agar/polyacrylamide composites for packaging applications. Int. J. Biol. Macromol. 2021, 182, 1301–1309. [Google Scholar] [CrossRef]
- Eratte, D.; Wang, B.; Dowling, K.; Barrow, C.J.; Adhikari, B.P. Complex coacervation with whey protein isolate and gum arabic for the microencapsulation of omega-3 rich tuna oil. Food Funct. 2014, 5, 2743–2750. [Google Scholar] [CrossRef]
- Shinde, U.A.; Nagarsenker, M.S. Characterization of gelatin-sodium alginate complex coacervation system. Indian J. Pharm. Sci. 2009, 71, 313. [Google Scholar]
- Roy, S.; Rhim, J.-W. Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocoll. 2020, 98, 105302. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Guo, X.; Ji, M.; Wang, J.; Cheng, C.; Chen, L.; Wen, C.; Zhang, Q. Physicochemical, antioxidant, in vitro release, and heat sealing properties of fish gelatin films incorporated with β-cyclodextrin/curcumin complexes for apple juice preservation. Food Bioprocess Technol. 2018, 11, 447–461. [Google Scholar]
- Kanatt, S.R.; Rao, M.; Chawla, S.; Sharma, A. Active chitosan–polyvinyl alcohol films with natural extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Noronha, C.M.; de Carvalho, S.M.; Lino, R.C.; Barreto, P.L.M. Characterization of antioxidant methylcellulose film incorporated with α-tocopherol nanocapsules. Food Chem. 2014, 159, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Martins, J.T.; Cerqueira, M.A.; Vicente, A.A. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll. 2012, 27, 220–227. [Google Scholar]
- Luo, N.; Varaprasad, K.; Reddy, G.V.S.; Rajulu, A.V.; Zhang, J. Preparation and characterization of cellulose/curcumin composite films. RSC Adv. 2012, 2, 8483–8488. [Google Scholar] [CrossRef]
- Ma, Q.; Ren, Y.; Wang, L. Investigation of antioxidant activity and release kinetics of curcumin from tara gum/polyvinyl alcohol active film. Food Hydrocoll. 2017, 70, 286–292. [Google Scholar]
- Atef, M.; Rezaei, M.; Behrooz, R. Characterization of physical, mechanical, and antibacterial properties of agar-cellulose bionanocomposite films incorporated with savory essential oil. Food Hydrocoll. 2015, 45, 150–157. [Google Scholar]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Smart edible films based on gelatin and curcumin. Food Hydrocoll. 2017, 66, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, M.N.; de Matos Fonseca, J.; Feldhaus, H.K.; Soares, L.S.; Valencia, G.A.; de Campos, C.E.M.; Di Luccio, M.; Monteiro, A.R. Physical and morphological properties of hydroxypropyl methylcellulose films with curcumin polymorphs. Food Hydrocoll. 2019, 97, 105217. [Google Scholar] [CrossRef]
- Lee, K.Y.; Shim, J.; Lee, H.G. Mechanical properties of gellan and gelatin composite films. Carbohydr. Polym. 2004, 56, 251–254. [Google Scholar] [CrossRef]
- Ghaderi, J.; Hosseini, S.F.; Keyvani, N.; Gómez-Guillén, M.C. Polymer blending effects on the physicochemical and structural features of the chitosan/poly (vinyl alcohol)/fish gelatin ternary biodegradable films. Food Hydrocoll. 2019, 95, 122–132. [Google Scholar] [CrossRef]
- Koc, F.E.; Altıncekic, T.G. Investigation of gelatin/chitosan as potential biodegradable polymer films on swelling behavior and methylene blue release kinetics. Polym. Bull. 2021, 78, 3383–3398. [Google Scholar] [CrossRef]
- Pramanik, N.; Mitra, T.; Khamrai, M.; Bhattacharyya, A.; Mukhopadhyay, P.; Gnanamani, A.; Basu, R.K.; Kundu, P.P. Characterization and evaluation of curcumin loaded guar gum/polyhydroxyalkanoates blend films for wound healing applications. RSC Adv. 2015, 5, 63489–63501. [Google Scholar] [CrossRef]
- Türe, H. Characterization of hydroxyapatite-containing alginate–gelatin composite films as a potential wound dressing. Int. J. Biol. Macromol. 2019, 123, 878–888. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Antioxidant and antimicrobial poly (vinyl alcohol)-based films incorporated with grapefruit seed extract and curcumin. J. Environ. Chem. Eng. 2021, 9, 104694. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of antimicrobial and antioxidant gelatin/curcumin composite films for active food packaging application. Colloids Surf. B Biointerfaces 2020, 188, 110761. [Google Scholar] [CrossRef]
- Ki, C.S.; Baek, D.H.; Gang, K.D.; Lee, K.H.; Um, I.C.; Park, Y.H. Characterization of gelatin nanofiber prepared from gelatin–formic acid solution. Polymer 2005, 46, 5094–5102. [Google Scholar]
- Rezaei, A.; Nasirpour, A. Encapsulation of curcumin using electrospun almond gum nanofibers: Fabrication and characterization. Int. J. Food Prop. 2018, 21, 1608–1618. [Google Scholar] [CrossRef] [Green Version]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between gelatin and sodium alginate: UV and FTIR studies. J. Dispers. Sci. Technol. 2019, 41, 690–698. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Manshad, A.K.; Mohammadi, A.H. Effects of Tragacanth Gum as a natural polymeric surfactant and soluble ions on chemical smart water injection into oil reservoirs. J. Mol. Struct. 2020, 1200, 127078. [Google Scholar] [CrossRef]
- Chen, X.; Zou, L.-Q.; Niu, J.; Liu, W.; Peng, S.-F.; Liu, C.-M. The stability, sustained release and cellular antioxidant activity of curcumin nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef] [Green Version]
- Ismail, E.; Sabry, D.; Mahdy, H.; Khalil, M. Synthesis and Characterization of some ternary metal complexes of curcumin with 1, 10-phenanthroline and their anticancer applications. J. Sci. Res. 2014, 6, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Hazirah, M.N.; Isa, M.; Sarbon, N. Effect of xanthan gum on the physical and mechanical properties of gelatin-carboxymethyl cellulose film blends. Food Packag. Shelf Life 2016, 9, 55–63. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Joseph, A.; Ejaz, M.; Maniruzzaman, M. Zinc oxide/clove essential oil incorporated type B gelatin nanocomposite formulations: A proof-of-concept study for 3D printing applications. Food Hydrocoll. 2020, 98, 105256. [Google Scholar] [CrossRef]
- Zhong, Q.-P.; Xia, W.-S. Physicochemical properties of edible and preservative films from chitosan/cassava starch/gelatin blend plasticized with glycerol. Food Technol. Biotechnol. 2008, 46, 262–269. [Google Scholar]
- Wang, L.-F.; Rhim, J.-W. Grapefruit seed extract incorporated antimicrobial LDPE and PLA films: Effect of type of polymer matrix. LWT 2016, 74, 338–345. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Zia, J.; Paul, U.C.; Heredia-Guerrero, J.A.; Athanassiou, A.; Fragouli, D. Low-density polyethylene/curcumin melt extruded composites with enhanced water vapor barrier and antioxidant properties for active food packaging. Polymer 2019, 175, 137–145. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Curcumin incorporated poly (butylene adipate-co-terephthalate) film with improved water vapor barrier and antioxidant properties. Materials 2020, 13, 4369. [Google Scholar]
- Salgado, P.R.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Exploration of the antioxidant and antimicrobial capacity of two sunflower protein concentrate films with naturally present phenolic compounds. Food Hydrocoll. 2012, 29, 374–381. [Google Scholar] [CrossRef]

| Biopolymer Ratios (w/w) | Particle Yield (%) |
|---|---|
| 1GE: 1SFTG | 46.57 ± 1.71 b |
| 2GE: 1SFTG | 60.06 ± 0.14 a |
| 1GE: 2SFTG | 14.33 ± 0.81 c |
| Film Samples | Thickness (mm) | WVP (g mm/kP Day m2) | Moisture Content (%) | Water Solubility (%) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|---|
| F1 (1GE: 1SFTG) | 0.21 ± 0.06 a | 0.6048 ± 0.12 a | 63.67 ± 0.014d | 96.73 ± 1.11d | 1.74 ± 0.21 b | 81.48 ± 2.91 a |
| C1 (1GE: 1SFTG/curcumin) | 0.21 ± 0.02 a | 0.4752 ± 0.15 a | 54.14 ± 0.026 c | 80.74 ± 1.21 c | 0.62 ± 0.06 a | 122.00 ± 11.81 b |
| F2 (2GE: 1SFTG) | 0.25 ± 0.04 a | 0.6912 ± 0.14 a | 50.22 ± 0.025 b | 78.80 ± 8.60 b | 1.77 ± 0.48 b | 98.87 ± 23.08 a |
| C2 (2GE: 1SFTG/curcumin) | 0.25 ± 0.04 a | 0.6048 ± 0.12 a | 42.62 ± 0.057 a | 35.93 ± 3.55 a | 0.17 ± 0.07 a | 109.58 ± 10.78 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amani, F.; Rezaei, A.; Akbari, H.; Dima, C.; Jafari, S.M. Active Packaging Films Made by Complex Coacervation of Tragacanth Gum and Gelatin Loaded with Curcumin; Characterization and Antioxidant Activity. Foods 2022, 11, 3168. https://doi.org/10.3390/foods11203168
Amani F, Rezaei A, Akbari H, Dima C, Jafari SM. Active Packaging Films Made by Complex Coacervation of Tragacanth Gum and Gelatin Loaded with Curcumin; Characterization and Antioxidant Activity. Foods. 2022; 11(20):3168. https://doi.org/10.3390/foods11203168
Chicago/Turabian StyleAmani, Fateme, Atefe Rezaei, Hajar Akbari, Cristian Dima, and Seid Mahdi Jafari. 2022. "Active Packaging Films Made by Complex Coacervation of Tragacanth Gum and Gelatin Loaded with Curcumin; Characterization and Antioxidant Activity" Foods 11, no. 20: 3168. https://doi.org/10.3390/foods11203168