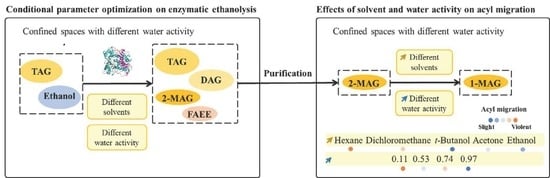
Preparation of 2-Arachidonoylglycerol by Enzymatic Alcoholysis: Effects of Solvent and Water Activity on Acyl Migration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of 2-MAG by Enzymatic Alcoholysis at a Controlled aw
2.3. Purification of Crude Product to Prepare High Purity 2-MAG
2.4. Acyl Migration of 2-MAG in Lipase-Inactivated System
2.5. Analysis of Alcoholysis Product by HPLC–RID
2.6. Statistical Analysis
3. Results and Discussion
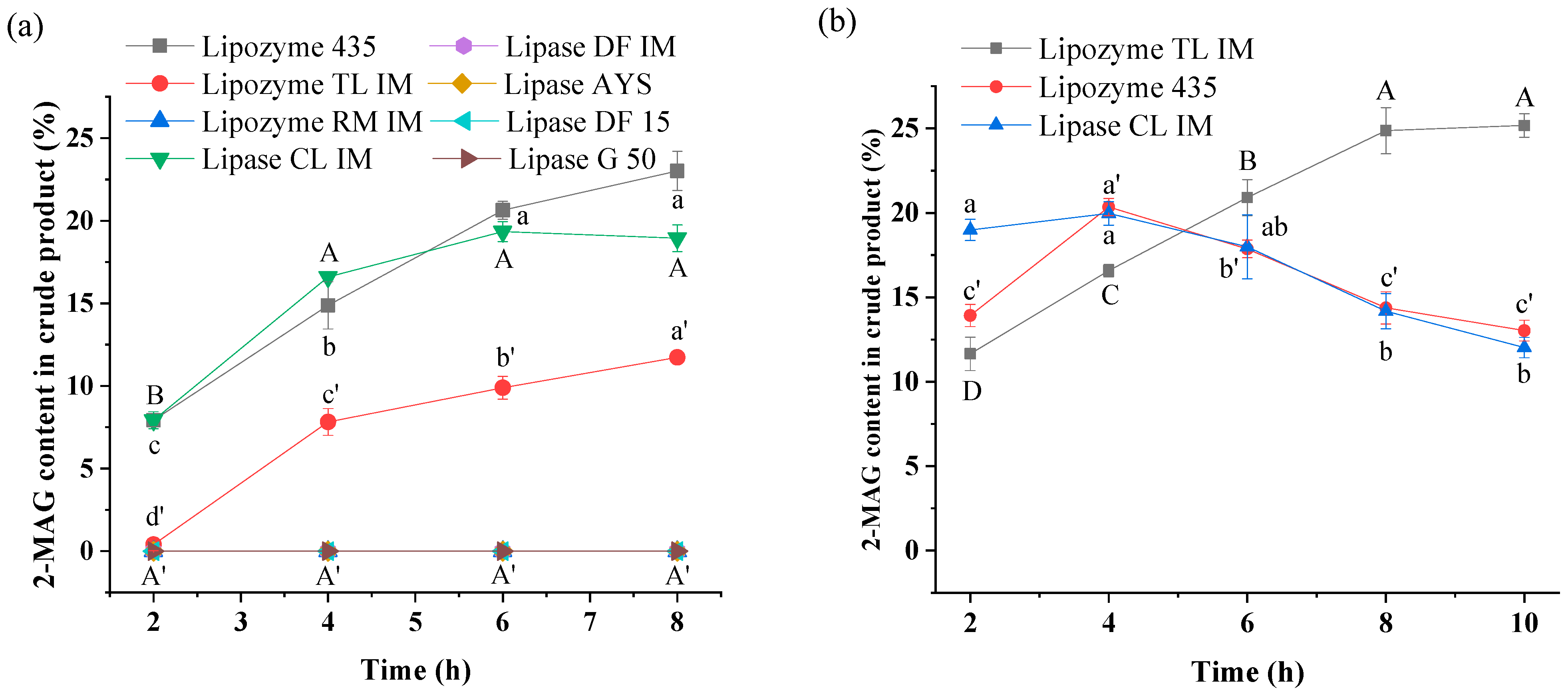
3.1. Effect of Lipase Type and Load on Alcoholysis
3.2. Optimization of Reaction Conditions for Enzymatic Alcoholysis
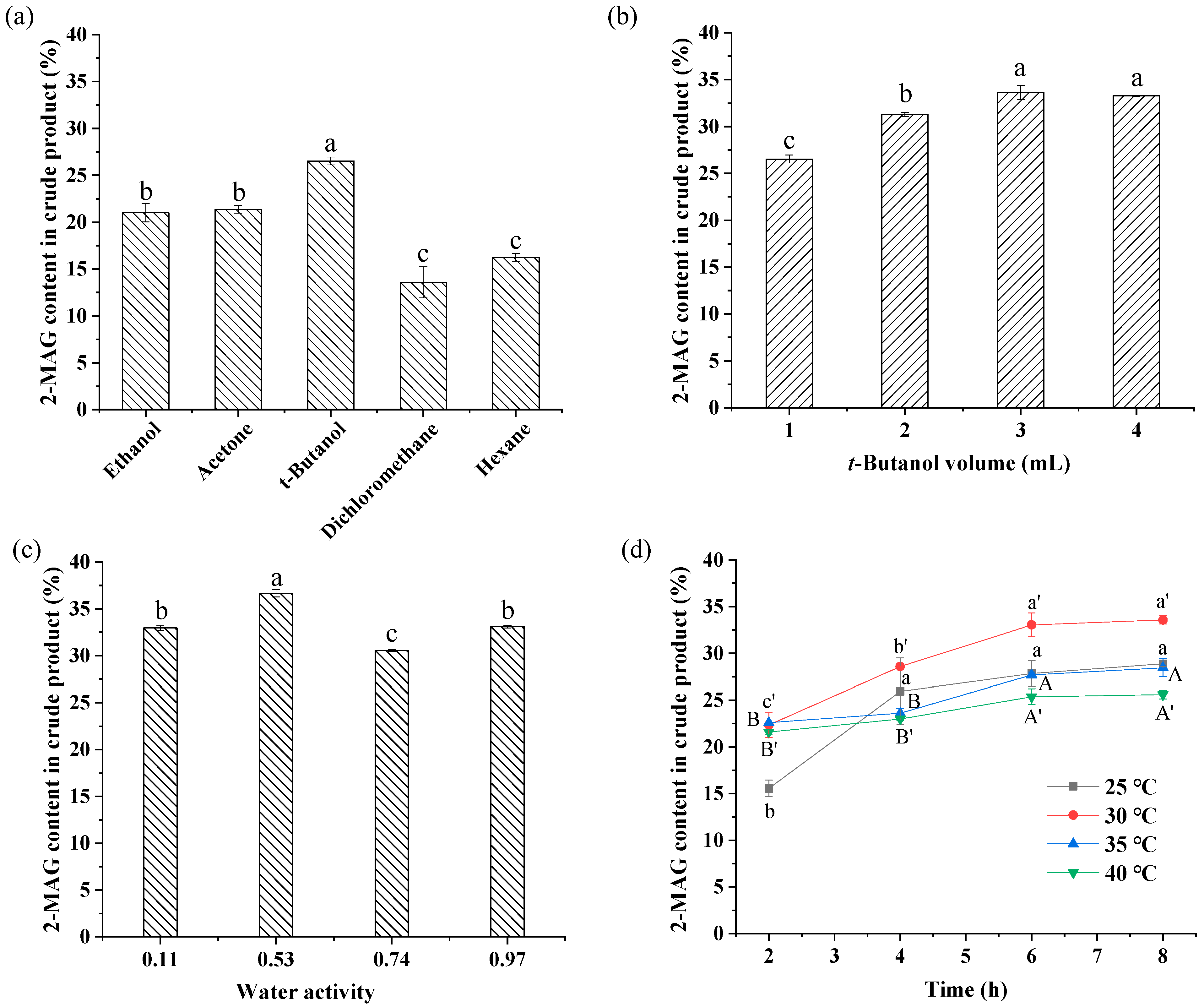
3.2.1. Effect of Solvent Type on Enzymatic Alcoholysis
3.2.2. Effect of Added Amount of t-Butanol on Enzymatic Alcoholysis
3.2.3. Effect of aw on Enzymatic Alcoholysis
3.2.4. Effect of Temperature and Time on Enzymatic Alcoholysis
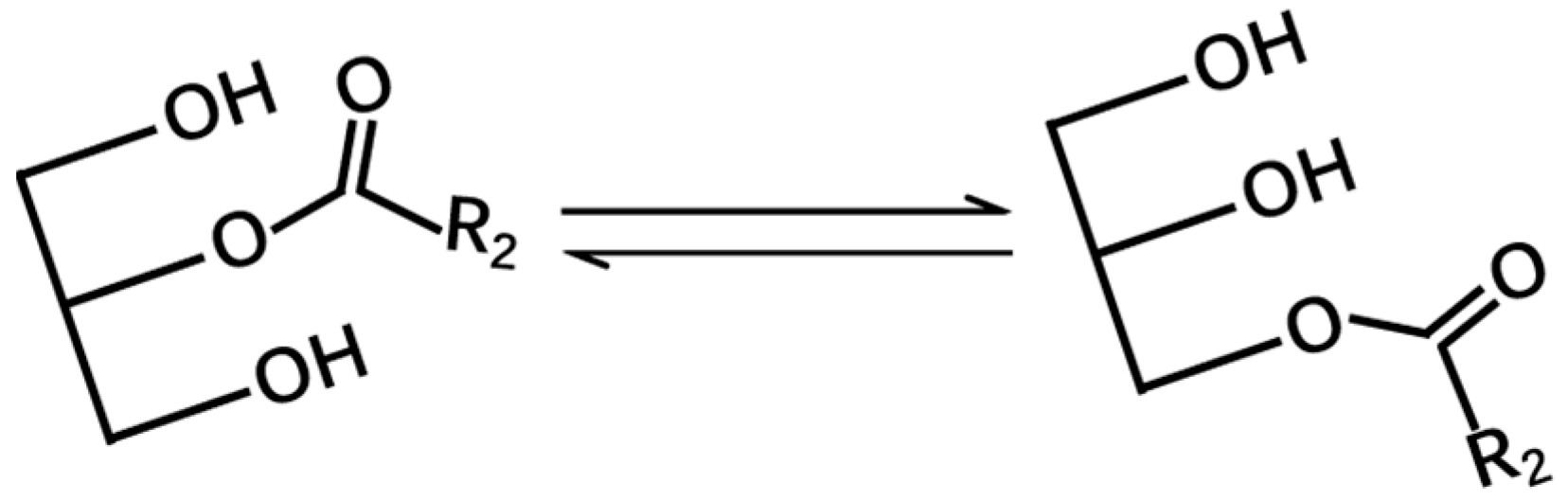
3.3. Effect of Solvent Type and aw on 2-MAG Isomerization in Catalyst-Free System
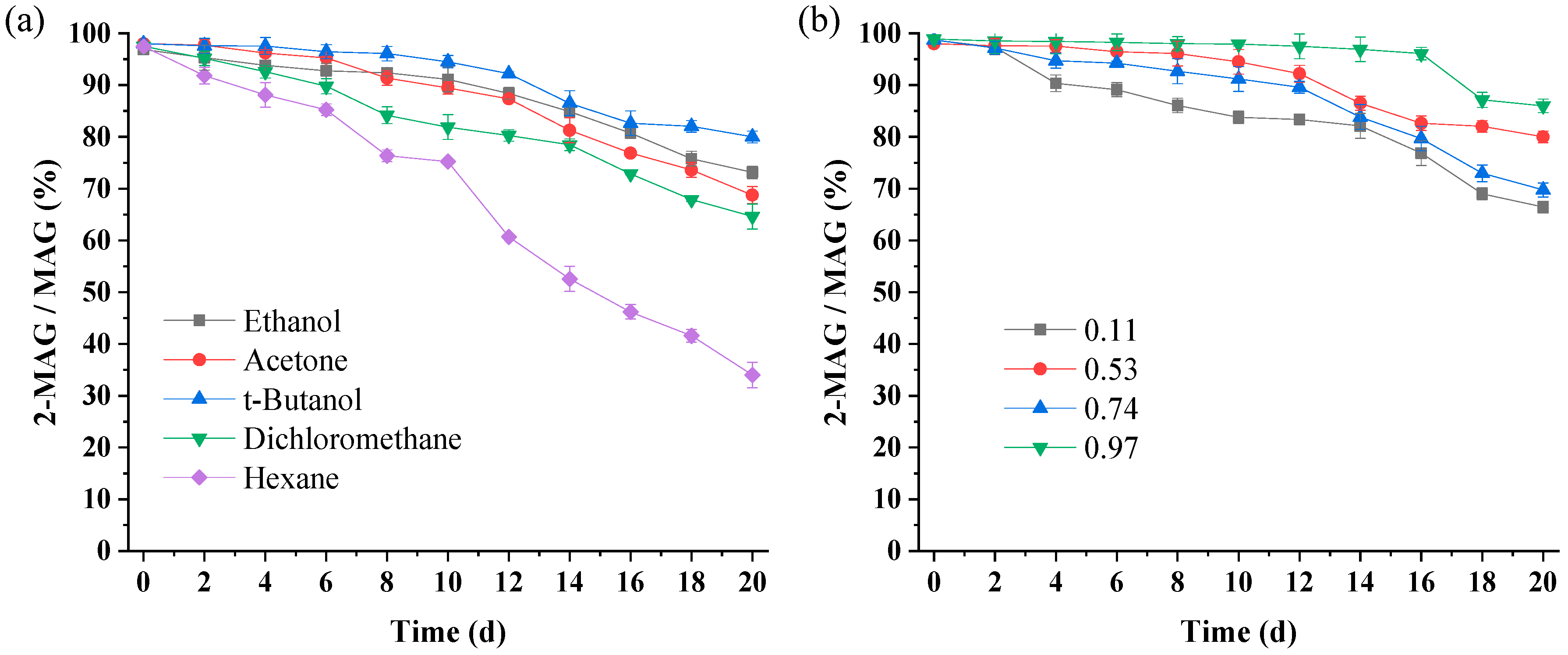
3.3.1. Effect of Solvent Type on 2-MAG Isomerization
3.3.2. Effect of aw on 2-MAG Isomerization
3.3.3. Acyl Migration of 2-MAG in Lipase-Catalyzed and Lipase-Inactivated Systems
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moreira, D.K.T.; Gandra, R.L.P.; Zuin, J.C.; Ract, J.N.R.R.; Ribeiro, A.P.B.; Juliana Alves Macedo, J.A.; Gambero, A.; Akil, E.; Torres, A.G.; Macedo, G.A. Synthesis and characterization of structured lipid rich in behenic acid by enzymatic interesterification. Food Bioprod. Process. 2020, 122, 303–310. [Google Scholar] [CrossRef]
- Mateos, P.S.; Navas, M.B.; Morcelle, S.R.; Ruscitti, C.; Matkovic, S.R.; Briand, L.E. Insights in the biocatalyzed hydrolysis, esterification and transesterification of waste cooking oil with a vegetable lipase. Catal. Today 2021, 372, 211–219. [Google Scholar] [CrossRef]
- Abed, S.M.; Wei, W.; Ali, A.H.; Korma, S.A.; Mousa, A.H.; Hassan, H.M.; Jin, Q.; Wang, X. Synthesis of 6structured lipids enriched with medium-chain fatty acids via solvent-free acidolysis of microbial oil catalyzed by Rhizomucor miehei lipase. LWT-Food Sci. Technol. 2018, 93, 306–315. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Xie, D.; Zou, S.; Jin, Q.; Wang, X. Synthesis and concentration of 2-monoacylglycerols rich in polyunsaturated fatty acids. Food Chem. 2018, 250, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Xu, F.; Zhang, N.; Wu, Y.; Ju, X.; Wang, L. Dietary a novel structured lipid synthesized by soybean oil and coconut oil alter fatty acid metabolism in C57BL/6J mice. Food Biosci. 2021, 44, 101396. [Google Scholar] [CrossRef]
- Ledesma, R.; Martínez-Pérez, R.B.; Curiel, D.A.; Fernández, L.M.; Silva, M.L.; Canales-Aguirre, A.A.; Rodríguez, J.A.; Mateos-Díaz, J.C.; Preza y Lerma, A.M.; Madrigal, M. Potential benefits of structured lipids in bulk compound chocolate: Insights on bioavailability and effect on serum lipids. Food Chem. 2022, 375, 131824. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, J.; Kannan, S.; Govindasamy, A. Structured form of DHA prevents neurodegenerative disorders: A better insight into the pathophysiology and the mechanism of DHA transport to the brain. Nutr. Res. 2021, 85, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Jin, Q.; Wang, X.; Akoh, C.C. High sn-2 docosahexaenoic acid lipids for brain benefits, and their enzymatic syntheses: A review. Engineering 2020, 6, 424–431. [Google Scholar] [CrossRef]
- Bedse, G.; Hill, M.N.; Patel, S. 2-Arachidonoylglycerol modulation of anxiety and stress adaptation: From grass roots to novel therapeutics. Biol. Psychiatry 2020, 88, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Clapper, J.R.; Fu, J.; D’Agostino, G.; Guijarro, A.; Thongkham, D.; Avanesian, A.; Astarita, G.; DiPatrizio, N.V.; Frontini, A.; et al. 2-Arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metab. 2012, 15, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Kokona, D.; Spyridakos, D.; Tzatzarakis, M.; Papadogkonaki, S.; Filidou, E.; Arvanitidis, K.I.; Kolios, G.; Lamani, M.; Makriyannis, A.; Malamas, M.S.; et al. The endocannabinoid 2-arachidonoylglycerol and dual ABHD6/MAGL enzyme inhibitors display neuroprotective and anti-inflammatory actions in the in vivo retinal model of AMPA excitotoxicity. Neuropharmacology 2021, 185, 108450. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Yang, L.; Wang, B.; Cui, L.; Li, C.; Zhuo, Y.; Zhang, L.; Zhang, S.; Zhang, Q.; Wang, X. The role of 2-arachidonoylglycerol in the regulation of the tumor-immune microenvironment in murine models of pancreatic cancer. Biomed. Pharmacother. 2019, 115, 108952. [Google Scholar] [CrossRef] [PubMed]
- Baggelaar, M.P.; Maccarrone, M.; van der Stelt, M. 2-Arachidonoylglycerol: A signaling lipid with manifold actions in the brain. Prog. Lipid Res. 2018, 71, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T. Lipase-catalyzed syntheses of monoacylglycerols. Enzym. Microb. Technol. 1995, 17, 578–586. [Google Scholar] [CrossRef]
- Stamatov, S.D.; Stawinski, J. Novel, regioselective transformation of an oxirane system. An efficient approach to the synthesis of endocannabinoid 2-arachidonoylglycerol. Tetrahedron Lett. 2002, 43, 1759–1761. [Google Scholar] [CrossRef]
- Duclos, R.I., Jr.; Johnston, M.; Vadivel, S.K.; Makriyannis, A.; Glaser, S.T.; Gatley, S.J. A methodology for radiolabeling of the endocannabinoid 2-arachidonoylglycerol (2-AG). J. Org. Chem. 2011, 76, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, M.; Wang, T.; Jin, Q.; Wang, X. An improved method for the synthesis of 2-arachidonoylglycerol. Process Biochem. 2014, 49, 1415–1421. [Google Scholar] [CrossRef]
- Kim, B.H.; Akoh, C.C. Recent research trends on the enzymatic synthesis of structured lipids. J. Food Sci. 2015, 80, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, S.K.; Whitten, K.M.; Makriyannis, A. Chemoenzymatic synthesis of 2-arachidonoylglycerol, an endogenous ligand forcannabinoid receptors. Tetrahedron Lett. 2011, 52, 1149–1150. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; Yang, Z.; Wang, X.; Wang, T. Effect of solvent on acyl migration of 2-monoacylglycerols in enzymatic ethanolysis. J. Agric. Food Chem. 2020, 68, 12358–12364. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Fruekilde, M.; Xu, X. Suppression of acyl migration in enzymatic production of structured lipids through temperature programming. Food Chem. 2005, 92, 101–107. [Google Scholar] [CrossRef]
- Compton, D.L.; Vermillion, K.E.; Laszlo, J.A. Acyl migration kinetics of 2-monoacylglycerols from soybean oil via 1H NMR. J. Am. Oil Chem. Soc. 2008, 84, 343–348. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z.; Lee, W.J.; Xie, X.; Li, A.; Wang, Y. Acyl migration occurrence of palm olein during interesterification catalyzed by sn-1,3 specific lipase. LWT-Food Sci. Technol. 2021, 142, 111023. [Google Scholar] [CrossRef]
- Li, W.; Du, W.; Li, Q.; Sun, T.; Liu, D. Study on acyl migration kinetics of partial glycerides: Dependence on temperature and water activity. J. Mol. Catal. B Enzym. 2010, 63, 17–22. [Google Scholar] [CrossRef]
- Cao, X.; Mangas-Sánchez, J.; Feng, F.; Adlercreutz, P. Acyl migration in enzymatic interesterification of triacylglycerols: Effects of lipases from Thermomyces lanuginosus and Rhizopus oryzae, support material, and water activity. Eur. J. Lipid Sci. Technol. 2016, 118, 1579–1587. [Google Scholar] [CrossRef]
- Li, W.; Du, W.; Li, Q.; Li, R.; Liu, D. Dependence on the properties of organic solvent: Study on acyl migration kinetics of partial glycerides. Bioresour. Technol. 2010, 101, 5737–5742. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Chen, F.; Liu, X.; Hu, J.; Zheng, L.; Jing, L.; Deng, Z. Trace water activity could improve the formation of 1,3-oleic-2-medium chain-rich triacylglycerols by promoting acyl migration in the lipase RM IM catalyzed interesterification. Food Chem. 2020, 313, 126130. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Du, W.; Liu, D. The pronounced effect of water activity on the positional selectivity of Novozym 435 during 1,3-diolein synthesis by esterification. Catal. Commun. 2010, 11, 356–358. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, S.; Akoh, C.C. Preparation and characterization of sn-2 polyunsaturated fatty acids-rich monoacylglycerols from menhaden oil and DHA-single cell oil. LWT-Food Sci. Technol. 2022, 156, 13012. [Google Scholar] [CrossRef]
- Muñío, M.M.; Esteban, L.; Robles, A.; Hita, E.; Jiménez, M.J.; González, P.A.; Camacho, B.; Molina, E. Synthesis of 2-monoacylglycerols rich in polyunsaturated fatty acids by ethanolysis of fish oil catalyzed by 1,3 specific lipases. Process Biochem. 2008, 43, 1033–1039. [Google Scholar] [CrossRef]
- Esteban, L.; Jimenez, M.J.; Hita, E.; Gonzalez, P.A.; Martin, L.; Robles, A. Production of structured triacylglycerols rich in pal mitic acid at sn-2 position and oleic acid at sn-1,3 positions as human milk fat substitutes by enzymatic acidolysis. Biochem. Eng. J. 2011, 54, 62–69. [Google Scholar] [CrossRef]
- Du, W.; Wang, L.; Liu, D. Improved methanol tolerance during Novozym 435-mediated methanolysis of SODD for biodiesel production. Green Chem. 2007, 9, 173–176. [Google Scholar] [CrossRef]
- Salihu, A.; Alam, M.Z. Solvent tolerant lipases: A review. Process Biochem. 2015, 50, 86–96. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Ma, Y.; Jin, Q.; Wang, X. Lipozyme 435-catalyzed synthesis of eicosapentaenoyl ethanolamide in a solvent-free system. J. Mol. Catal. B Enzym. 2015, 122, 233–239. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, L.; Liu, D.; Liu, H.; Du, W. Kinetics and mechanism of solvent influence on the lipase-catalyzed 1,3-diolein synthesis. ACS Omega 2020, 5, 24708–24716. [Google Scholar]
- Damstrup, M.L.; Jensen, T.; Sparsø, F.V.; Kiil, S.Z.; Jensen, A.D.; Xu, X. Solvent optimization for efficient enzymatic monoacyl-glycerol production based on a glycerolysis reaction. J. Am. Oil Chem. Soc. 2005, 82, 559–564. [Google Scholar] [CrossRef]
- Banik, S.D.; Nordblad, M.; Woodley, J.M.; Peters, G.H. A correlation between the activity of Candida antarctica lipase B and differences in binding free energies of organic solvent and substrate. ACS Catal. 2016, 6, 6350–6361. [Google Scholar] [CrossRef]
- Oh, J.E.; Lee, K.W.; Park, H.K.; Kim, J.Y.; Kwon, K.I.L.; Kim, J.W.; Kim, H.R.; Kim, I.N.H. Lipase-catalyzed acidolysis of olive oil with capric acid: Effect of water activity on incorporation and acyl migration. J. Agric. Food Chem. 2009, 57, 9280–9283. [Google Scholar] [CrossRef]
- Zulkeflee, S.A.; Sata, S.A.; Rohman, F.S.; Aziz, N. Modelling of immobilized Candida rugosa lipase catalysed esterification process in batch reactor equipped with temperature and water activity control system. Biochem. Eng. J. 2020, 16, 1107669. [Google Scholar] [CrossRef]
- Fureby, A.M.; Virto, C.; Adlercreutz, P.; Mattiasson, B. Acyl group migrations in 2-monoolein. Biocatal. Biotransform. 1996, 14, 89–111. [Google Scholar] [CrossRef]
- Mao, J.; Hu, Z.; Hu, J.; Zhu, X.; Xiong, H. A Density functional theory (DFT) study of the acyl migration occurring during lipase-catalyzed transesterifications. Int. J. Mol. Sci. 2019, 20, 3438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lee, W.J.; Wang, Y. Evaluation of enzymatic interesterification in structured triacylglycerols preparation: A concise review and prospect. Crit. Rev. Food Sci. Nutr. 2021, 61, 3145–3159. [Google Scholar] [CrossRef] [PubMed]
- Satyawali, Y.; Cauwenberghs, L.; Maesen, M.; Dejonghe, W. Lipase catalyzed solvent free synthesis of monoacylglycerols in various reaction systems and coupling reaction with pervaporation for in situ water removal. Chem. Eng. Process.-Process Intensif. 2021, 166, 108475. [Google Scholar] [CrossRef]
- Schmitke, J.L.; Wescott, C.R.; Klibanov, A.M. The mechanistic dissection of the plunge in enzymatic activity upon transition from water to anhydrous solvents. J. Am. Oil Chem. Soc. 1996, 118, 3360–3365. [Google Scholar] [CrossRef]
- Bi, Y.; Wang, Z.; Duan, Z.; Zhao, X.; Chen, X.; Nie, L. An insight into the solvent effect on the positional selectivity of the immobilized lipase from Burkholderia cepacia in 1,3-diolein synthesis. RSC Adv. 2015, 5, 23122–23124. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, K.; Wang, Y.; Huang, Z.; Wang, X. Preparation of 2-Arachidonoylglycerol by Enzymatic Alcoholysis: Effects of Solvent and Water Activity on Acyl Migration. Foods 2022, 11, 3213. https://doi.org/10.3390/foods11203213
Wang X, Liu K, Wang Y, Huang Z, Wang X. Preparation of 2-Arachidonoylglycerol by Enzymatic Alcoholysis: Effects of Solvent and Water Activity on Acyl Migration. Foods. 2022; 11(20):3213. https://doi.org/10.3390/foods11203213
Chicago/Turabian StyleWang, Xiaohan, Keying Liu, Yifan Wang, Zhuoneng Huang, and Xiaosan Wang. 2022. "Preparation of 2-Arachidonoylglycerol by Enzymatic Alcoholysis: Effects of Solvent and Water Activity on Acyl Migration" Foods 11, no. 20: 3213. https://doi.org/10.3390/foods11203213
APA StyleWang, X., Liu, K., Wang, Y., Huang, Z., & Wang, X. (2022). Preparation of 2-Arachidonoylglycerol by Enzymatic Alcoholysis: Effects of Solvent and Water Activity on Acyl Migration. Foods, 11(20), 3213. https://doi.org/10.3390/foods11203213