Biochemical and Molecular Profiling of Wild Edible Mushrooms from Huila, Angola
Abstract
:1. Introduction
2. Materials and Methods
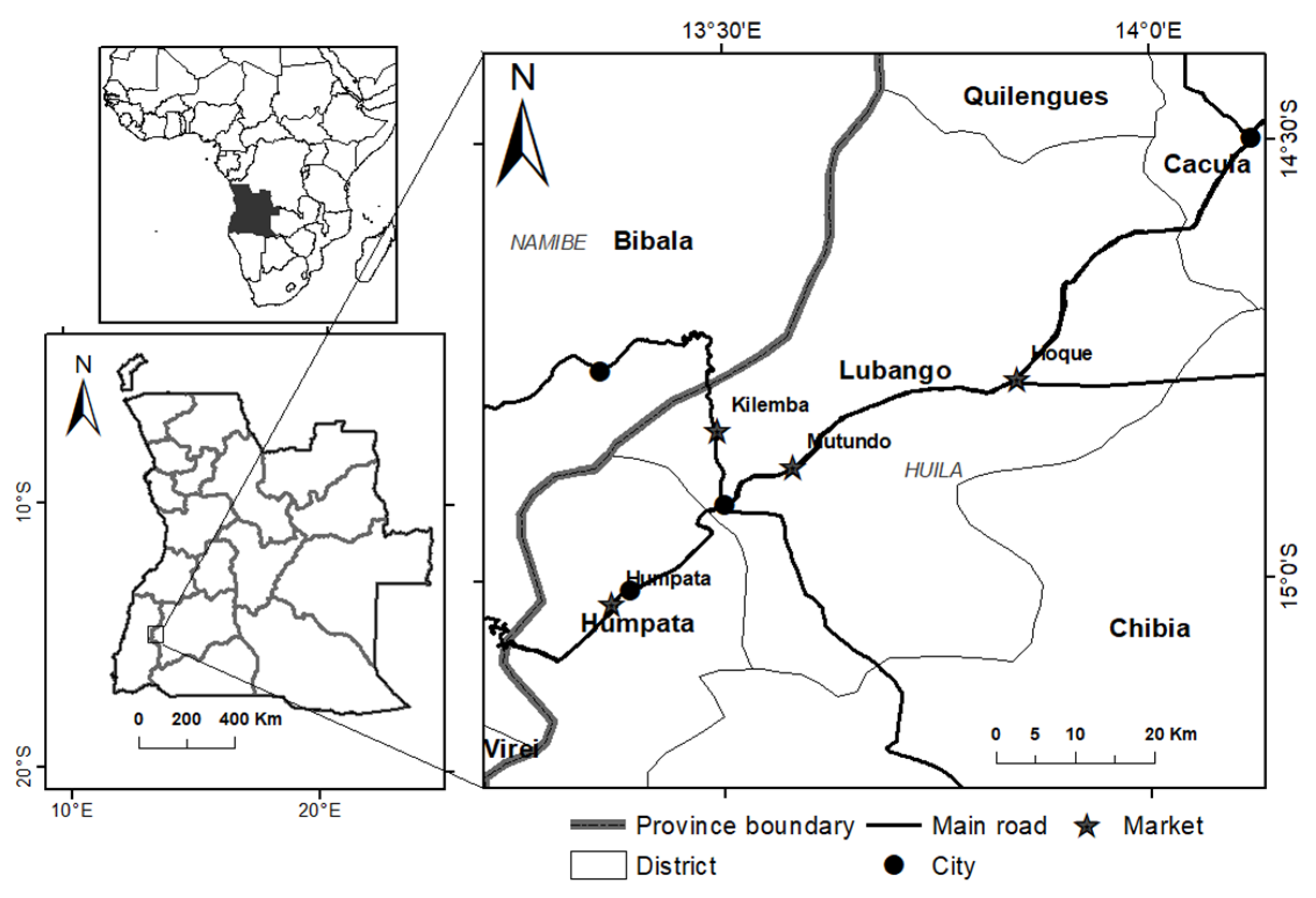
2.1. Markets and Mushroom Material
2.2. Morphological Characterization
2.3. Molecular Analysis
2.3.1. DNA Extraction, Amplification, and Sequencing
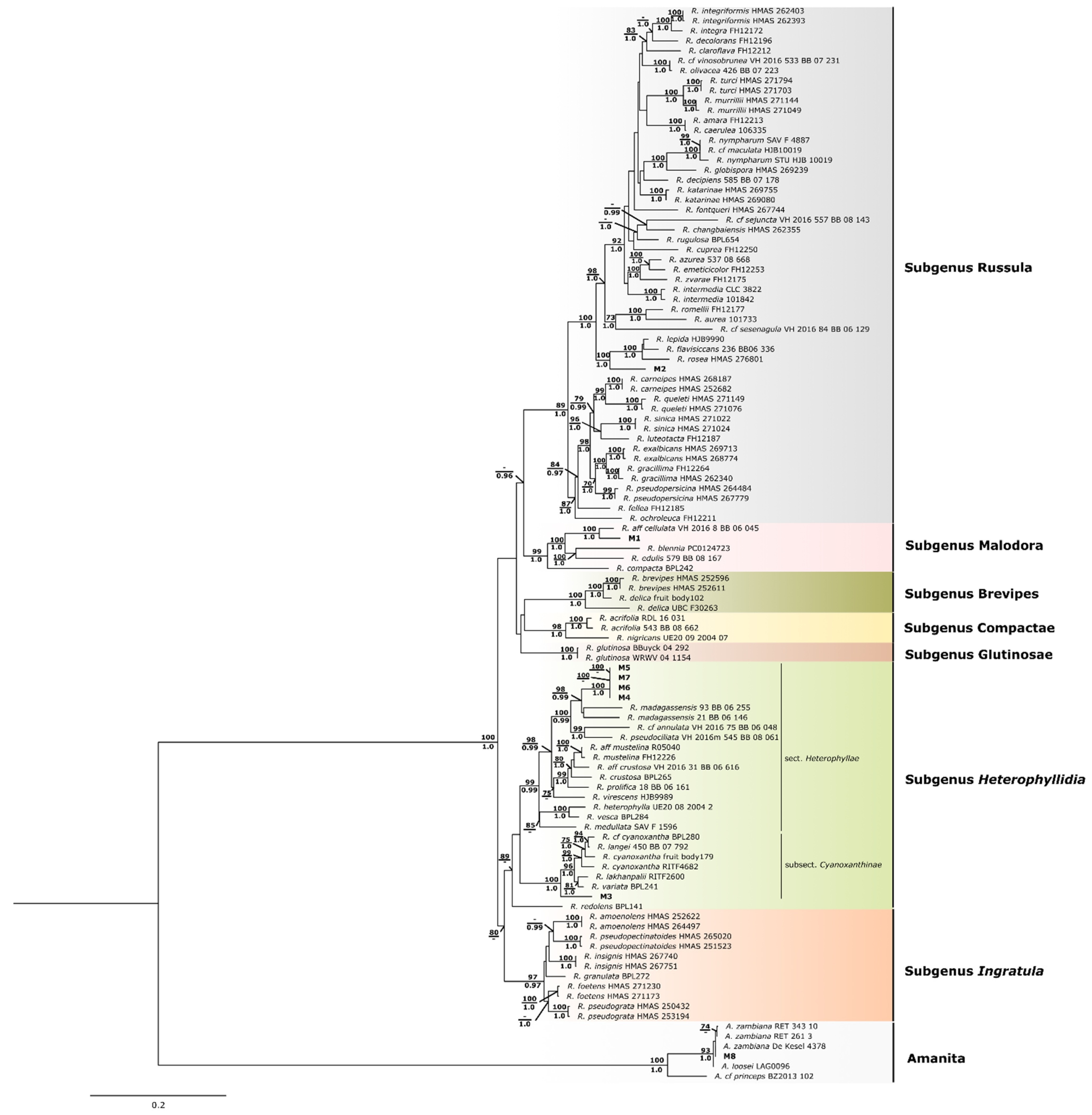
2.3.2. Phylogenetic Analyses
2.4. Extract Preparation
2.5. Chemical Characterization
2.5.1. Nutritional and Energetic Value
2.5.2. Hydrophilic Compounds
2.5.3. Lipophilic Compounds
2.6. Determination of Phenolic Acids and Related Compounds, and Bioactive Properties
2.6.1. Phenolic Acids and Related Compounds
2.6.2. Antioxidant Activity
2.6.3. Antimicrobial Activity
2.7. Data Analysis
3. Results and Discussion
3.1. Prices, Quantities Sold, and Socio-Economic Importance
3.2. Morphological Characterization and Molecular Identification of Sampled WEM
3.3. Chemical Characterization of Mushroom Species
3.4. Determination of Phenolic Acids and Related Compounds, and Bioactive Properties
3.4.1. Phenolic Acids and Related Compounds
3.4.2. Antioxidant Activity
3.4.3. Antimicrobial Activity
3.5. Multivariate Analysis of Chemical and Functional Characteristics of Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalac, P. Edible Mushrooms: Chemical Composition and Nutritional Value; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Chen, Y. Song Rong (Tricholoma matsutake) a Valuable Forrest Mushroom from China: Consumption, Development and Sustainability. In Forest Products, Livelihoods and Conservation: Case Studies of Non-Timber Forest Products System; Center for International Forestry Research: Bogor, Indonesia, 2004; Volume 1, pp. 78–93. [Google Scholar]
- Halling, R.E. Wild Edible Fungi: A Global Overview of Their Use and Importance to People. Non-Wood Forest Products 17. Econ. Bot. 2006, 60, 99–100. [Google Scholar] [CrossRef]
- Yun, W.; Hall, I.R. Edible Ectomycorrhizal Mushrooms: Challenges and Achievements. Can. J. Bot. 2004, 82, 1063–1073. [Google Scholar] [CrossRef]
- Harkonen, M. Mushroom Collecting in Tanzania and Hunan (Southern China): Inherited Wisdom and Folklore of Two Different Cultures. In Tropical Mycology; Elsevier: Amsterdam, The Netherlands, 2002; Volume 1, pp. 149–165. [Google Scholar]
- Kalac, P. Chemical Composition and Nutritional Value of European Species of Wild Growing Mushrooms: A Review. Food Chem. 2009, 113, 9–16. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C.F.R. Antioxidant Properties and Phenolic Profile of the Most Widely Appreciated Cultivated Mushrooms: A Comparative Study between in Vivo and in Vitro Samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Buswell, J.A. Mushroom Nutriceuticals. World J. Microbiol. Biotechnol. 1996, 12, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Sadler, M. Nutritional Properties of Edible Fungi. Nutr. Bull. 2003, 28, 305–308. [Google Scholar] [CrossRef]
- Chang, S.; Mshigeni, K. Mushroom and Their Human Health: Their Growing Significance as Potent Dietary Supplements; The University of Namibia: Windhoek, Namibia, 2001; 79p. [Google Scholar]
- Ferreira, I.; Barros, L.; Abreu, R. Antioxidants in Wild Mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, M.J.; Ferreira, I.C.F.R.; Froufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial Activity of Phenolic Compounds Identified in Wild Mushrooms, SAR Analysis and Docking Studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Alves, M.; Ferreira, I.F.R.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A Review on Antimicrobial Activity of Mushroom (Basidiomycetes) Extracts and Isolated Compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef] [Green Version]
- Zeleke, G.; Dejene, T.; Tadesse, W.; Agúndez, D.; Martín-Pinto, P. Ethnomycological Knowledge of Three Ethnic Groups in Ethiopia. Forests 2020, 11, 875. [Google Scholar] [CrossRef]
- Sitotaw, R.; Lulekal, E.; Abate, D. Ethnomycological Study of Edible and Medicinal Mushrooms in Menge District, Asossa Zone, Benshangul Gumuz Region, Ethiopia. J. Ethnobiol. Ethnomed. 2020, 16, 11. [Google Scholar] [CrossRef] [Green Version]
- Teke, N.A.; Kinge, T.R.; Bechem, E.; Nji, T.M.; Ndam, L.M.; Mih, A.M. Ethnomycological Study in the Kilum-Ijim Mountain Forest, Northwest Region, Cameroon. J. Ethnobiol. Ethnomed. 2018, 14, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okoro, I.; Achuba, F. Proximate and Mineral Analysis of Some Wild Edible Mushrooms. Afr. J. Biotechnol. 2012, 11, 7720–7724. [Google Scholar] [CrossRef]
- Nakalembe, I.; Kabasa, J.D.; Olila, D. Comparative Nutrient Composition of Selected Wild Edible Mushrooms from Two Agro-Ecological Zones, Uganda. Springerplus 2015, 4, 433. [Google Scholar] [CrossRef] [Green Version]
- Rasalanavho, M.; Moodley, R.; Jonnalagadda, S. Elemental Bioaccumulation and Nutritional Value of Five Species of Wild Growing Mushrooms from South Africa. Food Chem. 2020, 319, 126596. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, R.; Jigam, A.; Makun, H.; Egwim, E. Antioxidant Properties of Selected African Vegetables, Fruits and Mushrooms: A Review. In Mycotoxin and Food Safety in Developing Countries; Book on Demand: Rijeka, Croatia, 2013; pp. 203–249. [Google Scholar] [CrossRef]
- Gul, K.; Singh, A.; Jabeen, R. Nutraceuticals and Functional Foods: The Foods for the Future World. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Seifert, K.A. Progress towards DNA Barcoding of Fungi. Mol. Ecol. Resour. 2009, 9, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Li, G.J.; Zhao, R.L.; Zhang, C.L.; Lin, F. A Preliminary DNA Barcode Selection for the Genus Russula (Russulales, Basidiomycota). Mycology 2019, 10, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Malaisse, F. Se Nourrir En Forêt Claire Africaine. Approche Écologique et Nutritionnelle. Nat. Sci. Soc. 1999, 3, 88. [Google Scholar]
- Ndong, H.; Degreef, J.; de Kesel, A. Champignons Comestibles des Forêts Denses D’afrique Centrale—Taxonomie et Identification; ABC Taxa: Bruxelles, Belgium, 2011; Volume 10, 253p. [Google Scholar]
- De Kesel, A.; Kasongo, B.; Degreef, J. Champignons Comestibles du Haut-Katanga (D.R. Congo); ABC Taxa: Bruxelles, Belgium, 2017; Volume 17, 290p. [Google Scholar]
- Degreef, J.; de Kesel, A. The Edible Fungi of Tropical Africa Annotated Database. Available online: https://www.efta-online.org/ (accessed on 12 September 2022).
- Mlambo, A.; Maphosa, M. Miombo Woodland Mushrooms of Commercial Food Value: A Survey of Central Districts of Zimbabwe. J. Food Secur. 2017, 5, 51–57. [Google Scholar] [CrossRef]
- Degreef, J.; Kasongo, B.; Niyongabo, E.; de Kesel, A. Edible Mushrooms, a Vulnerable Ecosystem Service from African Miombo Woodlands. BASE 2020. [Google Scholar] [CrossRef]
- Beeli, M. Amanita-Amanitopsis. In Flore Iconographique des Champignons du Congo; National Botanic Garden of Belgium: Bruxelles, Belgium, 1935; Volume 1, pp. 1–4. [Google Scholar]
- Buyck, B. Exploring the Diversity of “Smooth Chanterelles” (Cantharellus, Cantharellales). Cryptogam Mycol. 2014, 35, 23–40. [Google Scholar] [CrossRef]
- Pacioni, G.; Sharp, C. Mackintoshia, a New Sequestrate Basidiomycete Genus from Zimbabwe. Mycotaxon 2000, 75, 225–228. [Google Scholar]
- Buyck, B. New Taxa of Tropical Russulae: Pseudoepitheliosinae Subsect. Nov. Mycotax 1990, 39, 317–327. [Google Scholar]
- Urso, V.; Signorini, M.; Tonini, M.; Bruschi, P. Wild Medicinal and Food Plants Used by Communities Living in Mopane Woodlands of Southern Angola: Results of an Ethnobotanical Field Investigation. J. Ethnopharmacol. 2016, 177, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Göhre, A.; Toto-Nienguesse, Á.B.; Futuro, M.; Neinhuis, C.; Lautenschläger, T. Plants from Disturbed Savannah Vegetation and Their Usage by Bakongo Tribes in Uíge, Northern Angola. J. Ethnobiol. Ethnomed. 2016, 12, 42. [Google Scholar] [CrossRef] [Green Version]
- Heinze, C.; Dundão, M.D.F.; Neinhuis, C.; Lautenschläger, T. Economic Potential of Selected Native Plants from Cuanza Norte, Northern Angola. Econ. Bot. 2019, 73, 96–111. [Google Scholar] [CrossRef]
- Aldaco, R.; Mditshwa, A.; Dar, A.A.; Catarino, L.; Kissanga, R.; Sales, J.; Moldão, M.; Alves, V.; Mendes, H.; Romeiras, M.M.; et al. Nutritional and Functional Properties of Wild Leafy Vegetables for Improving Food Security in Southern Angola. Front. Sustain. Food Syst. 2021, 5, 791705. [Google Scholar] [CrossRef]
- Valeria, U.; Maria, A.; Pierro, B. Survey of the Ethnobotanical Uses of Ximenia americana L. (Mumpeke) among Rural Communities in South Angola. J. Med. Plants Res. 2013, 7, 7–18. [Google Scholar] [CrossRef]
- Ndifon, E. Systematic Appraisal of Macrofungi (Basidiomycotina: Ascomycotina) Biodiversity of Southern Africa: Uses, Distribution, Checklists. J. Asia Pac. Biodivers. 2022, 15, 80–85. [Google Scholar] [CrossRef]
- Lautenschläger, T. Riquezas Naturais de Uíge—Uma Breve introdução Sobre o Estado Atual, a Utilização, a Ameaça e a Preservação da Biodiversidade; Technische Universität: Dresden, Germany, 2014; Volume 2. [Google Scholar]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR Protoc. Guide Methods Appl. 2013, 18, 315–322. [Google Scholar]
- Moncalvo, J.-M.; Lutzoni, F.M.; Rehner, S.A.; Johnson, J.; Vilgalys, R. Phylogenetic Relationships of Agaric Fungi Based on Nuclear Large Subunit Ribosomal DNA Sequences. Syst. Biol. 2000, 49, 278–305. [Google Scholar] [CrossRef] [Green Version]
- Matheny, P. Improving Phylogenetic Inference of Mushrooms with RPB1 and RPB2 Nucleotide Sequences (Inocybe; Agaricales). Mol. Phylogenet. Evol. 2005, 35, 1–20. [Google Scholar] [CrossRef]
- Altschul, S.; Madden, T.; Schaffer, A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Larsson, A. AliView: A Fast and Lightweight Alignment Viewer and Editor for Large Datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.; Huelsenbeck, J. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A.; Drummond, A.; Xie, D.; Baele, G.; Suchard, M. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [Green Version]
- Darriba, D.; Taboada, G.; Doallo, R.; Posada, D. JModelTest 2: More Models, New Heuristics and Parallel Computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, N. Further Analysis of the Data by Anaike’ S Information Criterion and the Finite Corrections. Commun. Stat. Theory Methods 1978, 7, 13–26. [Google Scholar] [CrossRef]
- Kostić, M.; Smiljković, M.; Petrović, J.; Glamočlija, J.; Barros, L.; Ferreira, I.C.F.R.; Ćirić, A.; Soković, M. Chemical, Nutritive Composition and a Wide Range of Bioactive Properties of Honey Mushroom Armillaria Mellea (Vahl: Fr.) Kummer. Food Funct. 2017, 8, 3239–3249. [Google Scholar] [CrossRef] [Green Version]
- AOAC Official Methods of Analysis of AOAC International, 20th ed.; AOAC: Gaithersburg, MD, USA, 2016.
- Spréa, R.M.; Fernandes, Â.; Calhelha, R.C.; Pereira, C.; Pires, T.C.S.P.; Alves, M.J.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical and Bioactive Characterization of the Aromatic Plant: Levisticum Officinale W.D.J. Koch: A Comprehensive Study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Use of UFLC-PDA for the Analysis of Organic Acids in Thirty-Five Species of Food and Medicinal Plants. Food Anal. Methods 2013, 6, 1337–1344. [Google Scholar] [CrossRef]
- Fernandes, Â.; Barros, L.; Antonio, A.L.; Barreira, J.C.M.; Oliveira, M.B.P.P.; Martins, A.; Ferreira, I.C.F.R. Using Gamma Irradiation to Attenuate the Effects Caused by Drying or Freezing in Macrolepiota Procera Organic Acids and Phenolic Compounds. Food Bioprocess Technol. 2014, 7, 3012–3021. [Google Scholar] [CrossRef] [Green Version]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Pterospartum Tridentatum, Gomphrena Globosa and Cymbopogon Citratus: A Phytochemical Study Focused on Antioxidant Compounds. Food Res. Int. 2014, 62, 684–693. [Google Scholar] [CrossRef] [Green Version]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; van Griensven, L.J.L.D. Antibacterial Effects of the Essential Oils of Commonly Consumed Medicinal Herbs Using an In Vitro Model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef] [Green Version]
- Aaron Villanueva, R.M.; Job Chen, Z. Ggplot2: Elegant Graphics for Data Analysis. Measurement 2019, 2, 160–167. [Google Scholar] [CrossRef]
- Buyck, B.; Zoller, S.; Hofstetter, V. Walking the Thin Line Ten Years Later: The Dilemma of above- versus below-Ground Features to Support Phylogenies in the Russulaceae (Basidiomycota). Fungal Divers. 2018, 89, 267–292. [Google Scholar] [CrossRef]
- Vera, M.; Adamčík, S.; Adamčíková, K.; Hampe, F.; Caboň, M.; Manz, C.; Ovrebo, C.; Piepenbring, M.; Corrales, A. Morphological and Genetic Diversification of Russula floriformis, sp. nov., along the Isthmus of Panama. Mycologia 2021, 113, 807–827. [Google Scholar] [CrossRef]
- Garcia, C. El Genero Russula En La Peninsula ibérica; Centro de Estudios Micológicos de Euskadi: Bilbau, Spain, 2011; 440p. [Google Scholar]
- Belgique, B. Nouveaux Taxons Infragénériques Dans Le Genre Russula Persoon En Afrique Centrale. JSTOR 1990, 60, 191–211. [Google Scholar]
- Ribeiro, B.; Lopes, R.; Andrade, P.B.; Seabra, R.M.; Gonc ßalves, R.F.; Baptista, P.; Quelhas, I.; Valentão, P. Comparative Study of Phytochemicals and Antioxidant Potential of Wild Edible Mushroom Caps and Stipes. Food Chem. 2008, 110, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Kostić, M.; Ivanov, M.; Fernandes, Â.; Pinela, J.; Calhelha, R.C.; Glamočlija, J.; Barros, L.; Ferreira, I.C.F.R.; Soković, M.; Ćirić, A. Antioxidant Extracts of Three Russula Genus Species Express Diverse Biological Activity. Molecules 2020, 25, 4336. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized Analysis of Organic Acids in Edible Mushrooms from Portugal by Ultra Fast Liquid Chromatography and Photodiode Array Detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Gąsecka, M.; Magdziak, Z.; Siwulski, M.; Mleczek, M. Profile of Phenolic and Organic Acids, Antioxidant Properties and Ergosterol Content in Cultivated and Wild Growing Species of Agaricus. Eur. Food Res. Technol. 2018, 244, 259–268. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Tocopherols Composition of Portuguese Wild Mushrooms with Antioxidant Capacity. Food Chem. 2010, 119, 1443–1450. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, B.; Rangel, J.; Valentão, P.; Baptista, P.; Seabra, R.M.; Andrade, P.B. Contents of Carboxylic Acids and Two Phenolics and Antioxidant Activity of Dried Portuguese Wild Edible Mushrooms. J. Agric. Food Chem. 2006, 54, 8530–8537. [Google Scholar] [CrossRef] [Green Version]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant Properties of Phenolic Compounds Occurring in Edible Mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Tripathy, S.; Rajoriya, A.; Mahapatra, A.; Gupta, N. Biochemical and Antioxidant Properties of Wild Edible Mushrooms Used for Food by Tribal of Eastern India. Int. J. Pharm. Sci. 2016, 8, 194–199. [Google Scholar]
- Thongbai, B.; Hyde, K.; Lumyong, S.; Raspé, O. High Undescribed Diversity of Amanita Section Vaginatae in Northern Thailand. Mycosphere 2018, 9, 462–494. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, S.; Tulloss, R.E.; Amalfi, M.; Moncalvo, J.M. Palaeotropical Origins, Boreotropical Distribution and Increased Rates of Diversification in a Clade of Edible Ectomycorrhizal Mushrooms (Amanita Section Caesareae). J. Biogeogr. 2015, 42, 351–363. [Google Scholar] [CrossRef]
- De Lange, R.; Adamčík, S.; Adamčíkova, K.; Asselman, P.; Borovička, J.; Delgat, L.; Hampe, F.; Verbeken, A. Enlightening the Black and White: Species Delimitation and UNITE Species Hypothesis Testing in the Russula albonigra Species Complex. IMA Fungus 2021, 12, 20. [Google Scholar] [CrossRef]
- Looney, B.P.; Ryberg, M.; Hampe, F.; Sánchez-García, M.; Matheny, P.B. Into and out of the Tropics: Global Diversification Patterns in a Hyperdiverse Clade of Ectomycorrhizal Fungi. Mol. Ecol. 2016, 25, 630–647. [Google Scholar] [CrossRef]
- Chen, B.; Song, J.; Chen, Y.; Zhang, J.; Liang, J. Morphological and Phylogenetic Evidence for Two New Species of Russula Subg. Heterophyllidia from Guangdong Province of China. MycoKeys 2021, 82, 139. [Google Scholar] [CrossRef] [PubMed]
- Buyck, B.; Wang, X.; Adamcikova, K.; Cabon, M.; Jancovicova, S.; Hofstetter, V.; Adamcik, S. One step closer to unravelling the origin of Russula: Subgenus Glutinosae subg. nov. Mycosphere 2020, 11, 285–305. [Google Scholar] [CrossRef]

| Samples | Species | Common Name | Ecology/Morphology |
|---|---|---|---|
| M1 | Russula aff. cellulata Buyck | Ombando (Umbundu) | Occurs in closed to open Miombo woodland, under the tree canopy, sometimes in moss-covered soil, mainly from March to May. Cap: brown cream or light brown, often cracked, 5–10 cm Ø. Stipe: whitish, 4–5 × 1.5–2.5 cm. Spores: hyaline, with small warts, very occasionally connected by fine lines, subglobolose 6.5–8 μm. Basidia: 50 × 10 μm. |
| M2 | Russula sp1 [R. gr. rosea Pers. (=R. lepida Fr.)] | Opembe (Nyaneka); Membe, Umputu (Umbundu) | Occurs in closed to open Miombo woodland, under the tree canopy, often near the base of the tree trunks, mainly in March to May. Cap: vinaceous or blood-red, 3.5–7.5 cm Ø. Stipe: white with red or rosaceous flush, 3–5 × 1.8–2 cm. Spores: with warts, some connected by fine lines, broadly ellipsoid, 10 × 9 μm. Basidia: 45–50 × 10 μm. |
| M3 | Russula sp2 (subgenus Heterophyllidia, section Heterophyllae, subsection Cyanoxanthinae) | Chelene preto [black chelene] (Umbundu/Nyaneka) | Occurs in closed to open Miombo woodland, under the tree canopy, mainly in March to May. Cap: dark purple with greenish tones, mucilaginous, 3–5 cm Ø. Stipe: whitish, 3.5–4 × 2 cm. Spores: with warts, ellipsoid 7 × 5.5 μm. Basidia: 9 × 5 μm. |
| M4 | Russula sp3. (R. aff madagassensis R. Heim) | Chelene amarelo [yellow chelene] (Umbundu/Nyaneka) | Occurs in open Miombo woodland and open rocky areas on sloping terrains, mainly in March to May. Cap: brownish yellow, mucilaginous, 5–6 cm Ø. Stipe: 3.5–4 × 1.5–2 cm. Spores: with warts, ellipsoid, 7–8 × 5 μm. Basidia: 55 × 10 μm. |
| M5 | Russula sp3. (R. aff madagassensis R. Heim) | Chelene amarelo [yellow chelene] (Umbundu/Nyaneka) | Occurs in open Miombo woodland and open rocky areas on sloping terrains, mainly in March to May. Cap: brownish yellow, viscid, 5–6 cm Ø. Stipe: 3.5–4 × 1.5–2 cm. Spores: sub-spherical, with small warts interconnected by narrow veins giving a reticulated aspect, 7–7.5 μm. Basidia: 50–55.5 × 12 μm. |
| M6 | Russula sp3. (R. aff madagassensis R. Heim) | Chelene vermelho [red chelene] (Umbundu/Nyaneka) | Occurs in open Miombo woodland, under the tree canopy, mainly in March to May. Cap: reddish or pink 5–6 cm Ø. Stipe: 3.5–4 × 1.5–2 cm. Spores: ornamented with small warts interconnected by narrow veins giving a reticulated aspect, spherical, 8–9 μm. Basidia: 50 × 10 μm. |
| M7 | Russula sp3. (R. aff madagassensis R. Heim) | Chelene vermelho [red chelene] (Umbundu/Nyaneka) | Occurs in open Miombo woodland, under the tree canopy, mainly in March to May. Cap: reddish or light pink, 5–6 cm Ø; Stipe: 3.5–4 × 1.5–2 cm. Spores: ornamented with small warts interconnected by narrow veins giving a reticulated aspect, spherical, 8–9 μm. Basidia: 50 × 10–12.5 μm. |
| M8 | Amanita loosei Beeli (=A. zambiana) | Ndenda (Umbundu); Ondenda (Nyaneka) | Occurs in closed to open Miombo woodland, under the tree canopy, often in rocky soil, mainly in December to May. Cap: brownish at the centre becoming paler towards the margin, 10–25 cm Ø, (can reach 30–40 cm in fresh). Stipe: white, 5–10 × 2–3 cm. Ring and volva: present. Spores: hyaline, smooth, spherical a sub-spherical, 12 μm. Basidia: 50 × 10 μm |
| M1 (Russula aff. cellulata) | M2 (Russula sp1) | M3 (Russula sp2) | M4 (Russula sp3) | M5 (Russula sp3) | M6 (Russula sp3) | M7 (Russula sp3) | M8 (Amanita loosei) | |
|---|---|---|---|---|---|---|---|---|
| Nutritional value (g/100 g dw) | ||||||||
| Fat | 1.99 ± 0.05 h | 2.17 ± 0.06 g | 5.24 ± 0.02 b | 2.43 ± 0.02 f | 3.70 ± 0.06 c | 3.16 ± 0.01 e | 3.51 ± 0.09 d | 8.20 ± 0.01 a |
| Proteins | 14.1 ± 0.5 d | 12.6 ± 0.4 e | 14.1 ± 0.1 d | 15.8 ± 0.1 bc | 16.2 ± 0.1 ab | 15.4 ± 0.1 c | 18.1 ± 0.3 a | 15.2 ± 0.2 c |
| Ash | 6.85 ± 0.03 f | 9.20 ± 0.01 e | 15.87 ± 0.01 a | 14.47 ± 0.03 b | 14.05 ± 0.01 c | 13.39 ± 0.04 d | 16.19 ± 0.04 a | 14.92 ± 0.06 b |
| Carbohydrates | 77.1 ± 0.4 a | 76.1 ± 0.4 b | 64.82 ± 0.05 f | 67.3 ± 0.1 d | 66.0 ± 0.1 e | 68.1 ± 0.1 c | 63.8 ± 1.4 f | 61.7 ± 0.1 g |
| Energy value (kcal/100 g dw) | 382.6 ± 0.3 a | 374.1 ± 0.2 b | 362.7 ± 0.1 c | 354.3 ± 0.2 d | 362.3 ± 0.2 c | 362.2 ± 0.1 c | 352.8 ± 0.4 d | 381.4 ± 0.2 a |
| Hydrophilic compounds (free sugars and organic acids, g/100 g dw) | ||||||||
| Frutose | nd | nd | nd | 0.16 ± 0.03 c | 0.10 ± 0.01 d | 0.23 ± 0.01 b | 0.28 ± 0.04 a | 0.05 ± 0.01 e |
| Mannitol | 20.48 ± 0.04 a | 16.49 ± 0.07 b | 12.45 ± 0.03 f | 13.67 ± 0.03 d | 14.29 ± 0.01 c | 13.01 ± 0.04 e | 6.71 ± 0.02 g | 4.88 ± 0.07 h |
| Trehalose | 0.77 ± 0.03 g | 0.83 ± 0.01 e | 1.28 ± 0.04 c | 1.38 ± 0.02 b | 0.71 ± 0.05 h | 1.08 ± 0.08 d | 0.77 ± 0.07 f | 6.50 ± 0.08 a |
| Total sugars | 21.25 ± 0.06 a | 17.32 ± 0.07 b | 13.74 ± 0.01 f | 15.21 ± 0.02 c | 15.09 ± 0.06 d | 14.32 ± 0.04 e | 7.76 ± 0.09 h | 11.43 ± 0.01 g |
| Oxalic | 0.022 ± 0.001 e | 0.029 ± 0.002 e | 0.105 ± 0.001 c | 0.115 ± 0.003 c | 0.106 ± 0.001 c | 0.49 ± 0.02 a | 0.150 ± 0.003 b | 0.057 ± 0.001 d |
| Quinic | 5.33 ± 0.05 b | 5.65 ± 0.06 a | 5.01 ± 0.01 c | 2.59 ± 0.01 f | 2.48 ± 0.01 g | 4.72 ± 0.01 d | 4.57 ± 0.01 e | 1.66 ± 0.01 h |
| Malic | 2.39 ± 0.05 f | 1.64 ± 0.01 g | 3.99 ± 0.01 d | 4.09 ± 0.03 c | 3.48 ± 0.01 e | 4.55 ± 0.01 a | 4.39 ± 0.02 b | 1.42 ± 0.01 h |
| Citric | 0.85 ± 0.01 f | 0.84 ± 0.01 f | 1.22 ± 0.01 e | 1.71 ± 0.01 c | 1.56 ± 0.01 d | 2.04 ± 0.01 b | 2.60 ± 0.06 a | 0.718 ± 0.007 g |
| Fumaric | tr | tr | tr | tr | tr | tr | tr | tr |
| Total organic acids | 8.59 ± 0.01 d | 8.16 ± 0.05 f | 10.34 ± 0.01 c | 8.51 ± 0.02 e | 7.63 ± 0.01 g | 11.80 ± 0.01 a | 11.71 ± 0.08 b | 3.86 ± 0.01 h |
| Lipophilic compounds (fatty acids, % and tocopherol, µg/100 g dw) | ||||||||
| C16:0 | 19.81 ± 0.03 d | 12.1 ± 0.1 e | 30.46 ± 0.02 a | 23.2 ± 0.3 c | 20.03 ± 0.04 d | 24.5 ± 0.1 b | 19.95 ± 0.06 d | 19.5 ± 0.1 d |
| C18:0 | 5.03 ± 0.04 g | 9.4 ± 0.1 b | 11.9 ± 0.1 a | 6.68 ± 0.09 d | 5.93 ± 0.01 e | 7.78 ± 0.1 c | 6.56 ± 0.01 d | 5.15 ± 0.05 f |
| C18:1n9c | 50.8 ± 0.1 c | 48.55 ± 0.05 d | 46.67 ± 0.08 e | 42.810.5 f | 48.9 ± 0.5 d | 53.3 ± 0.01 b | 50.3 ± 0.3 c | 55.80 ± 0.01 a |
| C18:2n6c | 17.4 ± 0.1 e | 26.85 ± 0.07 a | 1.87 ± 0.01 h | 19.04 ± 0.09 c | 20.7 ± 0.1 b | 8.6 ± 0.1 g | 18.7 ± 0.1 c,d | 15.7 ± 0.2 f |
| SFA (%) | 26.4 ± 0.1 f | 22.2 ± 0.1 g | 47.3 ± 0.1 a | 35.0 ± 0.5 b | 27.4 ± 0.7 e | 34.5 ± 0.1 c | 28.3 ± 0.1 d | 26.22 ± 0.1 f |
| MUFA (%) | 51.8 ± 0.1 c | 49.1 ± 0.1 g | 50.6 ± 0.1 e | 43.7 ± 0.6 h | 49.7 ± 0.5 f | 54.0 ± 0.1 b | 51.0 ± 0.3 d | 56.4 ± 0.1 a |
| PUFA (%) | 21.8 ± 0.1 c | 28.7 ± 0.2 a | 2.2 ± 0.1 h | 21.3 ± 0.1 d | 22.9 ± 0.1 b | 11.5 ± 0.1 g | 20.7 ± 0.2 e | 17.4 ± 0.1 f |
| α-Tocopherol | 36.9 ± 0.7 b | 22.8 ± 0.8 d | 23.1 ± 0.1 c | nd | nd | 21.8 ± 0.8 e | nd | 50.4 ± 0.8 a |
| β-Tocopherol | nd | 45.9 ± 0.4 | nd | nd | nd | nd | nd | nd |
| Total tocopherols | 36.9 ± 0.7 c | 68.7 ± 1.3 a | 23.1 ± 0.1 d | - | - | 21.8 ± 0.8 e | - | 50.4 ± 0.8 b |
| Morphotypes/Species | M1 (Russula aff. cellulata) | M2 (Russula sp1) | M3 (Russula sp2) | M4 (Russula sp3) | M5 (Russula sp3) | M6 (Russula sp3) | M7 (Russula sp3) | M8 (Amanita loosei) |
|---|---|---|---|---|---|---|---|---|
| Antioxidant activity (EC50 mg/mL) | ||||||||
| TBARS | 2.09 ± 0.05 c | 1.07 ± 0.08 e | 2.47 ± 0.04 a | 1.15 ± 0.03 d | 2.25 ± 0.06 b | 2.52 ± 0.05 a | 1.08 ± 0.09 e | 2.25 ± 0.08 b |
| Phenolic acids and related compounds (µg/g of extract) | ||||||||
| Protocatechuic acid | 9.1 ± 0.5 g | 120 ± 1 a | 2.89 ± 0.01 h | 56.7 ± 0.6 b | 46.3 ± 0.3 c | 33.5 ± 0.4 d | 19.8 ± 0.4 f | 31.9 ± 0.6 e |
| p-Hydroxybenzoic acid | 93.3 ± 0.6 f | 28.0 ± 0.4 h | 31.8 ± 0.9 g | 274.0 ± 0.1 c | 286.7 ± 0.2 b | 145.2 ± 0.5 d | 139.4 ± 0.9 e | 320.1 ± 0.4 a |
| p-Coumaric acid | 21.4 ± 0.3 f | 15 ± 1 g | 6.7 ± 0.2 h | 43.6 ± 0.4 c | 45.1 ± 0.5 b | 39.4 ± 0.6 d | 29.3 ± 0.6 e | 48.2 ± 0.5 a |
| Total | 123.8 ± 0.5 g | 163.3 ± 0.8 f | 41 ± 1 h | 374 ± 1 c | 378.1 ± 0.9 b | 218.1 ± 0.1 d | 188 ± 1 e | 400.3 ± 0.6 a |
| Cinnamic acid | 55.0 ± 0.7 b | 16.5 ± 0.3 c | 6.37 ± 0.07 d | 6.2 ± 0.1 d | 3.43 ± 0.01 e | 3.8 ± 0.7 e | 79 ± 3 a | 16.3 ± 0.9 c |
| Staphylococcus aureus (ATCC 11632) | Bacillus cereus (Clinical Isolate) | Listeria monocytogenes (NCTC 7973) | Escherichia coli (ATCC 25922) | Salmonella typhimurium (ATCC 13311) | Enterobacter cloacae (ATCC 35030) | ||
|---|---|---|---|---|---|---|---|
| M1 (Russula aff. cellulata) | MIC | 2 | 1 | 2 | 0.5 | 1 | 2 |
| MBC | 4 | 2 | 4 | 1 | 2 | 4 | |
| M2 (Russula sp1) | MIC | 1 | 1 | 2 | 0.5 | 1 | 1 |
| MBC | 2 | 2 | 4 | 1 | 2 | 2 | |
| M3 (Russula sp2 | MIC | 2 | 1 | 2 | 0.5 | 1 | 1 |
| MBC | 4 | 2 | 4 | 1 | 2 | 2 | |
| M4 (Russula sp3) | MIC | 1 | 0.5 | 1 | 0.5 | 1 | 2 |
| MBC | 2 | 1 | 2 | 1 | 2 | 4 | |
| M5 (Russula sp3) | MIC | 4 | 1 | 2 | 0.5 | 1 | 2 |
| MBC | 8 | 2 | 4 | 1 | 2 | 4 | |
| M6 (Russula sp3) | MIC | 1 | 0.5 | 2 | 0.5 | 2 | 2 |
| MBC | 2 | 1 | 4 | 1 | 4 | 4 | |
| M7 (Russula sp3) | MIC | 4 | 1 | 1 | 0.5 | 1 | 2 |
| MBC | 8 | 2 | 2 | 1 | 2 | 4 | |
| M8 (Amanita loosei) | MIC | 1 | 1 | 2 | 0.5 | 1 | 2 |
| MBC | 2 | 2 | 4 | 1 | 2 | 4 | |
| Streptomycin | MIC | 0.10 | 0.04 | 0.20 | 0.20 | 0.20 | 0.25 |
| MBC | 0.20 | 0.10 | 0.30 | 0.30 | 0.30 | 0.50 | |
| Ampicilin | MIC | 0.25 | 0.25 | 0.40 | 0.40 | 0.25 | 0.75 |
| MBC | 0.40 | 0.45 | 0.50 | 0.50 | 0.50 | 1.20 |
| Aspergillus fumigatus (Human Isolate) | Aspergillus niger (ATCC 6275) | Aspergillus versicolor (ATCC11730) | Penicillium funiculosum (ATCC 36839) | Trichoderma viride (IAM 5061) | Penicillium verrucosum var. cyclopium (Food Isolate) | ||
|---|---|---|---|---|---|---|---|
| M1 (Russula aff. cellulata) | MIC | 1 | 1 | 1 | 2 | 0.5 | 4 |
| MFC | 2 | 2 | 2 | 4 | 1 | 8 | |
| M2 (Russula sp1) | MIC | 0.5 | 1 | 1 | 1 | 0.5 | 1 |
| MFC | 1 | 2 | 2 | 2 | 1 | 2 | |
| M3 (Russula sp2 | MIC | 0.5 | 1 | 1 | 0.5 | 0.5 | 1 |
| MFC | 1 | 2 | 2 | 1 | 1 | 2 | |
| M4 (Russula sp3) | MIC | 0.5 | 2 | 1 | 0.5 | 0.5 | 1 |
| MFC | 1 | 4 | 2 | 1 | 1 | 2 | |
| M5 (Russula sp3) | MIC | 0.5 | 1 | 1 | 0.5 | 0.5 | 1 |
| MFC | 1 | 2 | 2 | 1 | 1 | 2 | |
| M6 (Russula sp3) | MIC | 0.5 | 1 | 1 | 1 | 0.5 | 4 |
| MFC | 1 | 2 | 2 | 2 | 1 | 8 | |
| M7 (Russula sp3) | MIC | 0.5 | 1 | 1 | 0.5 | 0.5 | 1 |
| MFC | 1 | 2 | 2 | 1 | 1 | 2 | |
| M8 (Amanita loosei) | MIC | 1 | 1 | 1 | 1 | 0.5 | 1 |
| MFC | 2 | 2 | 2 | 2 | 1 | 2 | |
| Ketoconazole | MIC | 0.25 | 0.20 | 0.20 | 0.20 | 2.50 | 0.20 |
| MFC | 0.50 | 0.50 | 0.50 | 0.50 | 3.50 | 0.30 | |
| Bifonazole | MIC | 0.15 | 0.15 | 0.10 | 0.20 | 0.20 | 0.10 |
| MFC | 0.20 | 0.20 | 0.20 | 0.25 | 0.25 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kissanga, R.; Liberal, Â.; Diniz, I.; Rodrigues, A.S.B.; Baptista-Ferreira, J.L.; Batista, D.; Ivanov, M.; Soković, M.; Ferreira, I.C.F.R.; Fernandes, Â.; et al. Biochemical and Molecular Profiling of Wild Edible Mushrooms from Huila, Angola. Foods 2022, 11, 3240. https://doi.org/10.3390/foods11203240
Kissanga R, Liberal Â, Diniz I, Rodrigues ASB, Baptista-Ferreira JL, Batista D, Ivanov M, Soković M, Ferreira ICFR, Fernandes Â, et al. Biochemical and Molecular Profiling of Wild Edible Mushrooms from Huila, Angola. Foods. 2022; 11(20):3240. https://doi.org/10.3390/foods11203240
Chicago/Turabian StyleKissanga, Raquel, Ângela Liberal, Inês Diniz, Ana S. B. Rodrigues, João L. Baptista-Ferreira, Dora Batista, Marija Ivanov, Marina Soković, Isabel C. F. R. Ferreira, Ângela Fernandes, and et al. 2022. "Biochemical and Molecular Profiling of Wild Edible Mushrooms from Huila, Angola" Foods 11, no. 20: 3240. https://doi.org/10.3390/foods11203240










