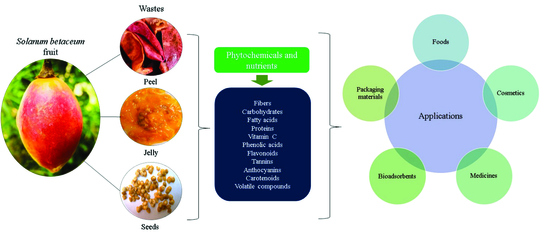
Solanum betaceum Fruits Waste: A Valuable Source of Bioactive Compounds to Be Used in Foods and Non-Foods Applications
Abstract
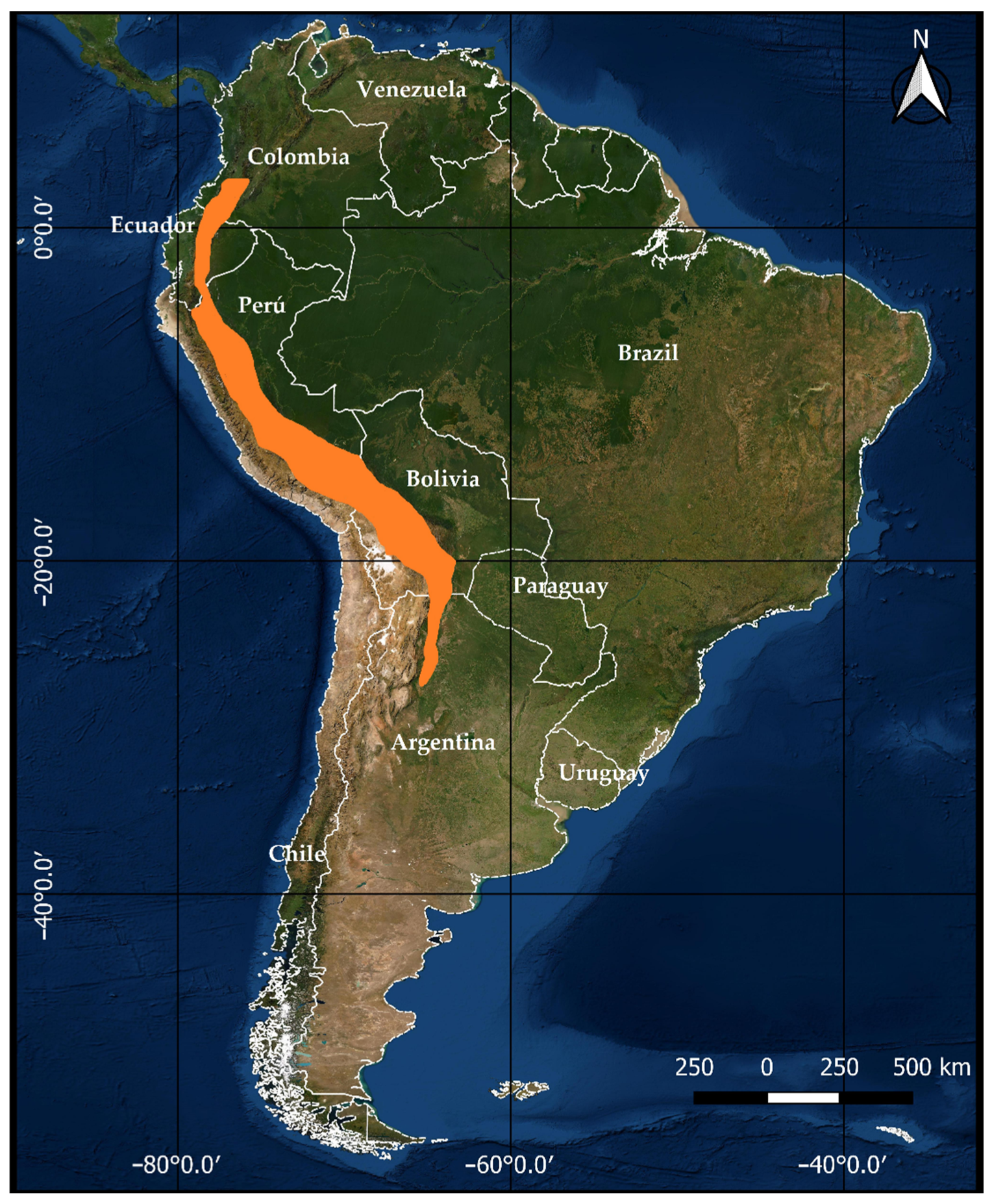
1. Introduction
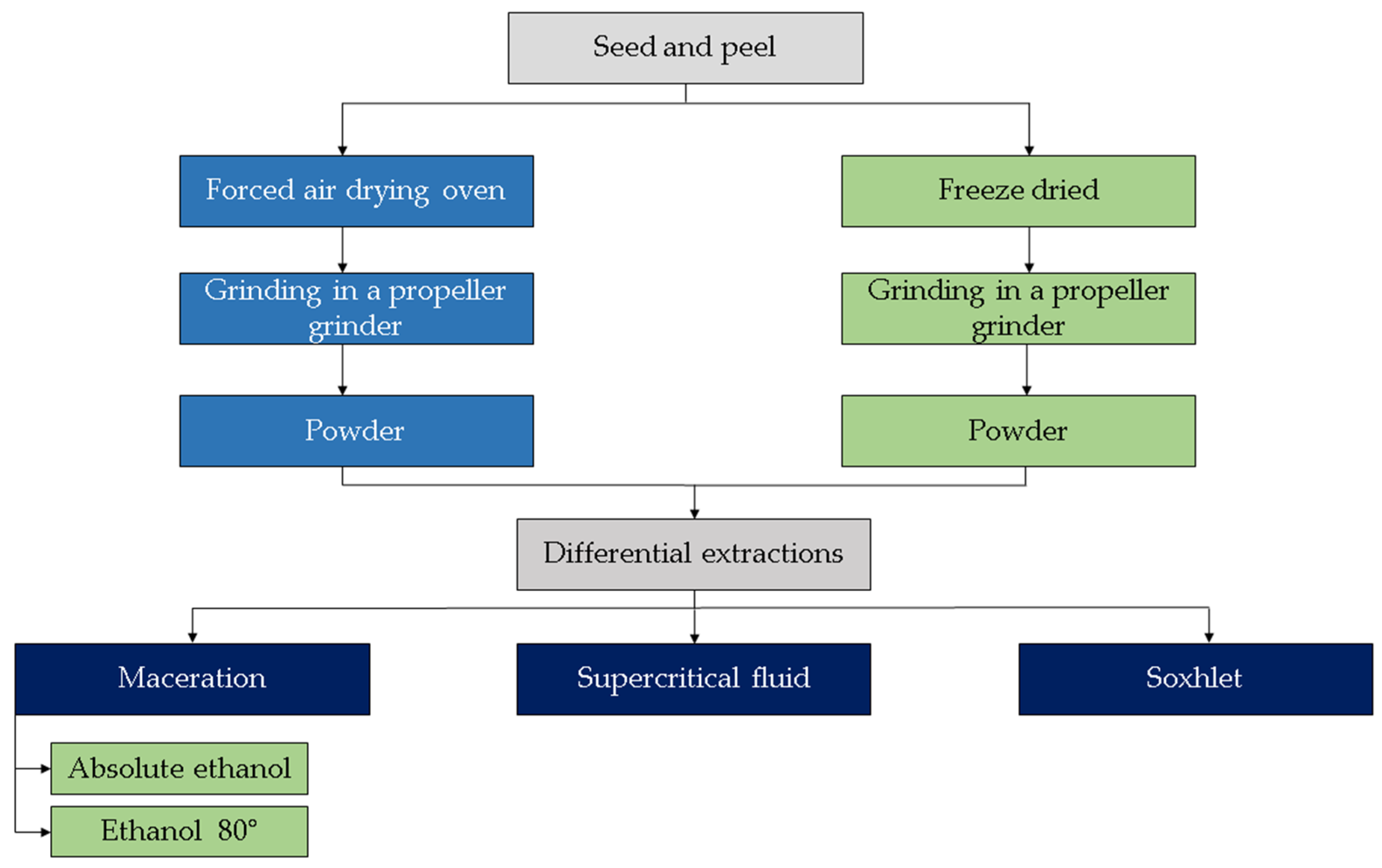
2. Nutritional and Phytochemical Composition of Powder Obtained from Waste of Red and Orange S. betaceum Fruits
2.1. Nutritional Composition
2.2. Phytochemical Composition
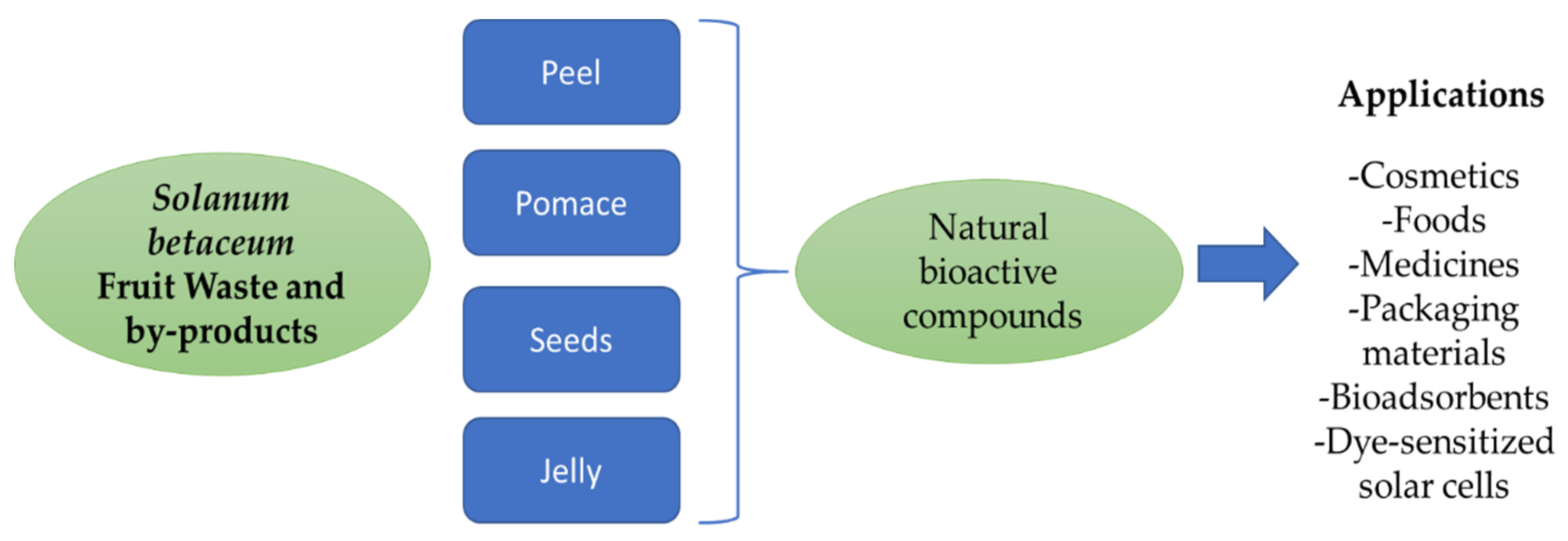
3. Functionally Active Ingredients from S. betaceum Wastes to Food and Cosmetic Products
3.1. Polyphenol-Enriched Extracts from Seed, Peel and Pomace of S. betaceum Fruits to Elaborate Food Formulations with Biological Activity
3.2. Polyphenol-Enriched Extracts from Seed, Peel, and Pomace of S. betaceum Fruits to Obtain Cosmetic Formulations with Biological Activity
3.3. Bioactive Carotenoid-Enriched Extracts from Seed and Peel of S. betaceum Fruits
3.4. Protein from Solanum betaceum Fruits
3.5. Oil from S. betaceum Seeds to Be Used in Food or in Cosmetics
3.6. Volatile Compounds (VC) in Peel of Freeze-Dried S. betaceum Fruits
3.7. Hydrocolloid from S. betaceum Pulp and Seed Mucilage
4. Innovations in Biodegradable Food Packaging Manufacture by Using Polyphenols, Pigments and Polysaccharides from S. betaceum Fruit Waste
5. Bioremediation: Bioadsorption of Toxic Metals by S. betaceum Peel Waste
6. Dye-Sensitized Solar Cells by S. betaceum Pulp and Seeds
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Food and Agricultural Organization (FAO). The State of Food and Agriculture 2019: Moving Forward on Food Loss and Waste Reduction; Food and Agricultural Organization: Rome, Italy, 2019. [Google Scholar]
- Hanson, C.; Lipinski, B.; Robertson, K.; Dias, D.; Gavilan, I.; Gréverath, P.; Ritter, P.; Fonseca, J.; Van Otterdijk, R.; Timmermans, T.; et al. Food Loss and Waste Accounting and Reporting Standard 2016; World Resources Institute: Washington, DC, USA, 2016. [Google Scholar]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef]
- Rolker, H.; Eisler, M.; Cardenas, L.; Deeney, M.; Takahashi, T. Food waste interventions in low-and-middle-income countries: A systematic literature review, Resour. Conserv. Recycl. 2022, 186, 106534. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Kumar, A.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour. Technol. 2017, 248, 2–12. [Google Scholar] [CrossRef] [PubMed]
- IUCN (International Union for Conservation of Nature and Natural Resources) Red List. Available online: https://www.iucnredlist.org/search?query=solanum%20betaceum&searchType=species (accessed on 23 September 2022).
- Ojeda, A.M.; Bermeo, S.P.; Bastidas, A.R.; Muñoz, C. Producción y Comercialización de Tamarillo (Cyphomandra betacea Sent), Para el Mercado Internacional; Escuela Superior Politécnica del Litoral: Quito, Ecuador, 2009. [Google Scholar]
- Márquez, C.J.; Otero, C.; Cortés, M. Changes physiological, textural, physicochemical and microestructural of the tree tomato (Cyphomandra betacea S.) at postharvest. Vitae 2007, 14, 9–16. [Google Scholar]
- Ministerio de Agricultura y Ganadería. Boletín Situacional: Tomate de Árbol; Coordinación General del Sistema de Información Nacional (CGSIN): Quito, Ecuador, 2018. [Google Scholar]
- Prohens, J.; Nuez, F. The tamarillo (Cyphomandra betacea). Small Fruits Rev. 2001, 1, 43–68. [Google Scholar] [CrossRef]
- Aitken, A.G.; Hewett, E.W. Fresh Facts: New Zealand Horticulture; New Zealand Institute for Plant & Food Research: Auckland, New Zealand, 2016; p. 14. [Google Scholar]
- Diep, T.T.; Rush, E.C.; Yoo, M.J.Y. Tamarillo (Solanum betaceum Cav.): A review of physicochemical and bioactive properties and potential applications. Food Rev. Int. 2020, 38, 1343–1367. [Google Scholar] [CrossRef]
- Meza, N.; Manzano Mendez, J. Characteristics of tree tomato (Cyphomandra betaceae [Cav] Sendtn) fruits based in aril coloration, at the Venezuelan Andes Zone. Rev. UDO Agríc. 2009, 9, 289–294. [Google Scholar]
- Rivas, M.; Gurni, A.; Vignale, D. Caracterizacion micrográfica de Solanum betaceum Cav. (Solanaceae), un cultivo andino medicinal. In Avances Sobre Plantas Medicinales Andinas; Vignale, N.D., Pochetino, M.L., Eds.; CYTED: Cambridge, UK, 2009; pp. 207–218. [Google Scholar]
- Orqueda, M.E.; Rivas, M.; Zampini, I.C.; Alberto, M.R.; Torres, S.; Cuello, S.; Sayago, J.; Thomas-Valdes, S.; Jiménez-Aspee, F.; Schmeda-Hirschmann, G.; et al. Chemical and functional characterization of seed, pulp and skin powder from chilto (Solanum betaceum), an Argentine native fruit. Phenolic fractions affect key enzymes involved in metabolic syndrome and oxidative stress. Food Chem. 2017, 216, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Orqueda, M.E.; Torres, S.; Zampini, I.C.; Cattaneo, F.; Di Pardo, A.F.; Valle, E.M.; Jimenez-Aspee, F.; Schmeda-Hirschmann, G.; Isla, M.I. Integral use of Argentinean Solanum betaceum red fruits as functional food ingredient to prevent metabolic syndrome: Effect of in vitro simulated gastroduodenal digestion. Heliyon 2020, 6, e03387. [Google Scholar] [CrossRef] [PubMed]
- Orqueda, M.E.; Torres, S.; Verón, H.; Pérez, J.; Rodriguez, F.; Zampini, I.C.; Isla, M.I. Physicochemical, microbiological, functional and sensory properties of frozen pulp of orange and orange-red chilto (Solanum betaceum Cav.) fruits. Sci. Hortic. 2021, 276, 109736. [Google Scholar] [CrossRef]
- Vasco, C.; Avila, J.; Ruales, J.; Svanberg, U.; Kamal-Eldin, A. Physical and chemical characteristics of golden-yellow and purple-red varieties of tamarillo fruit (Solanum betaceum Cav.). Int. J. Food Sci. Nutr. 2009, 60, 278–288. [Google Scholar] [CrossRef]
- Diep, T.T.; Pook, C.; Yoo, M.J.Y. Physicochemical properties and proximate composition of tamarillo (Solanum betaceum Cav.) fruits from New Zealand. J. Food Compos. Anal. 2020, 92, 103563. [Google Scholar] [CrossRef]
- Diep, T.T.; Pook, C.; Rush, E.C.; Yoo, M.J.Y. Quantification of carotenoids, α-tocopherol, and ascorbic acid in amber, mulligan, and laird’s large cultivars of New Zealand tamarillos (Solanum betaceum Cav.). Foods 2020, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Quezada, P.G.; Raigón, M.D.; Riofrío-Cuenca, T.; García-Martínez, M.D.; Plazas, M.; Burneo, J.I.; Prohens, J. Diversity for chemical composition in a collection of different varietal types of tree tomato (Solanum betaceum Cav.), an Andean exotic fruit. Food Chem. 2015, 169, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Espin, S.; Gonzalez-Manzano, S.; Taco, V.; Poveda, C.; Ayuda-Durán, B.; Gonzalez-Paramas, A.M.; Santos-Buelga, C. Phenolic composition and antioxidant capacity of yellow and purple-red Ecuadorian cultivars of tree tomato (Solanum betaceum Cav.). Food Chem. 2016, 194, 1073–1080. [Google Scholar] [CrossRef]
- Diep, T.T.; Yoo, M.J.Y.; Pook, C.; Sadooghy-Saraby, S.; Gite, A.; Rush, E. Volatile Components and Preliminary Antibacterial Activity of Tamarillo (Solanum betaceum Cav.). Foods 2021, 10, 2212. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Heatherbell, D.A. Identification of anthocyanins and distribution of flavonoids in tamarillo fruit (Cyphomandra betaceae (Cav.) Sendt.). J. Sci. Food Agric. 1974, 25, 1221–1228. [Google Scholar] [CrossRef]
- Torres, A.; Guinand, J.; Pérez, S. Development of an energy drink including tree tomato (Solanum betaceum) pulp. Rev. Venez. Cienc. Tecnol. Aliment. 2015, 6, 57–68. [Google Scholar]
- Fernandino, C.M.; Nepomuceno, A.T.; Fonseca, H.C.; Bastos, R.A.; Lima, J.P.D. Physicochemical properties of tamarillo pulp (Solanum betaceum) and its applicability in the production of ice cream. Braz. J. Food Technol. 2021, 24, e2020090. [Google Scholar] [CrossRef]
- Gannasin, S.P.; Mustafa, S.; Adzahan, N.M.; Muhammad, K. In vitro prebiotic activities of tamarillo (Solanum betaceum Cav.) hydrocolloids. J. Funct. Foods 2015, 19, 10–19. [Google Scholar] [CrossRef]
- do Nascimento, G.E.; Corso, C.R.; de Paula Werner, M.F.; Baggio, C.H.; Iacomini, M.; Cordeiro, L.M. Structure of an arabinogalactan from the edible tropical fruit tamarillo (Solanum betaceum) and its antinociceptive activity. Carbohydr. Polym. 2015, 116, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Gannasin, S.P.; Adzahan, N.M.; Hamzah, M.Y.; Mustafa, S.; Muhammad, K. Physicochemical properties of tamarillo (Solanum betaceum Cav.) hydrocolloid fractions. Food Chem. 2015, 182, 292–301. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, G.E.; Iacomini, M.; Cordeiro, L.M. A comparative study of mucilage and pulp polysaccharides from tamarillo fruit (Solanum betaceum Cav.). Plant Physiol. Biochem. 2016, 104, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Orqueda, M.E.; Mendez, D.; Martínez-Abad, A.; Zampini, I.C.; Torres, S.; Isla, M.I.; Lopez-Rubio, A.; Fabra, M.J. Feasibility of active biobased films produced using red chilto wastes to improve the protection of fresh salmon fillets via a circular economy approach. Food Hydrocoll. 2022, 133, 107888. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Lucci, P.; Núñez, O.; Tundis, R.; Balzano, M.; Frega, N.G.; Conte, L.; Moret, S.; Filatova, D.; Moyano, E.; et al. Native Colombian Fruits and Their by-Products: Phenolic Profile, Antioxidant Activity and Hypoglycaemic Potential. Foods 2019, 8, 89. [Google Scholar] [CrossRef]
- Ramakrishnan, Y.; Khoddami, A.; Gannasin, S.P.; Muhammad, K. Tamarillo (Cyphomandra betacea) seed oils as a potential source of essential fatty acid for food, cosmetic and pharmacuetical industries. Acta Hortic. 2013, 1012, 1415–1421. [Google Scholar] [CrossRef]
- Dorado Achicanoy, D.; Hurtado Benavides, A.; Martínez-Correa, H.A. Study of supercritical CO2 extraction of tamarillo (Cyphomandra betacea) seed oil containing high added value compounds. Electrophoresis 2018, 39, 1917–1925. [Google Scholar] [CrossRef]
- Ali Hassan, S.H.; Bakar, M.F. Antioxidative and anticholinesterase activity of Cyphomandra betacea fruit. Sci. World J. 2013, 2013, 278071. [Google Scholar] [CrossRef]
- Diep, T.; Pook, C.; Yoo, M. Phenolic and anthocyanin compounds and antioxidant activity of tamarillo (Solanum betaceum Cav.). Antioxidants 2020, 9, 169. [Google Scholar] [CrossRef]
- Hurtado, N.H.; Morales, A.L.; González-Miret, M.L.; Escudero-Gilete, M.L.; Heredia, F.J. Colour, pH stability and antioxidant activity of anthocyanin rutinosides isolated from tamarillo fruit (Solanum betaceum Cav.). Food Chem. 2009, 117, 88–93. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Bobbio, P.A.; Bobbio, F.O. Carotenoid composition and vitamin A value of the Brazilian fruit Cyphomandra betacea. Food Chem. 1983, 12, 61–65. [Google Scholar] [CrossRef]
- de Rosso, V.V.; Mercadante, A.Z. HPLC–PDA–MS/MS of anthocyanins and carotenoids from dovyalis and tamarillo fruits. J. Agric. Food Chem. 2007, 55, 9135–9141. [Google Scholar] [CrossRef] [PubMed]
- Mertz, C.; Gancel, A.; Gunata, Z.; Alter, P.; Dhuique-Mayer, C.; Vailant, F.; Perez, A.M.; Ruales, J.; Brat, P. Phenolic compounds, carotenoids and antioxidant activity of three tropical fruits. J. Food Compos. Anal. 2009, 22, 381. [Google Scholar] [CrossRef]
- Ordóñez, R.M.; Cardozo, M.L.; Zampini, I.C.; Isla, M.I. Evaluation of antioxidant activity and genotoxicity of alcoholic and aqueous beverages and pomace derived from ripe fruits of Cyphomandra betacea Sendt. J. Agric. Food Chem. 2010, 58, 331–333. [Google Scholar] [CrossRef]
- Castro-Vargas, H.I.; Benelli, P.; Ferreira, S.R.S.; Parada-Alfonso, F. Supercritical fluid extracts from tamarillo (Solanum betaceum Sendtn) epicarp and its application as protectors against lipid oxidation of cooked beef meat. J. Supercrit. Fluids 2013, 76, 17–23. [Google Scholar] [CrossRef]
- Sánchez, W.F.; Murillo, E.; Méndez, J.J. Antioxidant potential of agroindustrial residues from three high consumption fruits in Tolima. Sci. Tech. 2010, 46, 138–143. [Google Scholar]
- Diep, T.T.; Yoo, M.J.Y.; Rush, E. Tamarillo Polyphenols Encapsulated-Cubosome: Formation, Characterization, Stability during Digestion and Application in Yoghurt. Antioxidants 2022, 11, 520. [Google Scholar] [CrossRef]
- Orqueda, M.E.; Zampini, I.C.; Bravo, K.; Osorio, E.; Isla, M.I. Potential use of native fruits waste from Argentina as nonconventional sources of cosmetic ingredients. J. Cosmet. Dermatol. 2022, in press. [Google Scholar] [CrossRef]
- Pazmiño, D.; Fernández, D.; López, O.D.; Iraizoz, A. Evaluación de diferentes combinaciones de polímeros en la microencapsulación de licopenos procedentes de residuos de tomate de árbol (Solanum betaceum). Rev. Bionat. 2022, 7, 29. [Google Scholar] [CrossRef]
- García, J.M.; Giuffrida, D.; Dugo, P.; Mondello, L.; Osorio, C. Development and characterization of carotenoid-rich microencapsulates from tropical fruit by-products and yellow tamarillo (Solanum betaceum Cav.). Powder Technol. 2018, 339, 702–709. [Google Scholar] [CrossRef]
- Mertz, C.; Brat, P.; Caris-Veyrat, C.; Gunata, Z. Characterization and thermal lability of carotenoids and vitamin C of tamarillo fruit (Solanum betaceum Cav.). Food Chem. 2010, 119, 653–659. [Google Scholar] [CrossRef]
- Ordóñez, R.; Zampini, I.; Rodriguez, F.; Cattaneo, F.; Sayago, J.; Isla, M.I. Radical Scavenging capacity and antimutagenic properties of purified proteins from Solanum betaceum fruits and Solanum tuberosum tubers. J. Agric. Food Chem. 2011, 59, 8655–8660. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, R.M.; Sayago, J.E.; Zampini, I.C.; Rodriguez, F.; Cattaneo, F.; Isla, M.I. Bioactive proteins from edible plants of Solanum genus. Curr. Top. Pept. Protein Res. 2012, 12, 75–79. [Google Scholar]
- Cunnane, S.; Anderson, M. Pure linoleate deficiency in the rat: Influence on growth, accumulation of n-6 polyunsaturates, and (1–14C) linoleate oxidation. J. Lipid Res. 1997, 38, 805–812. [Google Scholar] [CrossRef]
- Cruz, R.; Chavez, S.G.; Fernández-Jeri, A.B. Actividad antioxidante y ácidos grasos de aceite de semillas de siete frutas nativas de la región Amazonas, Perú. Inf. Tecnol. 2021, 32, 141–148. [Google Scholar] [CrossRef]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Ramadan, M.F. Fruit Oils: Chemistry and Functionality; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Tabaszewska, M.; Antoniewska, A.; Rutkowska, J.; Skoczylas, Ł.; Słupski, J.; Skoczeń-Słupska, R. Bioactive components, volatile profile and in vitro antioxidative properties of Taxus baccata L. Red Arils. Molecules 2021, 26, 4474. [Google Scholar] [CrossRef]
- Bernstein, L.R.; Tanner, T.; Godfrey, C.; Noll, B. Chemistry and pharmacokinetics of gallium maltolate, a compound with high oral gallium bioavailability. Met. Based Drugs 2000, 7, 33–47. [Google Scholar] [CrossRef]
- Reffitt, D.M.; Burden, T.J.; Seed, P.T.; Wood, J.; Thompson, R.P.; Powell, J.J. Assessment of iron absorption from ferric trimaltol. Ann. Clin. Biochem. 2000, 37, 457–466. [Google Scholar] [CrossRef]
- Gannasin, S.P.; Ramakrishnan, Y.; Adzahan, N.M.; Muhammad, K. Functional and preliminary characterisation of hydrocolloid from tamarillo (Solanum betaceum Cav.) puree. Molecules 2012, 17, 6869–6885. [Google Scholar] [CrossRef] [PubMed]
- Gannasin, S.P.; Adzahan, N.M.; Mustafa, S.; Muhammad, K. Techno-functional properties and in vitro bile acid-binding capacities of tamarillo (Solanum betaceum Cav.) hydrocolloids. Food Chem. 2016, 196, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.A.; Orqueda, M.E.; Gómez-Mascaraque, L.G.; Isla, M.I.; López-Rubio, A. Crosslinked electrospun zein-based food packaging coatings containing bioactive chilto fruit extracts. Food Hydrocoll. 2019, 95, 496–505. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Villabona-Ortiz, Á.; Garcés-Jaraba, L. Adsorption of heavy metals in waste water using biological materials. TecnoLógicas 2015, 18, 109–123. [Google Scholar] [CrossRef]
- Cabrera Andrade, L.F. Bioadsorción de Iones de Plomo y Cromo Procedentes de Aguas Residuales Utilizando la Cáscara del Tomate de Árbol (Solanum betaceum). Bachelor’s Thesis, Universidad Politécnica Salesiana, Cuenca, Ecuador, 2017. [Google Scholar]
- Iqbal, M.Z.; Ali, S.R.; Khan, S. Progress in dye sensitized solar cell by incorporating natural photosensitizers. Sol. Energy 2019, 181, 490–509. [Google Scholar] [CrossRef]
- Susanti, D.; Nafi, M.; Purwaningsih, H.; Fajarin, R.; Kusuma, G.E. The preparation of dye sensitized solar cell (DSSC) from TiO2 and tamarillo extract. Procedia Chem. 2014, 9, 3–10. [Google Scholar] [CrossRef]

| Nutritional and Phytochemical Composition | Orange, Yellow and Golden Yellow Fruits | Red and Purple Red Fruits | ||||
|---|---|---|---|---|---|---|
| Orange Fruits (Argentina) [16] | New Zealand (Yellow) [13] | Ecuador (Golden-Yellow) [19] | Argentina (Red) [17] | New Zealand (Red) [13] | Ecuador (Purple-Red) [19] | |
| Total phenolic (mg GAE/100 g FW) | 89.1 | 117.0 | 125.0 | 68.1 | 191.0 | 187.0 |
| Flavone and flavonol (mg QE/100 g FW) | 20.4 | - | - | 25.1 | - | - |
| Anthocyanins (mg C-3GE/100 g FW) | 0.2 | ND | ND | 12.7 | 82.0 | 38.0 |
| Ascorbic acid (mg AA/100 g FW) | 15.2 | 31.0 | 17.0 | 15.4 | 29.8 | 16.0 |
| Carotenoids (g β carotene/100 g FW) | 0.2 | - | 3.4 | 0.2 | - | 5.2 |
| Total sugar (g GE/100 g FW) | 1.1 | 3.4 | - | 2.1 | 3.5 | - |
| Glucose (g/100 g FW) | 0.1 | 0.8 | 1.7 | 0.2 | 0.8 | 1.4 |
| Fructose (g/100 g FW) | 0.3 | 0.9 | 1.6 | 0.3 | 0.9 | 1.4 |
| Sucrose (g/100 g FW) | 1.0 | 1.6 | 1.9 | 1.5 | 1.7 | 1.7 |
| Total Protein (g/100 g FW) | 2.4 | 1.9 | 2.4 | 2.2 | 2.0 | 2.2 |
| Fat (g/100 g FW) | 0.02 | 0.5 | 0.7 | 0.04 | 0.4 | 0.6 |
| Fiber (g/100 g FW) | 2.1 | 3.2 | - | 4.2 | 3.3 | - |
| Potassium (mg/100 g FW) | 637.8 | 292.0 | 398.0 | 251.8 | 321.0 | 379.0 |
| Iron (mg/100 g FW) | 0.2 | 0.4 | 0.2 | - | 0.5 | 0.4 |
| Magnesium (mg/100 g FW) | 21.54 | 20.0 | 16.0 | 15.5 | 21.0 | 14.0 |
| Phytochemical Content of S. betaceum Wastes | Red and Orange Fruit Peel (Argentina) | Red and Orange Fruit Seeds (Argentina) | Purple and Golden Yellow Peel (Ecuador) | Purple and Golden Yellow Seed-Jelly (Ecuador) | Red Fruit Peel (Colombia) | Red Fruit Jelly (Colombia) | Fruit Peel (Malaysia) | Fruit Seeds (Colombia) | Yellow, Red and Purple Fruits Peel (New Zealand) |
|---|---|---|---|---|---|---|---|---|---|
| Total phenolics (mg GAE/100 g) | 408.9 [17] 523.8 [16] | 623.6 [17] 179 [16] | 620 [19] a 387 [19] a | 152 [19] a 94 [19] a | 240–489 [36] | 1583.8 [37] 1673.28 [37] 2225.1 [37] | |||
| Flavone and flavonols (mg QE/100 g) | 195.3 [17] 265.7 [16] | 180.5 [17] 175.6 [16] | 129–336 [36] | ||||||
| Condensed tannins (mg procyanidin B2/100 g) | 75.7 [17] ND [16] | 265.0 [17] ND [16] | |||||||
| Anthocyanins (mg C-3GE/100 g) | 62.5 [17] 1.01 [16] | 65.2 [17] ND [16] | 0.20 1 [38] | 20.03 1 [38] | 0.61–1.36 [36] | 1.24 [37] 155.82 [37] 259.18 [37] | |||
| Ascorbic acid (mg AA/100 g) | 43.5 [17] 51.1 [16] | 45.2 [17] 56.8 [16] | 22 [21] a 18 [21] a 19 [21] a | ||||||
| Carotenoids (g β carotene/100 g) | ND [17] 1.37 [16] | ND [17] 0.53 [16] | 0.01–0.02 [36] | 0.23 [21] a 0.15 [21] a 0.15 [21] a | |||||
| Total sugar (g GE/100 g) | 8.9 [17] 2.23 [16] | 20.5 [17] 11.9 [16] | 3.6 [20] 9.7 [20] 6.9 [20] | ||||||
| Glucose (g/100 g) | 0.6 [17] 0.9 [16] | 0.9 [17] 0.7 [16] | 1.3 [20] 3.6 [20] 2.5 [20] | ||||||
| Fructose (g/100 g) | 1.2 [17] 2.7 [16] | 3.9 [17] 1.9 [16] | 1.8 [20] 5.1 [20] 3.6 [20] | ||||||
| Sucrose (g/100 g) | 4.2 [17] 7.8 [16] | 8.5 [17] 6.8 [16] | |||||||
| Total Protein (g/100 g) | 10.5 [17] 8.8 [16] | 21.9 [17] 20.9 [16] | |||||||
| Fat (g/100 g) | 0.8 [17] 0.2 [16] | 0.4 [17] 0.3 [16] | 17.4 2 [35] | ||||||
| Fiber (g/100 g) | 23.2 [17] 23.4 [16] | 29.5 [17] 28.42 [16] |
| Type of Fatty Acid | % of Fatty Acid |
|---|---|
| Oleic (C18:1 n-9) | 17.20 ± 0.60 [33] 14.93 ± 0.44 [34] 15.11–17.94 [35] |
| Linoleic (C18:2) | 58.30 ± 1.00 [33] 70.47 ± 0.35 [34] 66.67–72.09 [35] |
| Linolenic (C18:3) | 1.73 ± 0.04 [34] 1.53–3.50 [35] |
| Palmitic (C16:0) | 9.41 ± 0.15 [34] 7.70–11.02 [35] |
| Palmitoleic (C16:1) | 0.54 ± 0.05 [34] 0.19–0.25 [35] |
| Stearic (C18) | 2.23 ± 0.12 [34] 1.42–3.50 [35] |
| Arachidic (C20:0) | 0.23 ± 0.06 [34] |
| Phellonic (C22:0) | 0.22 ± 0.01 [34] |
| Lignoceric (C24:0) | 0.23 ± 0.14 [34] |
| S. betaceum by-products | Identification of Phenolic Compounds |
|---|---|
| Seeds and peel polyphenol enriched extract | Caffeoyl hexoside |
| Dicaffeoylquinic acid derivative | |
| Caffeoyl quinic acid | |
| Caffeoyl quinic acid hexoside | |
| Dihydrokaempferol pentoside | |
| Caffeoylquinic acid | |
| 3-Caffeoyl quinic acid | |
| Apigenin pentoside | |
| Hydroxy rosmarinic acid | |
| Synapoyl hexoside | |
| Rosmarinic acid dihexoside | |
| Quercetin rhamnoside | |
| Rutin | |
| Rosmarinic acid hexoside | |
| Isorinic acid hexoside | |
| Malonyl rosmarinic acid hexoside | |
| Quercetin hexoside | |
| Kaempferol rutinoside | |
| Quercetin glucuronide | |
| Rosmarinic acid | |
| Isorinic acid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isla, M.I.; Orqueda, M.E.; Moreno, M.A.; Torres, S.; Zampini, I.C. Solanum betaceum Fruits Waste: A Valuable Source of Bioactive Compounds to Be Used in Foods and Non-Foods Applications. Foods 2022, 11, 3363. https://doi.org/10.3390/foods11213363
Isla MI, Orqueda ME, Moreno MA, Torres S, Zampini IC. Solanum betaceum Fruits Waste: A Valuable Source of Bioactive Compounds to Be Used in Foods and Non-Foods Applications. Foods. 2022; 11(21):3363. https://doi.org/10.3390/foods11213363
Chicago/Turabian StyleIsla, María Inés, María Eugenia Orqueda, María Alejandra Moreno, Sebastián Torres, and Iris Catiana Zampini. 2022. "Solanum betaceum Fruits Waste: A Valuable Source of Bioactive Compounds to Be Used in Foods and Non-Foods Applications" Foods 11, no. 21: 3363. https://doi.org/10.3390/foods11213363
APA StyleIsla, M. I., Orqueda, M. E., Moreno, M. A., Torres, S., & Zampini, I. C. (2022). Solanum betaceum Fruits Waste: A Valuable Source of Bioactive Compounds to Be Used in Foods and Non-Foods Applications. Foods, 11(21), 3363. https://doi.org/10.3390/foods11213363