Bacillus coagulans Alleviates Intestinal Damage Induced by TiO2 Nanoparticles in Mice on a High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Measurement of the Blood Glucose
2.3. Analysis of the Lipid Profiles in Serum
2.4. Assessment of Pro-Inflammatory Cytokines
2.5. Evaluation of Antioxidant Enzyme
2.6. Analysis of Gut Microbiota
2.7. Quantification of Short-Chain Fatty Acids
2.8. Metabolomic Analysis
2.9. Statistical Analysis
3. Results
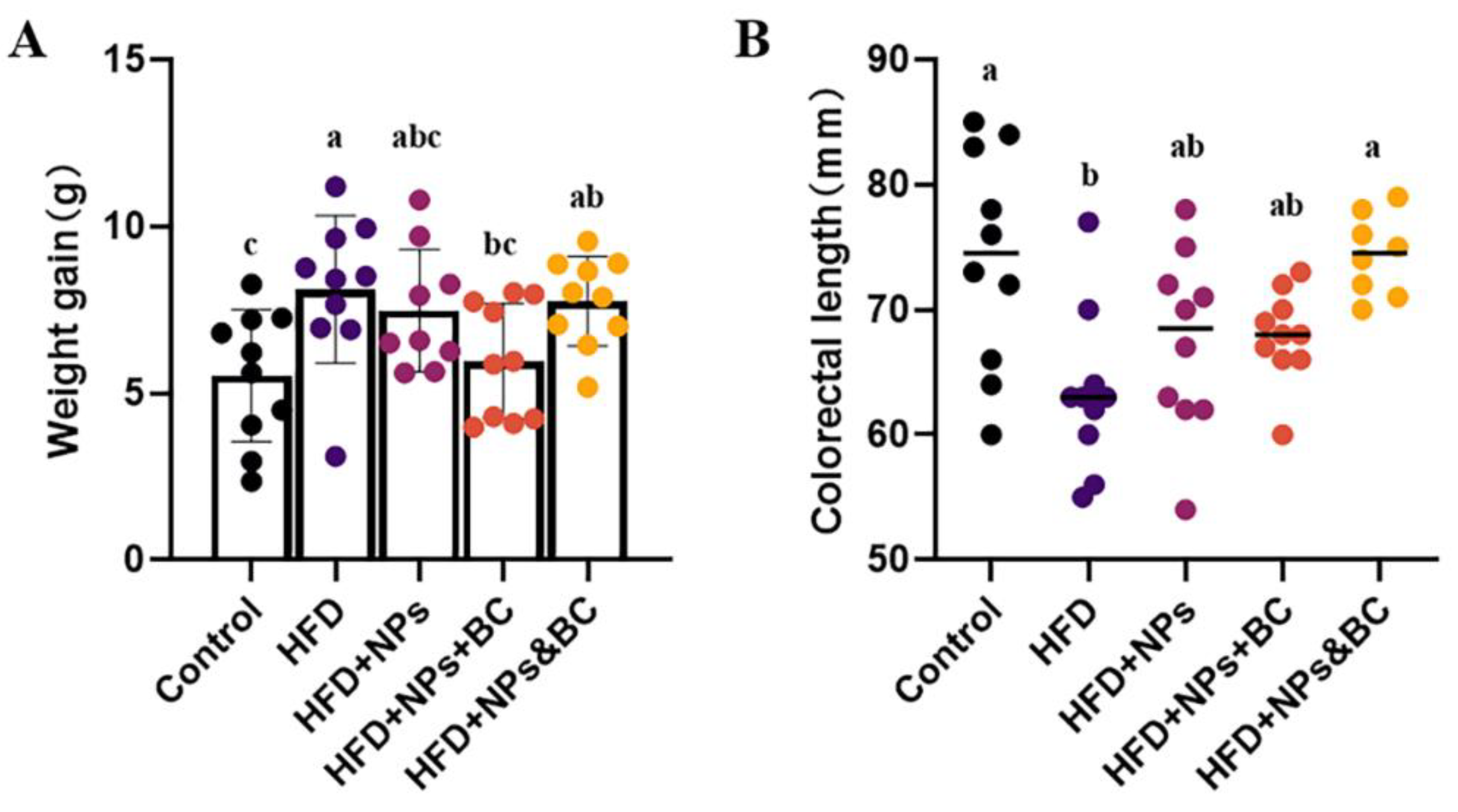
3.1. Effects on Body Weight and Colorectal Length
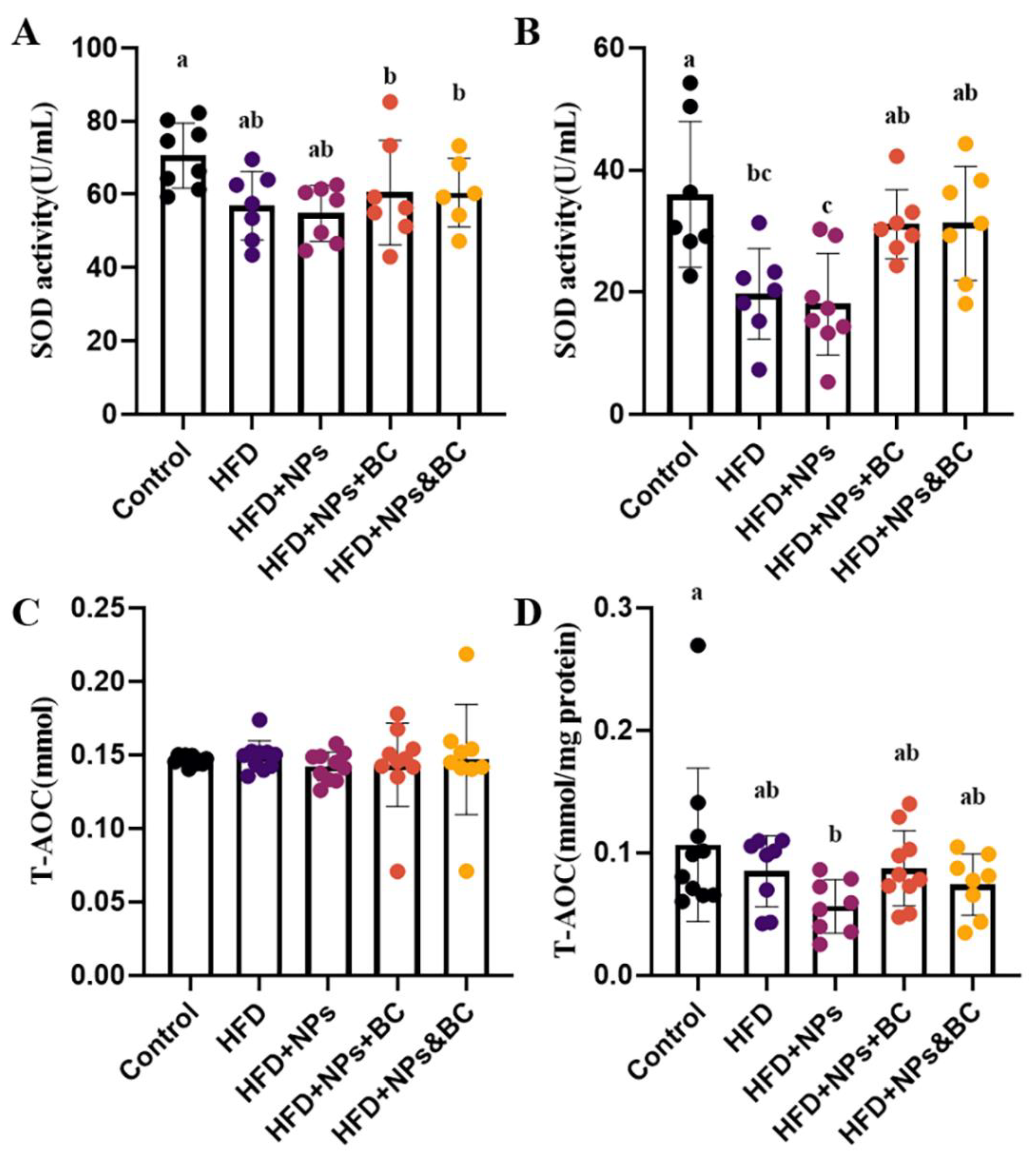
3.2. Effects on Inflammatory Response and Oxidative Stress
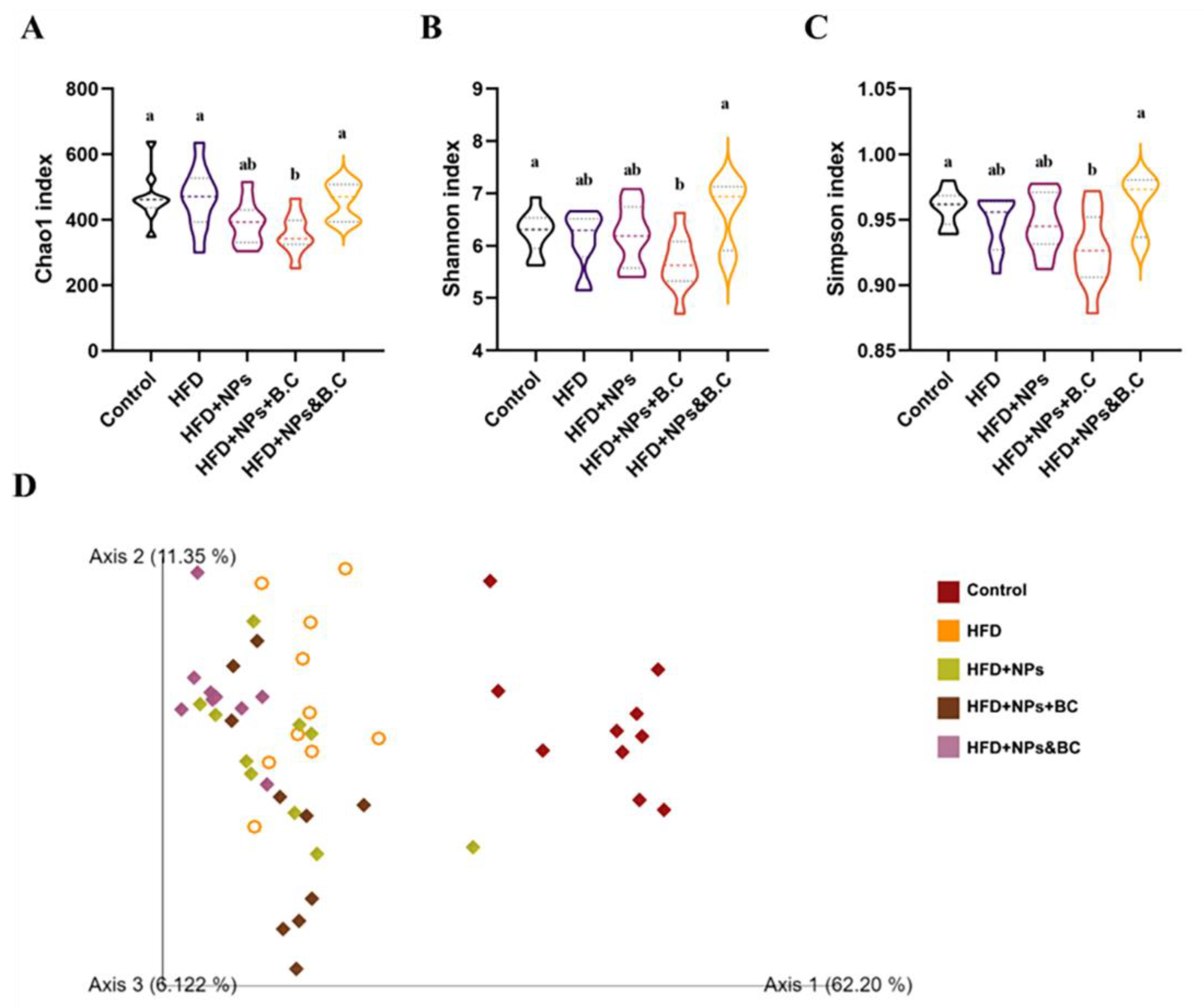
3.3. Effects on the Diversity of Gut Microbiota
3.4. Effects on the Composition of Gut Microbiota
3.5. Effects on the Production of Short-Chain Fatty Acids
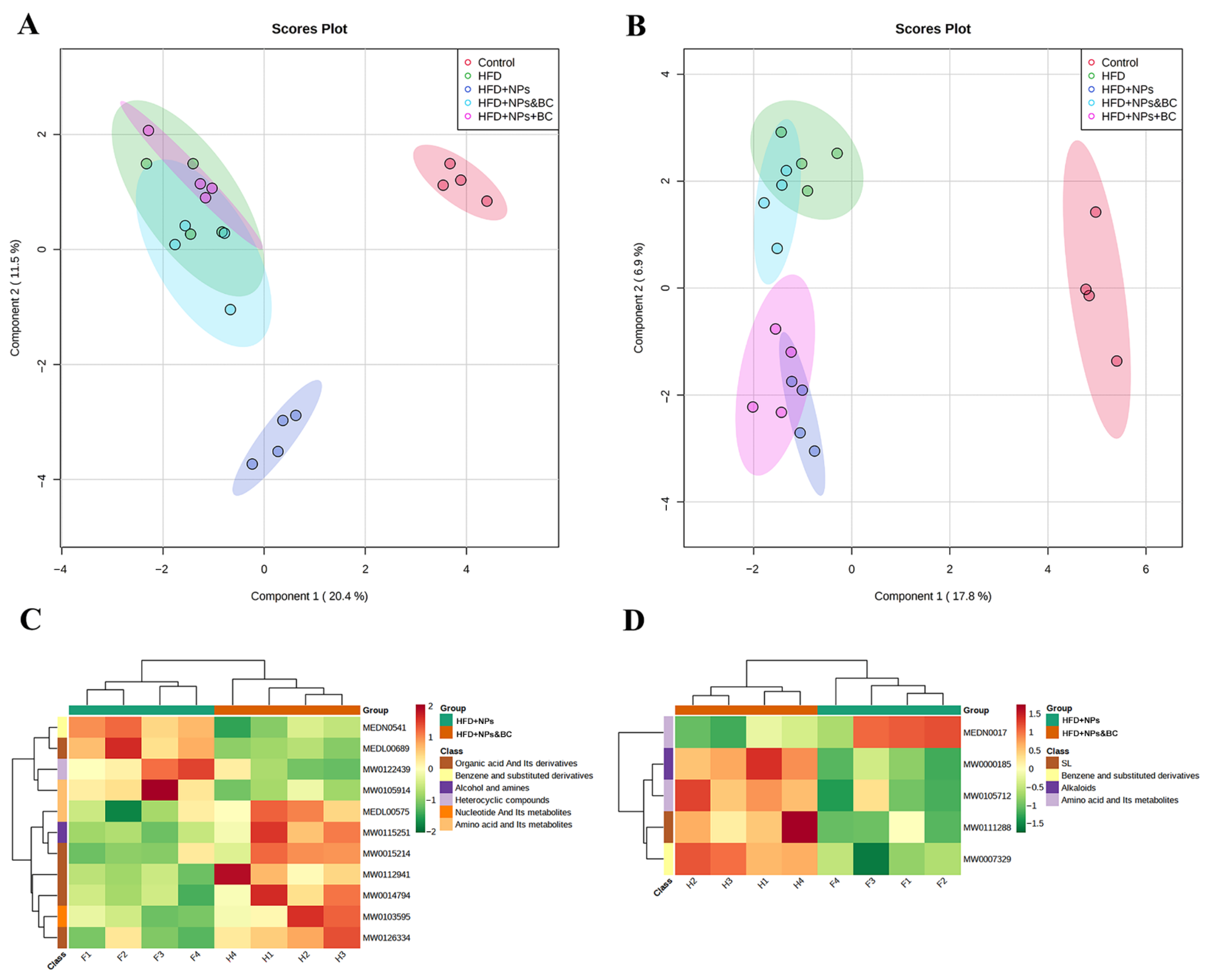
3.6. Effects on Fecal Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baranowska-Wójcik, E.; Szwajgier, D.; Winiarska-Mieczan, A. A review of research on the impact of E171/TiO2 NPs on the digestive tract. J. Trace Elements Med. Biol. 2022, 72. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Wójcik, E. Factors Conditioning the Potential Effects TiO2 NPs Exposure on Human Microbiota: A Mini-Review. Biol. Trace Element Res. 2021, 199, 4458–4465. [Google Scholar] [CrossRef] [PubMed]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J.; Xiao, H.; Demokritou, P. Physicochemical and colloidal aspects of food matrix effects on gastrointestinal fate of ingested inorganic nanoparticles. Adv. Colloid Interface Sci. 2017, 246, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Heringa, M.B.; Geraets, L.; Van Eijkeren, J.C.H.; Vandebriel, R.J.; De Jong, W.H.; Oomen, A.G. Risk assessment of titanium dioxide nanoparticles via oral exposure, including toxicokinetic considerations. Nanotoxicology 2016, 10, 1515–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Fernandez, M.J.F.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; Husoy, T.; et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e06585. [Google Scholar] [CrossRef]
- Limage, R.; Tako, E.; Kolba, N.; Guo, Z.; García-Rodríguez, A.; Marques, C.N.H.; Mahler, G.J. TiO2 Nanoparticles and Commensal Bacteria Alter Mucus Layer Thickness and Composition in a Gastrointestinal Tract Model. Small 2020, 16, e2000601. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.J.; Ainley, C.C.; Harvey, R.S.J.; Mason, I.M.; Kendall, M.D.; Sankey, E.A.; Dhillon, A.P.H.; Thompson, R.P. Characterisation of inorganic microparticles in pigment cells of human gut associated lymphoid tissue. Gut 1996, 38, 390–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brun, E.; Barreau, F.; Veronesi, G.; Fayard, B.; Sorieul, S.; Chanéac, C.; Carapito, C.; Rabilloud, T.; Mabondzo, A.; Herlin-Boime, N.; et al. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part. Fibre Toxicol. 2014, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Tan, Y.; Li, F.; Wang, H.; Lin, Y.; Lu, F.; Zhao, H. Intestinal Microecology of Mice Exposed to TiO2 Nanoparticles and Bisphenol A. Foods 2022, 11, 1696. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, K.M.A.; El-Amir, Y.O. Protective effects of thymoquinone and avenanthramides on titanium dioxide nanoparticles induced toxicity in Sprague-Dawley rats. Pathol.-Res. Pract. 2017, 213, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Rasmi, Y.; Hajaghazadeh, M.; Karimipour, M. Hepatoprotective effect of thymol against subchronic toxicity of titanium dioxide nanoparticles: Biochemical and histological evidences. Environ. Toxicol. Pharmacol. 2018, 58, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.A.; Morón, B.; Becker, H.M.; Lang, S.; Atrott, K.; Spalinger, M.R.; Scharl, M.; Wojtal, K.A.; Fischbeck-Terhalle, A.; Frey-Wagner, I.; et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: Role of the NLRP3 inflammasome. Gut 2017, 66, 1216–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Han, Y.; Gu, M.; Du, H.; Song, M.; Zhu, X.; Ma, G.; Pan, C.; Wang, W.; Zhao, E.; et al. Foodborne Titanium Dioxide Nanoparticles Induce Stronger Adverse Effects in Obese Mice than Non-Obese Mice: Gut Microbiota Dysbiosis, Colonic Inflammation, and Proteome Alterations. Small 2020, 16, e2001858. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.-L.; Rainteau, D.; Bridonneau, C.; da Costa, G.; Van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C.; et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 2018, 9, 2802. [Google Scholar] [CrossRef] [Green Version]
- Konuray, G.; Erginkaya, Z. Potential Use of Bacillus coagulans in the Food Industry. Foods 2018, 7, 92. [Google Scholar] [CrossRef] [Green Version]
- Majeed, M.; Majeed, S.; Arumugam, S.; Ali, F.; Beede, K. Comparative evaluation for thermostability and gastrointestinal survival of probiotic Bacillus coagulans MTCC 5856. Biosci. Biotechnol. Biochem. 2020, 85, 962–971. [Google Scholar] [CrossRef]
- Shinde, T.; Perera, A.P.; Vemuri, R.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Shastri, S.; Southam, B.; Eri, R.; Stanley, R. Synbiotic Supplementation Containing Whole Plant Sugar Cane Fibre and Probiotic Spores Potentiates Protective Synergistic Effects in Mouse Model of IBD. Nutrients 2019, 11, 818. [Google Scholar] [CrossRef] [Green Version]
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Majeed, S.; Ali, F. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: A randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr. Res. 2018, 62, 1218. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Sivakumar, A.; Ali, F.; Pande, A.; Majeed, S.; Karri, S.K. Bacillus coagulans MTCC 5856 supplementation in the management of diarrhea predominant Irritable Bowel Syndrome: A double blind ran-domized placebo controlled pilot clinical study. Nutr. J. 2016, 15, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Arumugam, S.; Pande, A.; Majeed, S.; Ali, F. A Double-Blind, Place-bo-Controlled, Parallel Study Evaluating the Safety of Bacillus coagulans MTCC 5856 in Healthy Individuals. J. Clin. Toxicol. 2016, 6, 283. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Sivakumar, A.; Ruiter, T.E.-d.; Booij-Veurink, J.; de Vries, Y.P.; Ali, F. Eval-uation of genetic and phenotypic consistency of Bacillus coagulans MTCC 5856: A commercial probiotic strain. World J. Microbiol. Biotechnol. 2016, 32, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Nie, G.; Yang, F.; Chen, J.; Zhuang, Y.; Dai, X.; Liao, Z.; Yang, Z.; Cao, H.; Xing, C.; et al. Molybdenum and cadmium co-induce oxidative stress and apoptosis through mitochondria-mediated pathway in duck renal tubular epithelial cells. J. Hazard. Mater. 2019, 383, 121157. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Parvez, S.; Sayeed, I.; Haque, R.; Bin-Hafeez, B.; Raisuddin, S. Biomarkers of oxidative stress: A comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci. Total Environ. 2003, 309, 105–115. [Google Scholar] [CrossRef]
- Gao, M.; Liu, Y.; Song, Z. Effects of polyethylene microplastic on the phytotoxicity of di-n-butyl phthalate in lettuce (Lactuca sativa L. var. ramosa Hort). Chemosphere 2019, 237, 124482. [Google Scholar] [CrossRef]
- Momeni, H.R.; Eskandari, N. Effect of curcumin on kidney histopathological changes, lipid peroxidation and total antioxidant capacity of serum in sodium arsenite-treated mice. Exp. Toxicol. Pathol. 2017, 69, 93–97. [Google Scholar] [CrossRef]
- Mrozinska, S.; Kapusta, P.; Gosiewski, T.; Sroka-Oleksiak, A.; Ludwig-Słomczyńska, A.H.; Matejko, B.; Kiec-Wilk, B.; Bulanda, M.; Malecki, M.T.; Wolkow, P.P.; et al. The Gut Microbiota Profile According to Glycemic Control in Type 1 Diabetes Patients Treated with Personal Insulin Pumps. Microorganisms 2021, 9, 155. [Google Scholar] [CrossRef]
- Barreau, F.; Tisseyre, C.; Ménard, S.; Ferrand, A.; Carriere, M. Titanium dioxide particles from the diet: Involvement in the genesis of inflammatory bowel diseases and colorectal cancer. Part. Fibre Toxicol. 2021, 18, 26. [Google Scholar] [CrossRef]
- Liang, Y.; Li, C.; Liu, B.; Zhang, Q.; Yuan, X.; Zhang, Y.; Ling, J.; Zhao, L. Protective effect of extracorporeal membrane oxygenation on intestinal mucosal injury after cardiopulmonary resuscitation in pigs. Exp. Ther. Med. 2019, 18, 4347–4355. [Google Scholar] [CrossRef]
- Polinska, B.; Matowicka-Karna, J.; Kemona, H. The cytokines in inflammatory bowel disease. Postep. Hig. I Med. Dosw. 2009, 63, 389–394. [Google Scholar] [PubMed]
- Moldoveanu, A.C.; Diculescu, M.; Braticevici, C.F. Cytokines in inflammatory bowel disease. Rom. J. Intern. Med. = Rev. Roum. De Med. Interne 2015, 53, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.-L.; Sun, R.; Qiao, X.-J.; Xu, C.-C.; Shang, X.-Y.; Niu, W.-N. Protective effect of glutamine on intestinal injury and bacterial community in rats exposed to hypobaric hypoxia environment. World J. Gastroenterol. 2014, 20, 4662–4674. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, H.; Li, Y. Effects of Bacillus subtilis on Epithelial Tight Junctions of Mice with Inflammatory Bowel Disease. J. Interf. Cytokine Res. 2016, 36, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, S.; Zhou, D.; Zhou, S.; Jia, G. Effects of oral exposure to titanium dioxide nanoparticles on gut microbiota and gut-associated metabolism in vivo. Nanoscale 2019, 11, 22398–22412. [Google Scholar] [CrossRef]
- Moldoveanu, B.; Otmishi, P.; Jani, P.; Walker, J.; Sarmiento, X.; Guardiola, J.; Saad, M.; Yu, J. Inflammatory mechanisms in the lung. J. Inflamm. Res. 2009, 2, 1–11. [Google Scholar]
- Abbasi-Oshaghi, E.; Mirzaei, F.; Pourjafar, M. NLRP3 inflammasome, oxidative stress, and apoptosis induced in the intestine and liver of rats treated with titanium dioxide nanoparticles: In vivo and in vitro study. Int. J. Nanomed. 2019, 14, 1919–1936. [Google Scholar] [CrossRef] [Green Version]
- Mu, W.; Wang, Y.; Huang, C.; Fu, Y.; Li, J.; Wang, H.; Jia, X.; Ba, Q. Effect of Long-Term Intake of Dietary Titanium Dioxide Nanoparticles on Intestine Inflammation in Mice. J. Agric. Food Chem. 2019, 67, 9382–9389. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, H.; Zhou, M.; Duan, Y.; Li, N.; Gong, X.; Hu, R.; Hong, M.; Hong, F. Signaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticles. J. Biomed. Mater. Res. Part A 2011, 96, 221–229. [Google Scholar] [CrossRef]
- Kongseng, S.; Yoovathaworn, K.; Wongprasert, K.; Chunhabundit, R.; Sukwong, P.; Pissuwan, D. Cytotoxic and inflammatory responses of TiO2 nanoparticles on human peripheral blood mononuclear cells. J. Appl. Toxicol. 2016, 36, 1364–1373. [Google Scholar] [CrossRef]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory Links Between High Fat Diets and Diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Tang, J.; Wang, L.; Liu, R.; Giesy, J.P. Combined cytotoxicity of polystyrene nanoplastics and phthalate esters on human lung epithelial A549 cells and its mechanism. Ecotoxicol. Environ. Saf. 2021, 213, 112041. [Google Scholar] [CrossRef]
- Thomson, A.; Hemphill, D.; Jeejeebhoy, K. Oxidative Stress and Antioxidants in Intestinal Disease. Dig. Dis. 1998, 16, 152–158. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.M.; Alrokayan, S.; Alhadlaq, H. Oxidative stress mediated cytotoxicity and apoptosis response of bismuth oxide (Bi2O3) nanoparticles in human breast cancer (MCF-7) cells. Chemosphere 2019, 216, 823–831. [Google Scholar] [CrossRef]
- Sohm, B.; Immel, F.; Bauda, P.; Pagnout, C. Insight into the primary mode of action of TiO2 nanoparticles on Escherichia coli in the dark. Proteomics 2015, 15, 98–113. [Google Scholar] [CrossRef]
- Kong, H.; Song, J.; Jang, J. Photocatalytic Antibacterial Capabilities of TiO2−Biocidal Polymer Nanocomposites Synthesized by a Surface-Initiated Photopolymerization. Environ. Sci. Technol. 2010, 44, 5672–5676. [Google Scholar] [CrossRef]
- Schwarzfischer, M.; Rogler, G. The Intestinal Barrier—Shielding the Body from Nano- and Microparticles in Our Diet. Metabolites 2022, 12, 223. [Google Scholar] [CrossRef]
- Tleyjeh, I.M.; Routh, J.; Qutub, M.O.; Lischer, G.; Liang, K.V.; Baddour, L.M. Lactobacillus gasseri causing Fournier’s gangrene. Scand. J. Infect. Dis. 2004, 36, 501–503. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef]
- Rohrhofer, J.; Zwirzitz, B.; Selberherr, E.; Untersmayr, E. The Impact of Dietary Sphingolipids on Intestinal Microbiota and Gastrointestinal Immune Homeostasis. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Wei, X.; Jiang, S.; Zhao, X.; Li, H.; Lin, W.; Li, B.; Lu, J.; Sun, Y.; Yuan, J. Community-Metabolome Correlations of Gut Microbiota from Child-Turcotte-Pugh of A and B Patients. Front. Microbiol. 2016, 7, 1856. [Google Scholar] [CrossRef]




| Groups, n = 10 | Treatments * | Duration |
|---|---|---|
| Control | Normal diet | 8 weeks |
| HFD | High-fat diet | 8 weeks |
| HFD + NPs | High-fat diet + TiO2 NPs | 8 weeks |
| HFD + NPs + BC | High-fat diet + TiO2 NPs + B. coagulans | 8 weeks |
| HFD + NPs&BC | High-fat diet + TiO2 NPs, followed by a high-fat diet + B. coagulans | High-fat diet + TiO2 NPs for 4 weeks, followed by a high-fat diet + B. coagulans MTCC 5856 for 4 weeks |
| Group | Domain | Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|---|---|
| Control | 1 | 22 | 60 | 123 | 171 | 244 | 93 |
| HFD | 1 | 23 | 54 | 117 | 173 | 275 | 111 |
| HFD + NPs | 1 | 13 | 17 | 37 | 55 | 138 | 81 |
| HFD + NPs + BC | 1 | 13 | 19 | 41 | 56 | 145 | 80 |
| HFD + NPs&BC | 1 | 13 | 18 | 43 | 72 | 163 | 109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; Yang, C.; Zhang, B.; Chen, D.; Lu, F.; Zhao, H. Bacillus coagulans Alleviates Intestinal Damage Induced by TiO2 Nanoparticles in Mice on a High-Fat Diet. Foods 2022, 11, 3368. https://doi.org/10.3390/foods11213368
Shi Q, Yang C, Zhang B, Chen D, Lu F, Zhao H. Bacillus coagulans Alleviates Intestinal Damage Induced by TiO2 Nanoparticles in Mice on a High-Fat Diet. Foods. 2022; 11(21):3368. https://doi.org/10.3390/foods11213368
Chicago/Turabian StyleShi, Qingying, Chen Yang, Bingjie Zhang, Dongxiao Chen, Fuping Lu, and Huabing Zhao. 2022. "Bacillus coagulans Alleviates Intestinal Damage Induced by TiO2 Nanoparticles in Mice on a High-Fat Diet" Foods 11, no. 21: 3368. https://doi.org/10.3390/foods11213368






