A New Strategy Based on LC-Q TRAP-MS for Determining the Distribution of Polyphenols in Different Apple Varieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Sample Preparation
2.4. HPLC Conditions
2.5. ESI-Q TRAP-MS/MS
2.6. LIT Experiments
2.7. QQQ Experiments
2.8. Data Processing and Statistical Analysis
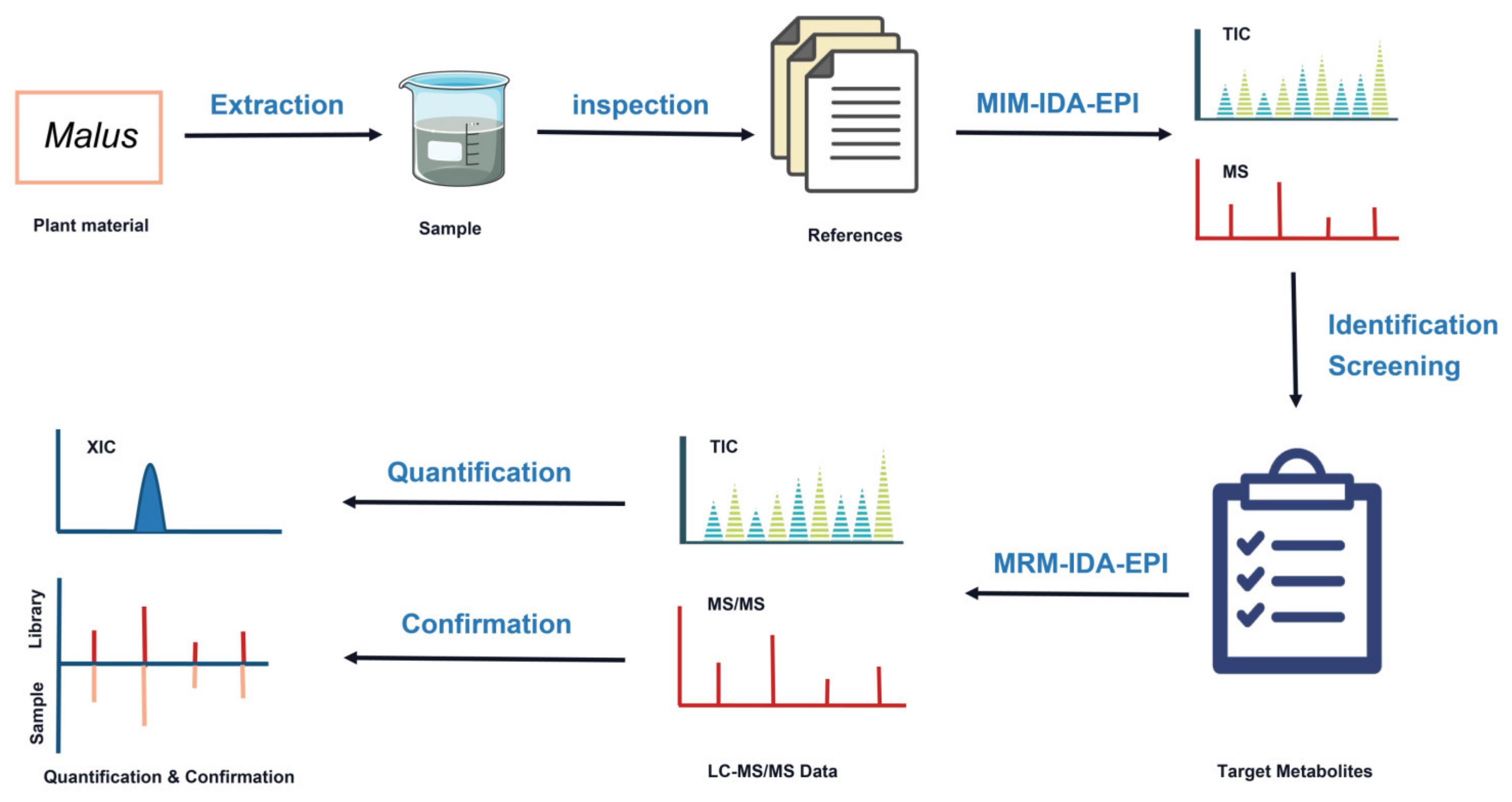
3. Results and Discussion
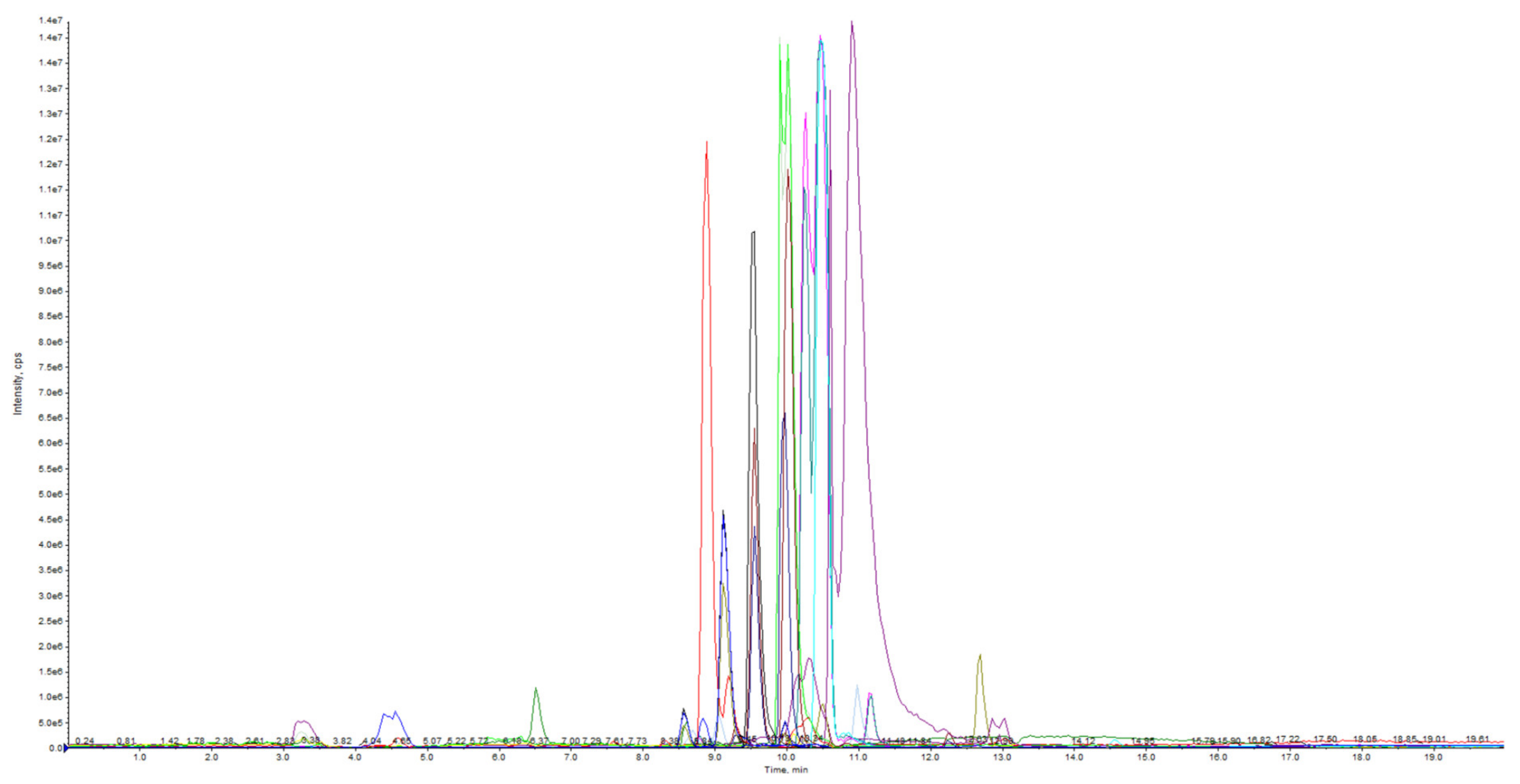
3.1. Optimization of Chromatographic Conditions for LC-Q TRAP-MS
3.2. Identification of Apple Polyphenol Components and MS2 Spectral Tag (MS2T) Library Construction
3.3. Targeted Quantification and Validation of Apple Polyphenols
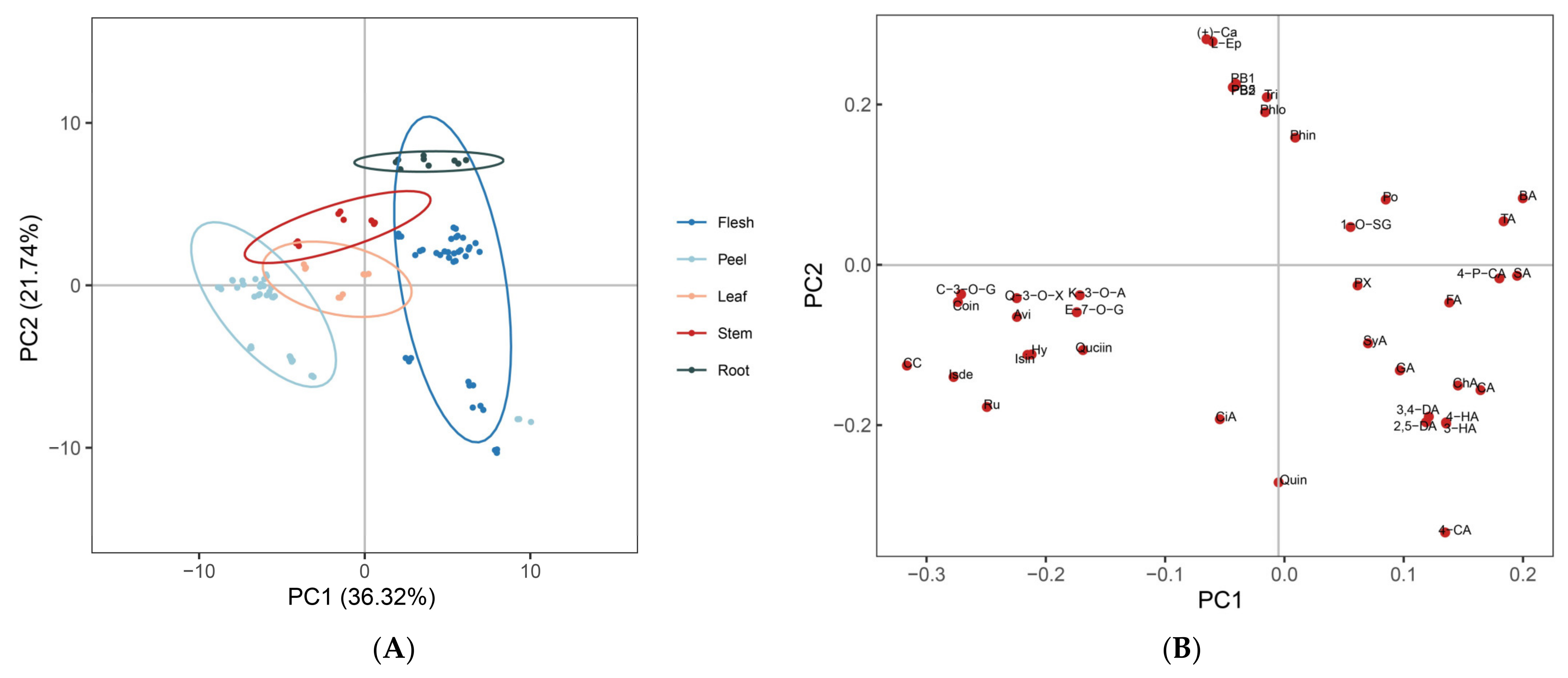
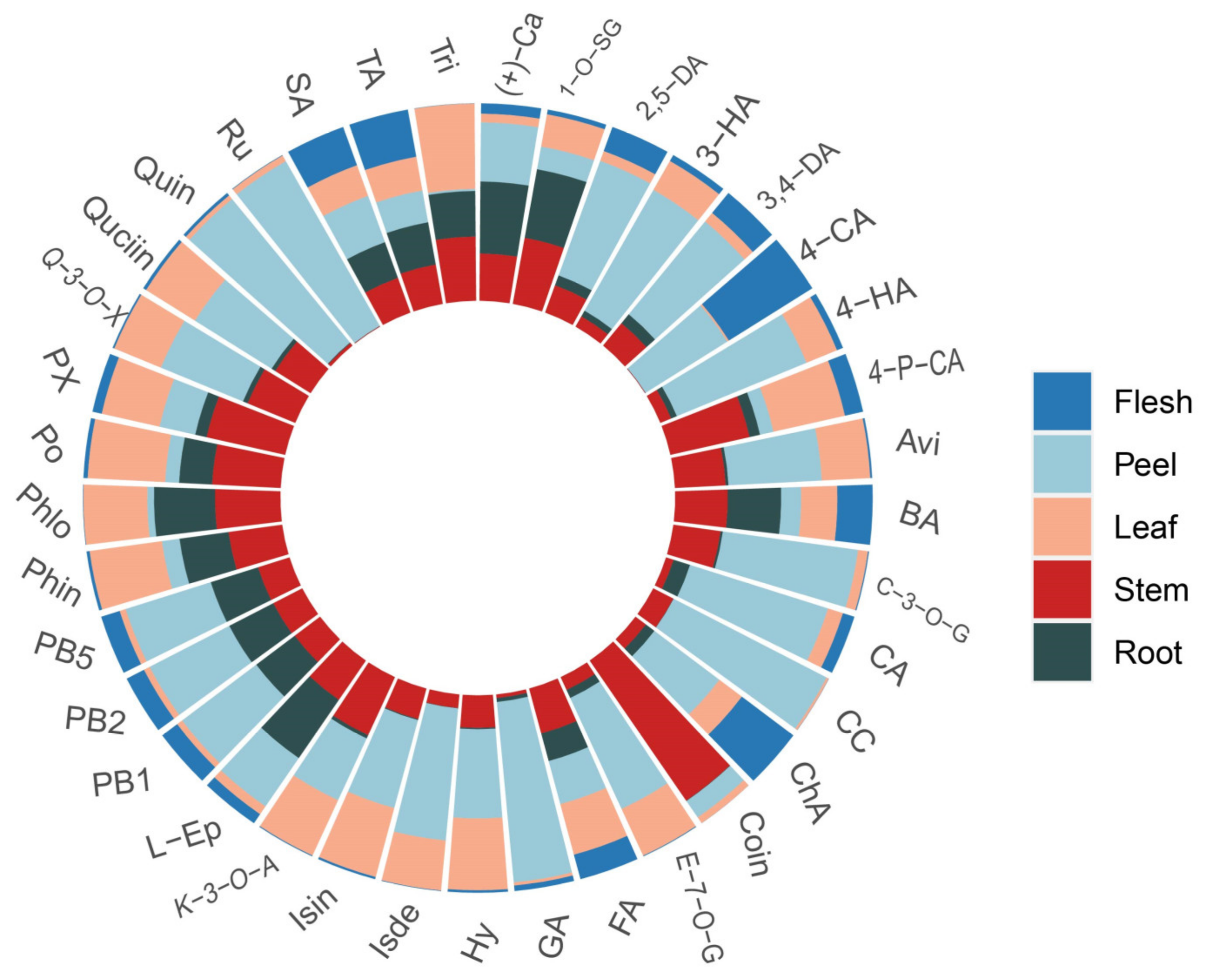
3.4. Distribution of Polyphenolic Compounds in Different Varieties and Tissues of Apple
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petkovsek, M.M.; Slatnar, A.; Stampar, F.; Veberic, R. The influence of organic/integrated production on the content of phenolic compounds in apple leaves and fruits in four different varieties over a 2-year period. J. Sci. Food Agric. 2010, 90, 2366–2378. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Flores, M.I.; Romero-González, R.; Martínez Vidal, J.L.; Garrido Frenich, A. Evaluation of the Presence of Phenolic Compounds in Different Varieties of Apple by Ultra-High-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Food Anal. Methods 2015, 8, 696–709. [Google Scholar] [CrossRef]
- Hertog, M.G.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995, 155, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Pedreschi, R.; Yuan, J.; Kawas, J.R.; Chew, B.; Dowd, S.E.; Noratto, G. Apple consumption is associated with a distinctive microbiota, proteomics and metabolomics profile in the gut of Dawley Sprague rats fed a high-fat diet. PLoS ONE 2019, 14, e0212586. [Google Scholar] [CrossRef] [PubMed]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Sowa, A.; Zgórka, G.; Szykuła, A.; Franiczek, R.; Żbikowska, B.; Gamian, A.; Sroka, Z. Analysis of Polyphenolic Compounds inExtracts from Leaves of Some Malus domestica Cultivars: Antiradical and Antimicrobial Analysis of These Extracts. Biomed. Res. Int. 2016, 2016, 6705431. [Google Scholar] [CrossRef] [Green Version]
- Roopchand, D.E.; Krueger, C.G.; Moskal, K.; Fridlender, B.; Lila, M.A.; Raskin, I. Foodcompatible method for the efficient extraction and stabilization of cranberry pomace polyphenols. Food Chem. 2013, 141, 3664–3669. [Google Scholar] [CrossRef] [Green Version]
- Korkina, L.G.; Pastore, S.; Luca, C.; Kostyuk, V.A. Metabolism of Plant Polyphenols in the Skin: Beneficial Versus Deleterious Effects. Curr. Drug Metab. 2008, 9, 710–729. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 188. [Google Scholar] [CrossRef]
- Van der Sluis, A.A.; Dekker, M.; de Jager, A.; Jongen Wim, M.F. Activity and concentration of polyphenolic antioxidants in apple: Effect of cultivar, harvest year, and storage conditions. J. Agric. Food Chem. 2001, 49, 3606e13. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, Y.J.; Kim, D.O.; Lee, H.J.; Lee, C.Y. Major phenolics in apple and their contribution to the total antioxidant capacity. J. Agric. Food Chem. 2003, 51, 6516–6520. [Google Scholar] [CrossRef]
- Carbone, K.; Giannini, B.; Picchi, V.; Lo Scalzo, R.; Cecchini, F. Phenolic composition and free radical scavenging activity of different apple varieties in relation to the cultivar, tissue type and storage. Food Chem. 2011, 127, 493–500. [Google Scholar] [CrossRef]
- Bai, L.; Guo, S.; Liu, Q.; Cui, X.; Zhang, X.; Zhang, L.; Yang, X.; Hou, M.; Ho, C.T.; Bai, N. Characterization of nine polyphenols in fruits of Malus pumila Mill by high-performance liquid chromatography. J. Food Drug Anal. 2016, 24, 293–298. [Google Scholar] [CrossRef]
- Mari, A.; Tedesco, I.; Nappo, A.; Russo, G.L.; Malorni, A.; Carbone, V. Phenolic compound characterization and antiproliferative activity of ‘Annurca’ apple, a southern Italian cultivar. Food Chem. 2010, 123, 157–164. [Google Scholar] [CrossRef]
- Barbara, Ł.; Trampczynska, A.; Paczesna, J. Cultivar variation in apple peel and whole fruit phenolic composition. Sci. Hortic. 2009, 121, 176–181. [Google Scholar]
- Bottcher, C.; von Roepenack-Lahaye, E.; Schmidt, J.; Schmotz, C.; Neumann, S.; Scheel, D.; Clemens, S. Metabolome analysis of biosynthetic mutants reveals a diversity of metabolic changes and allows identification of a large number of new compounds in Arabidopsis. Plant Physiol. 2008, 147, 2107–2120. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, Q.; Zheng, Y.; Zhou, P.; Xu, X.; Liu, X.; Zhao, L.; Liu, H. Development of a mass spectrometry-based pseudotargeted metabolomics strategy to analyze hormone-stimulated gastric cancer cells. J. Pharm. Biomed. Anal. 2020, 180, 113041. [Google Scholar] [CrossRef]
- Shao, Y.; Zhu, B.; Zheng, R.; Zhao, X.; Yin, P.; Lu, X.; Jiao, B.; Xu, G.; Yao, Z. Development of urinary pseudotargeted LC-MS-based metabolomics method and its application in hepatocellular carcinoma biomarker discovery. J. Proteome Res. 2015, 6, 14, 906–916. [Google Scholar] [CrossRef]
- Chen, S.; Kong, H.; Lu, X.; Li, Y.; Yin, P.; Zeng, Z.; Xu, G. Pseudotargeted metabolomics method and its application in serum biomarker discovery for hepatocellular carcinoma based on ultra high-performance liquid chromatography/triple quadrupole mass spectrometry. Anal. Chem. 2013, 85, 8326–8333. [Google Scholar] [CrossRef]
- Rojo, D.; Barbas, C.; Rupeérez, F. LC-MS metabolomics of polar compounds. Bioanalysis 2012, 4, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Bantscheff, M.; Schirle, M.; Sweetman, G.; Rick, J.; Kuster, B. Quantitative mass spectrometry in proteomics: A critical review. Anal. Bioanal. Chem. 2007, 389, 1017–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, E.; Tautenhahn, R.; Neumann, S.; Gropl, C. Critical assessment of alignment procedures for LC-MS proteomics and metabolomics measurements. BMC Bioinf. 2008, 9, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, B.; Chen, B.; Liu, A.; Zhu, W.; Yao, S. Liquid chromatography-mass spectrometric multiple reaction monitoring-based strategies for expanding targeted profiling towards quantitative metabolomics. Curr. Drug Metab. 2012, 13, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Z.; Yu, R.; Zheng, T.; Sun, D.; Zhou, Y.; Tang, J.; Zhu, H.; Niu, L.; Cui, L.; Du, R.; et al. A Novel Strategy for Unveiling Spatial Distribution Pattern of Gallotannins in Paeonia rockii and Paeonia ostii Based on LC-QTRAP-MS. Metabolites 2022, 12, 326. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhang, J.; Tang, Z.W.; Wang, Y.P.; Gao, T.T.; Liu, X.M.; Ma, F.W.; Li, C. Tissue distribution and changes in dopamine during development and stress responses in Malus germplasm. J. Integr. Agric. 2022, 21, 710–724. [Google Scholar] [CrossRef]
- Sun, L.; Tao, S.; Zhang, S. Characterization and Quantification of Polyphenols and Triterpenoids in Thinned Young Fruits of Ten Pear Varieties by UPLC-Q TRAP-MS/MS. Molecules 2019, 24, 159. [Google Scholar] [CrossRef] [Green Version]
- Hager, J.W.; Le Blanc, J.C. High-performance liquid chromatography-tandem mass spectrometry with a new quadrupole/linear ion trap instrument. J. Chromatogr. A 2003, 1020, 3–9. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, W.; Zhang, B.; Cai, Y.; Han, Y. Evaluation of chlorogenic acid accumulation in cultivated and wild apples. J. Food Compost. Anal. 2021, 104, 104156. [Google Scholar] [CrossRef]
- Usenik, V.; Mikuli-Petkovšek, M.; Solar, A.; Štampar, F. Flavonols of leaves in relation to apple scab resistance. J. Plant Dis. Prot. 2004, 111, 137–144. [Google Scholar] [CrossRef]
- Hock, B.; Elstner, E. Der Einfluß von Schadstoffen und Schadwirkungen auf Pflanzen. J. Soil Sci. Plant Nut. 1988, 150, 124. [Google Scholar]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Holderbaum, D.F.; Kon, T.; Guerra, M.P. Dynamics of total phenolic content in different apple tissues and genotypes: Impacts and relevance for breeding programs. Sci. Hortic. 2014, 168, 58–63. [Google Scholar] [CrossRef]
- Illiano, A.; Pinto, G.; Carrera, M.A.; Palmese, A.; Di Novella, R.; Casoria, P.; Amoresano, A. LC-MS/MS-Based Quantification Method of Polyphenols for Valorization of Ancient Apple Cultivars from Cilento. ACS Food Sci. Technol. 2022, 2, 647–654. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq, S.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Mei, Y.; Sun, H.; Du, G.; Wang, X.; Lyu, D. Exogenous chlorogenic acid alleviates oxidative stress in apple leaves by enhancing antioxidant capacity. Sci. Hortic. 2020, 274, 109676. [Google Scholar] [CrossRef]
- Wang, L.J.; Li, J.H.; Gao, J.J.; Feng, X.X.; Shi, Z.X.; Gao, F.Y.; Xu, X.L.; Yang, L.Y. Inhibitory effect of chlorogenic acid on fruit russeting in ‘Golden Delicious’ apple. Sci. Hortic. 2014, 178, 14–22. [Google Scholar] [CrossRef]
- Zhang, D.; Bi, W.; Kai, K.; Ye, Y.; Liu, J. Effect of chlorogenic acid on controlling kiwifruit postharvest decay caused by Diaporthe sp. LWT 2020, 132, 109805. [Google Scholar] [CrossRef]
- Smailagić, D.; Banjac, N.; Ninković, S.; Savić, J.; Ćosić, T.; Pěnčík, A.; Ćalić, D.; Bogdanović, M.; Trajković, M.; Stanišić, M. New Insights into the Activity of Apple Dihydrochalcone Phloretin: Disturbance of Auxin Homeostasis as Physiological Basis of Phloretin Phytotoxic Action. Front. Plant Sci. 2022, 13, 875528. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073–2085. [Google Scholar] [CrossRef] [PubMed]

| Metabolite Name | RT (min) | Q1 | Q3 | DP | CE | Metabolite Name | RT (min) | Q1 | Q3 | DP | CE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4-P-coumaroylquinic acid | 9.46 | 337 | 173 | −50 | −20 | Caffeic acid | 10.14 | 179 | 134.9 | −73.13 | −21 |
| 1-O-Sinapoyl-β-D-glucose | 8.62 | 385 | 223 | −90 | −20 | Chlorogenic acid | 8.95 | 353 | 191 | −64 | −22 |
| 3,4-Dihydroxybenzoic acid | 9.03 | 153 | 109 | −30 | −20 | Cinnamic acid | 6.53 | 147 | 103 | −20 | −15 |
| 4-caffeoylquinic acid | 4.10 | 353 | 173 | −50 | −20 | Ferulic acid | 11.35 | 193 | 148.9 | −67.52 | −20.33 |
| 4-Hydroxycinnamic acid | 11.08 | 163 | 119 | −60 | −20 | Gallic acid | 11.47 | 169 | 125 | −27 | −19 |
| Avicularin | 10.46 | 433 | 300 | −60 | −35 | Hyperoside | 10.06 | 463 | 300 | −48 | −37 |
| Benzoic acid | 9.84 | 121.9 | 92 | −52.02 | −16.06 | Phlorizin | 10.61 | 435 | 273 | −175 | −23 |
| Isoquercitrin | 10.00 | 463 | 300 | −20 | −35 | Polydatin | 10.13 | 389 | 227 | −188 | −20 |
| Isoquercitroside | 9.97 | 463.38 | 271 | −90 | −39 | Procyanidin B1 | 9.67 | 577 | 407 | −50 | −30 |
| Kaempferol 3-O-arabinoside | 10.92 | 417 | 285 | −90 | −25 | procyanidin B5 | 9.22 | 577 | 289 | −70 | −35 |
| L-Epicatechin | 9.58 | 289 | 245 | −125.1 | −25.79 | Procyanidin B2 | 9.07 | 577 | 289 | −60 | −35 |
| Eriodictyol-7-O-glucoside | 11.07 | 449 | 287 | −60 | −50 | Terephthalic acid | 3.32 | 165 | 121 | −90 | −15 |
| Phloretin xyloglucoside | 10.77 | 567 | 273 | −40 | −35 | 3−Hydroxycinnamic acid | 11.08 | 163 | 119 | −40 | −20 |
| Quercetin | 12.62 | 301 | 151 | −123 | −28 | Phloretin | 13.08 | 275 | 169 | 20 | 20 |
| Quercetin 3-O-β-D-xylopyranoside | 10.48 | 433 | 301 | −90 | −30 | Cosmosiin | 8.39 | 433 | 271 | 60 | 25 |
| Quercitrin | 10.49 | 447 | 300 | −90 | −35 | Cyanidin 3-O-glucoside | 8.29 | 449 | 287 | 10 | 27 |
| Salicylic acid | 10.15 | 137.3 | 92.8 | −99.31 | −25.85 | Rutin | 9.51 | 611 | 303 | 80 | 25 |
| Syringic acid | 6.24 | 197.9 | 153 | −53.16 | −17.29 | Trilobatin | 10.67 | 437.3 | 275.1 | 80 | 15 |
| (+)-Catechin | 9.58 | 289 | 203 | −35.99 | −26.8 | Cyanidin Chloride | 9.63 | 287 | 213 | 115 | 43 |
| 2,5-Dihydroxybenzoic acid | 9.07 | 153 | 109 | −67.97 | −27.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Bai, Z.; Zhu, H.; Zheng, T.; Chen, X.; Li, P.; Zhang, J.; Ma, F. A New Strategy Based on LC-Q TRAP-MS for Determining the Distribution of Polyphenols in Different Apple Varieties. Foods 2022, 11, 3390. https://doi.org/10.3390/foods11213390
Wang M, Bai Z, Zhu H, Zheng T, Chen X, Li P, Zhang J, Ma F. A New Strategy Based on LC-Q TRAP-MS for Determining the Distribution of Polyphenols in Different Apple Varieties. Foods. 2022; 11(21):3390. https://doi.org/10.3390/foods11213390
Chicago/Turabian StyleWang, Minyan, Zhangzhen Bai, Huili Zhu, Tiantian Zheng, Xiujiao Chen, Pengmin Li, Jing Zhang, and Fengwang Ma. 2022. "A New Strategy Based on LC-Q TRAP-MS for Determining the Distribution of Polyphenols in Different Apple Varieties" Foods 11, no. 21: 3390. https://doi.org/10.3390/foods11213390






