Effect of Limited Enzymatic Hydrolysis on Structural and Functional Properties of Elaeagnus mollis Oil Meal Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of EMO Meal Protein Isolates
2.3. Preparation of EMO Meal Protein Hydrolysates
2.4. Estimation of the Degree of Hydrolysis (DH)
2.5. Structural Properties of Hydrolysates
2.5.1. Sodium Dodecyl Sulfide Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.5.2. Analysis of Sulfhydryl and Disulfide Contents
2.5.3. Surface Hydrophobicity
2.5.4. Intrinsic Fluorescence and Ultra-Violet (UV) Spectroscopy
2.5.5. Fourier-Transform Infrared (FTIR) Spectroscopy
2.6. Functional Properties
2.6.1. Solubility
2.6.2. Water and Oil-Holding Capacities
2.6.3. Determination of Foaming Capacity and Foaming Stability
2.6.4. Determination of Emulsifying Capacity and Emulsifying Stability
2.7. Statistical Analyses
3. Results and Discussion
3.1. SDS-PAGE Analysis
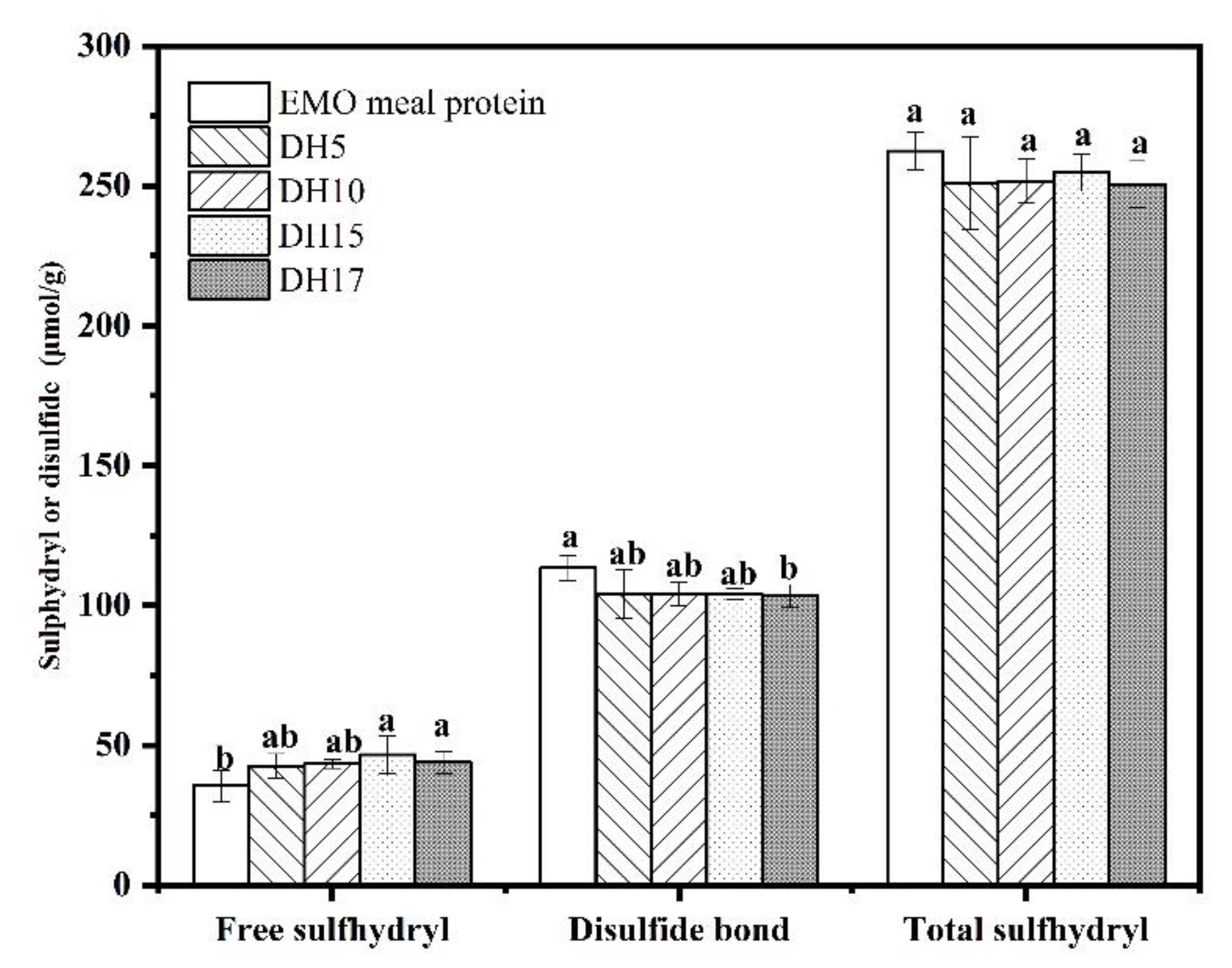
3.2. SHF and S-S Content
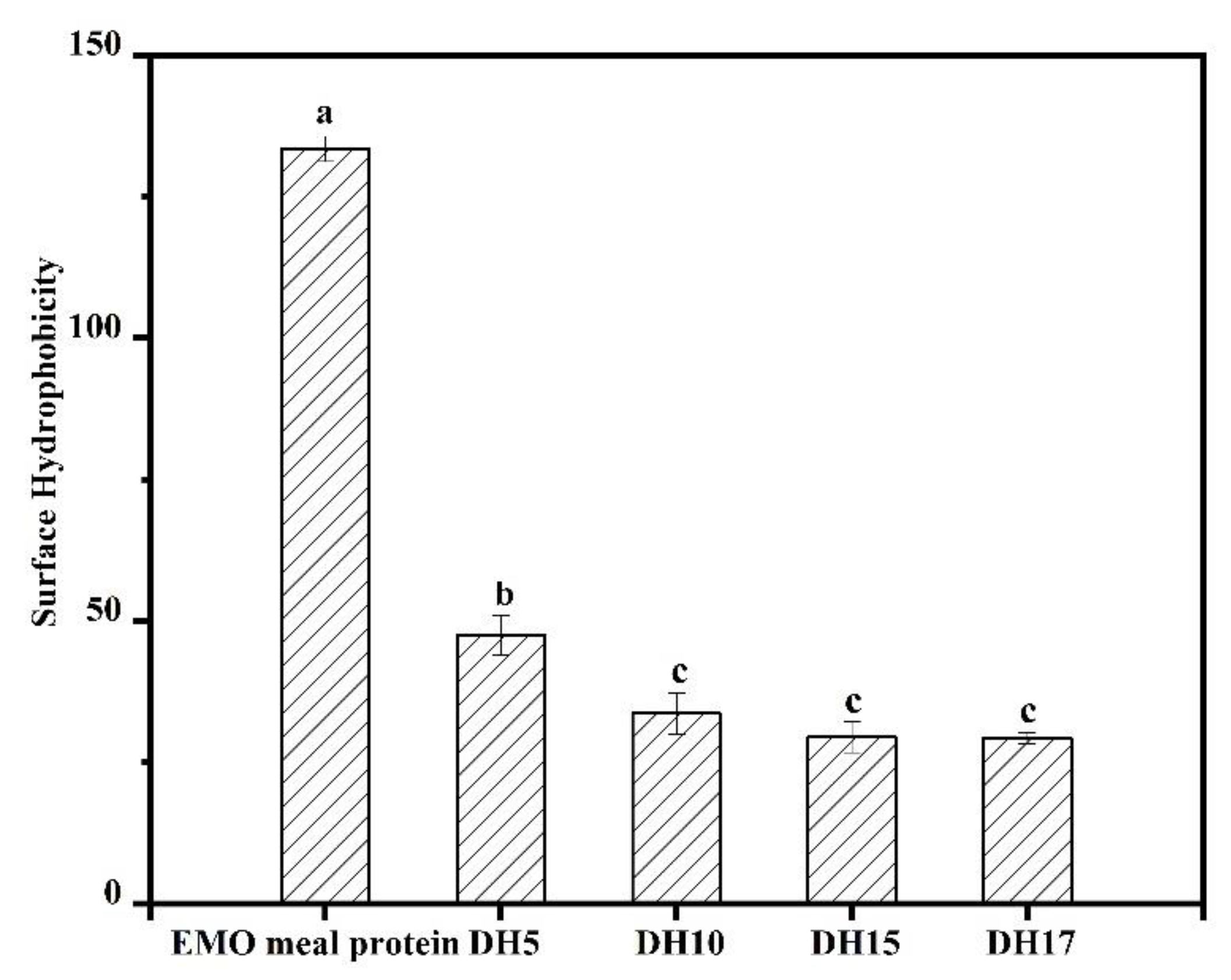
3.3. Surface Hydrophobicity
3.4. Intrinsic Fluorescence and Ultraviolet (UV) Spectra
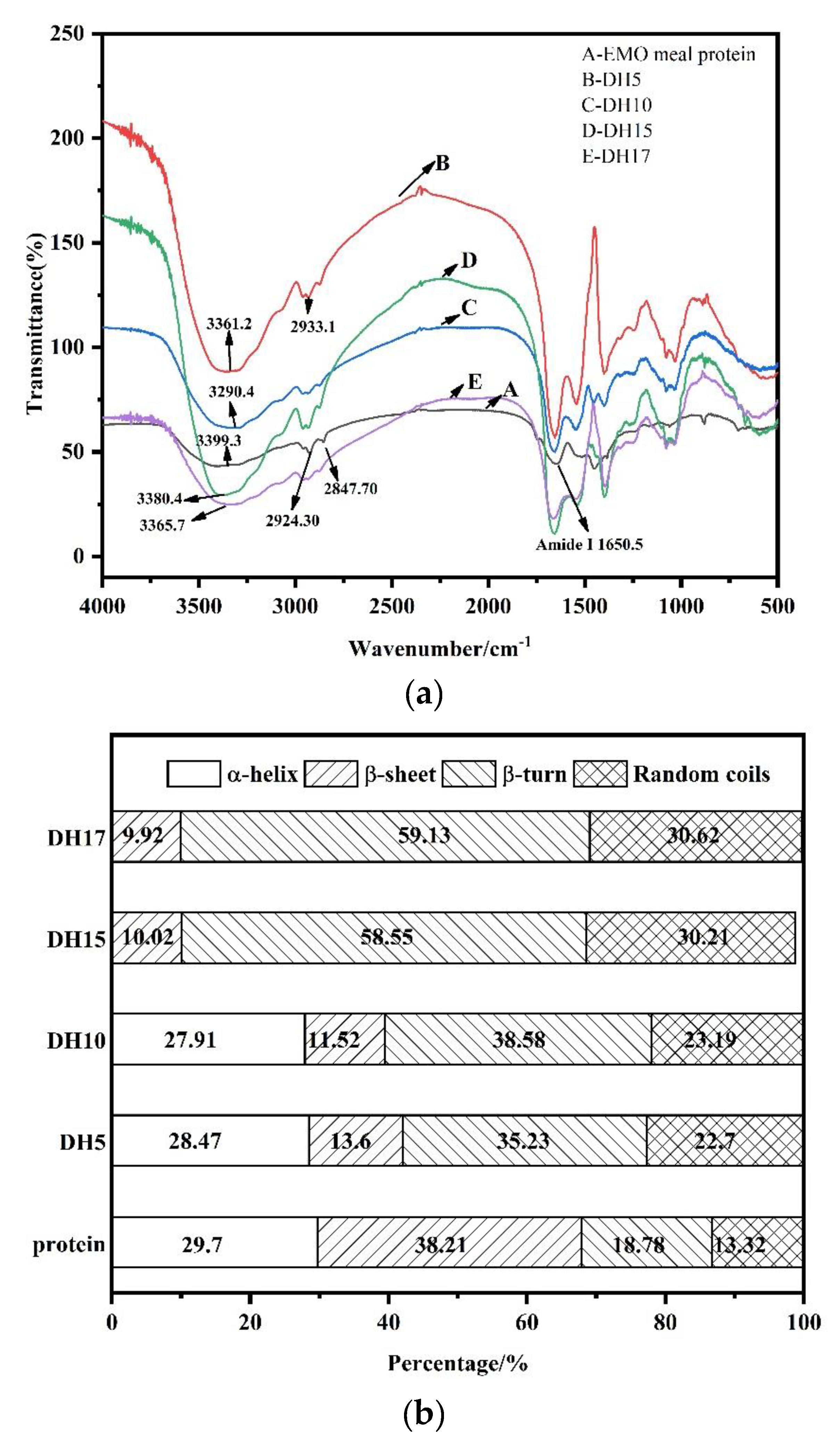
3.5. FTIR Spectrum Analysis
3.6. Analysis of Functional Properties
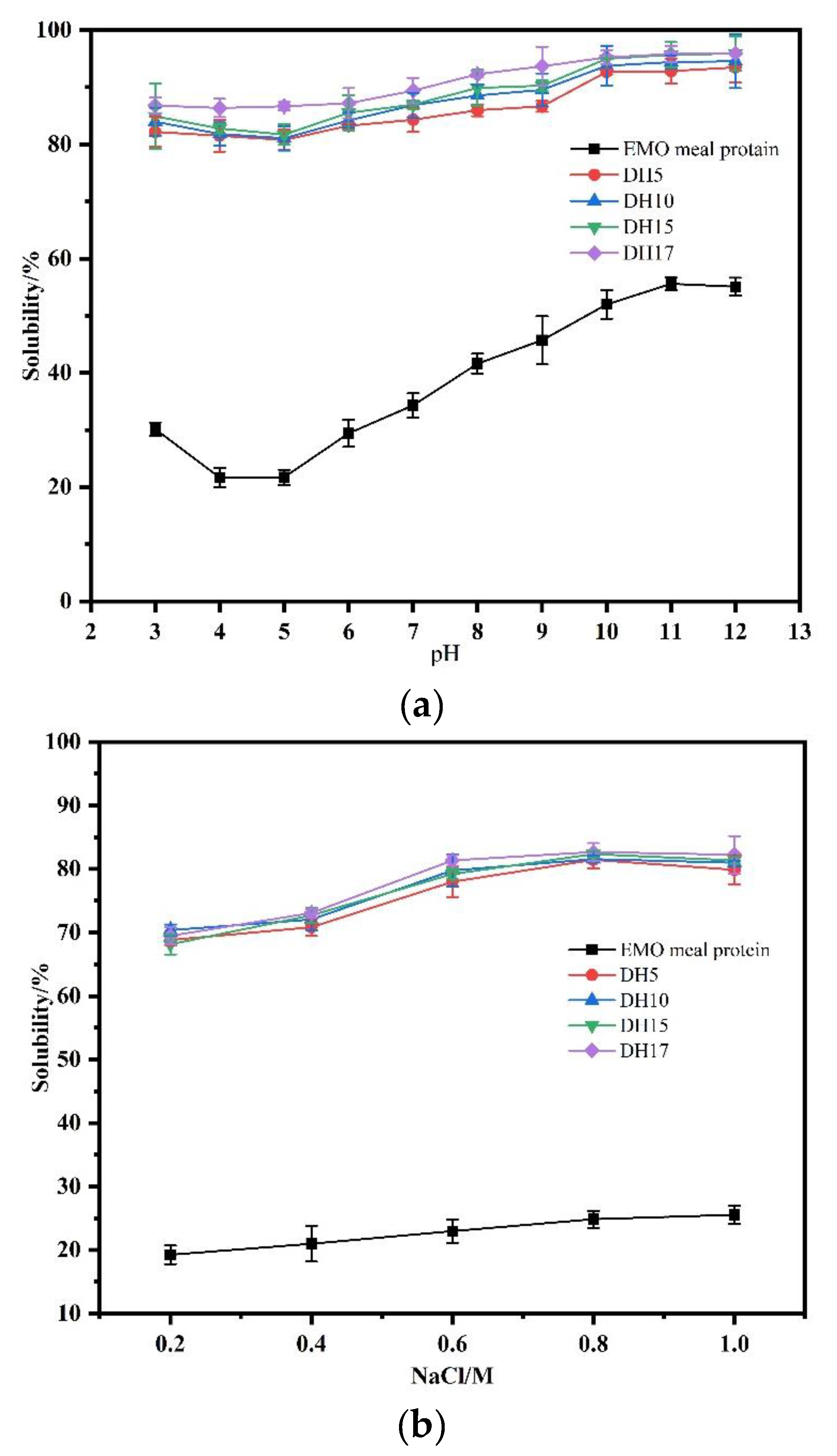
3.6.1. Solubility
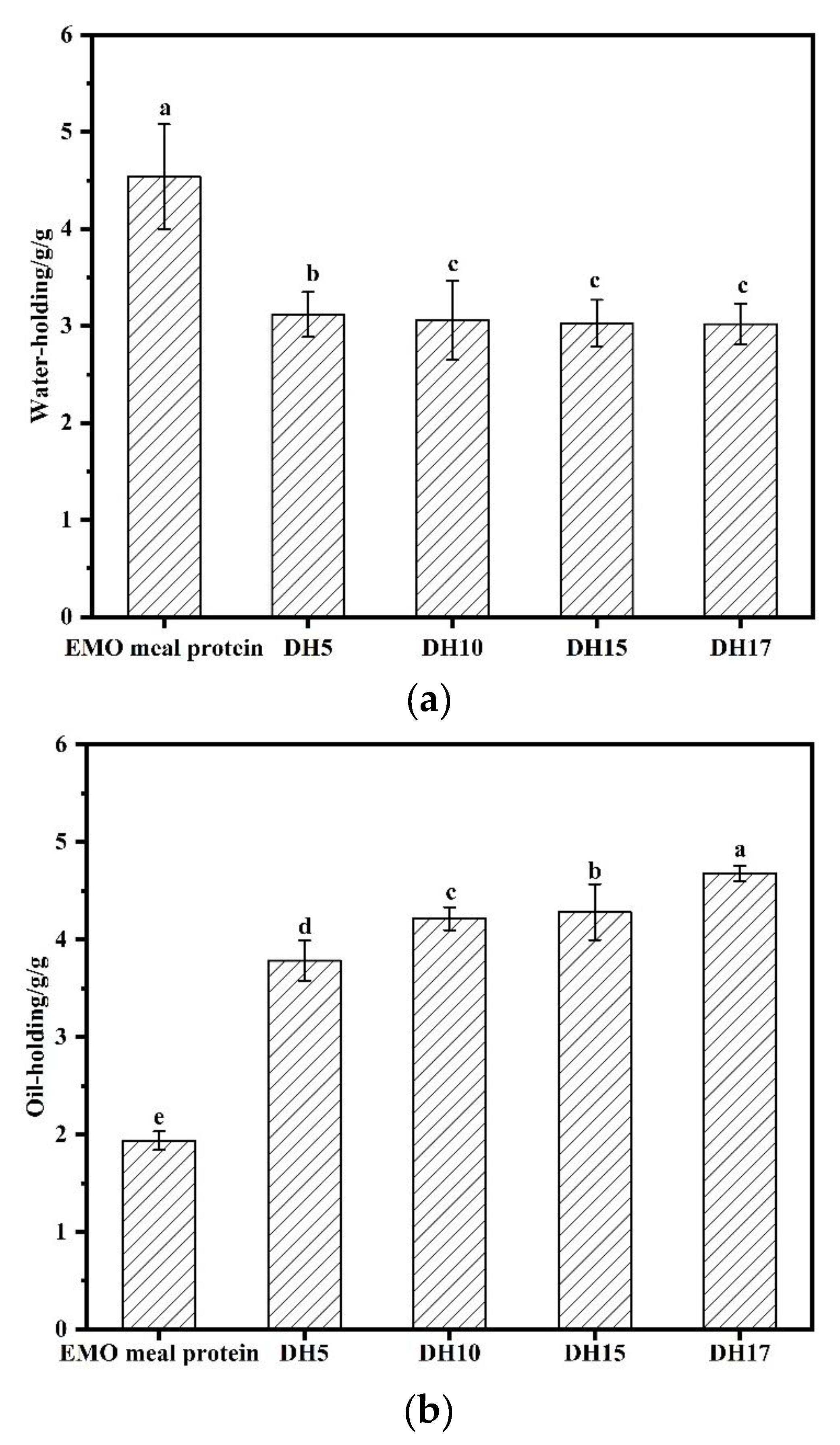
3.6.2. Water and Oil-Holding Capacities
3.6.3. Foaming Capacity and Foam Stability
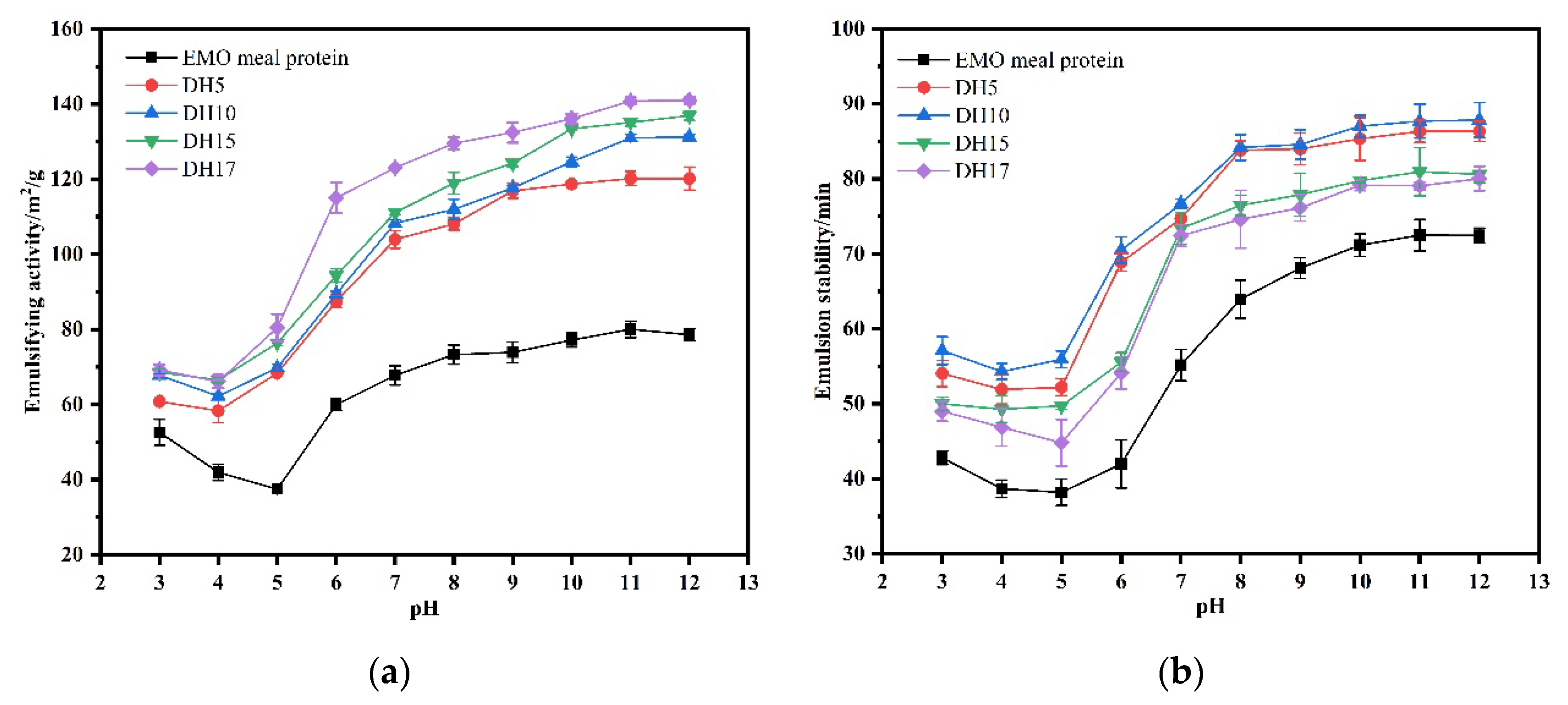
3.6.4. Emulsifying Capacity and Emulsifying Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moghadam, M.; Salami, M.; Mohammadian, M.; Emam-Djomeh, Z.; Jahanbani, R.; Moosavi-Movahedi, A.A. Physicochemical and bio-functional properties of walnut proteins as affected by trypsin-mediated hydrolysis. Food Biosci. 2020, 36, 100611. [Google Scholar] [CrossRef]
- Jin, F.; Wang, Y.; Tang, H.; Regenstein, J.M.; Wang, F. Limited hydrolysis of dehulled walnut (Juglans regia L.) proteins using trypsin: Functional properties and structural characteristics. LWT 2020, 133, 110035. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Sahin, A.W.; Arendt, E.K.; Zannini, E. Enzymatic Hydrolysis of Pulse Proteins as a Tool to Improve Techno-Functional Properties. Foods 2022, 11, 1307. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Huang, J.; Woo, M.W.; Hu, J.; Xiong, H.; Zhao, Q. Effect of cold and hot enzyme deactivation on the structural and functional properties of rice dreg protein hydrolysates. Food Chem. 2021, 345, 128784. [Google Scholar] [CrossRef] [PubMed]
- Nisov, A.; Ercili-Cura, D.; Nordlund, E. Limited hydrolysis of rice endosperm protein for improved techno-functional properties. Food Chem. 2020, 302, 125274. [Google Scholar] [CrossRef] [PubMed]
- García Arteaga, V.; Apéstegui Guardia, M.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Effect of enzymatic hydrolysis on molecular weight distribution, techno-functional properties and sensory perception of pea protein isolates. Innov. Food Sci. Emerg. Technol. 2020, 65, 102449. [Google Scholar] [CrossRef]
- Avramenko, N.A.; Low, N.H.; Nickerson, M.T. The effects of limited enzymatic hydrolysis on the physicochemical and emulsifying properties of a lentil protein isolate. Food Res. Int. 2013, 51, 162–169. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Liu, C.; Luo, L.; Chen, J.; Luo, S.; McClements, D.J.; Wu, L. Effect of limited enzymatic hydrolysis on structure and emulsifying properties of rice glutelin. Food Hydrocoll. 2016, 61, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Li, J.; Liu, Y. Effects of partial hydrolysis on the structural, functional and antioxidant properties of oat protein isolate. Food Funct. 2020, 11, 3144–3155. [Google Scholar] [CrossRef]
- Guo, C.; Qiao, J.; Zhang, S.; Li, M.; Li, J.; Hatab, S. Elaeagnus mollis Oil Attenuates Non-alcoholic Fatty Disease in High-fat Diet Induced Obese Mice via Modifying the Expression of Lipid Metabolismrelated Genes. J. Oleo Sci. 2019, 68, 893–908. [Google Scholar] [CrossRef]
- Rutherfurd, S.M. Methodology for determining degree of hydrolysis of proteins in hydrolysates: A review. J. AOAC Int. 2010, 93, 1515–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.B.; Lu, Q.Y. Characterizing the structural and surface properties of proteins isolated before and after enzymatic demulsification of the aqueous extract emulsion of peanut seeds. Food Hydrocoll. 2015, 47, 51–60. [Google Scholar] [CrossRef]
- Classics Lowry, O.; Rosebrough, N.; Farr, A.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Li, M.; Wen, X.; Peng, Y.; Wang, Y.; Wang, K.; Ni, Y. Functional properties of protein isolates from bell pepper (Capsicum annuum L. var. annuum) seeds. LWT 2018, 97, 802–810. [Google Scholar] [CrossRef]
- López, D.N.; Ingrassia, R.; Busti, P.; Bonino, J.; Delgado, J.F.; Wagner, J.; Boeris, V.; Spelzini, D. Structural characterization of protein isolates obtained from chia (Salvia hispanica L.) seeds. LWT 2018, 90, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Du, M.; Xie, J.; Gong, B.; Xu, X.; Tang, W.; Li, X.; Li, C.; Xie, M. Extraction, physicochemical characteristics and functional properties of Mung bean protein. Food Hydrocoll. 2018, 76, 131–140. [Google Scholar] [CrossRef]
- Jain, S.; Anal, A.K. Optimization of extraction of functional protein hydrolysates from chicken egg shell membrane (ESM) by ultrasonic assisted extraction (UAE) and enzymatic hydrolysis. LWT Food Sci. Technol. 2016, 69, 295–302. [Google Scholar] [CrossRef]
- Huang, T.; Bu, G.; Chen, F. The influence of composite enzymatic hydrolysis on the antigenicity of β-conglycinin in soy protein hydrolysates. J. Food Biochem. 2018, 42, e12544. [Google Scholar] [CrossRef]
- Zhang, Y.; Romero, H.M. Exploring the structure-function relationship of Great Northern and navy bean (Phaseolus vulgaris L.) protein hydrolysates: A study on the effect of enzymatic hydrolysis. Int. J. Biol. Macromol. 2020, 162, 1516–1525. [Google Scholar] [CrossRef]
- Guo, X.; Sun, X.; Zhang, Y.; Wang, R.; Yan, X. Interactions between soy protein hydrolyzates and wheat proteins in noodle making dough. Food Chem. 2018, 245, 500–507. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Li, S. Physicochemical and functional properties of Chinese quince seed protein isolate. Food Chem. 2019, 283, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Huang, L.; Tao, X.; Su, J.; Chen, B.; Zhao, M.; Zhao, Q.; Van der Meeren, P. Adjustment of the structural and functional properties of okara protein by acid precipitation. Food Biosci. 2020, 37, 100677. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Effects of divergent ultrasound pretreatment on the structure of watermelon seed protein and the antioxidant activity of its hydrolysates. Food Chem. 2019, 299, 125165. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, C.; Ma, W.; Wang, Z.; Yu, C.; Du, M. Effects of ultrasound treatment on the physicochemical and emulsifying properties of proteins from scallops (Chlamys farreri). Food Hydrocoll. 2019, 89, 707–714. [Google Scholar] [CrossRef]
- Carullo, D.; Donsì, F.; Ferrari, G. Influence of high-pressure homogenization on structural properties and enzymatic hydrolysis of milk proteins. LWT 2020, 130, 109657. [Google Scholar] [CrossRef]
- Shen, P.; Zhou, F.; Zhang, Y.; Yuan, D.; Zhao, Q.; Zhao, M. Formation and characterization of soy protein nanoparticles by controlled partial enzymatic hydrolysis. Food Hydrocoll. 2020, 105, 105844. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Z.; Li, Y.; Meng, X.; Sui, X.; Qi, B.; Zhou, L. Relationship between surface hydrophobicity and structure of soy protein isolate subjected to different ionic strength. Int. J. Food Prop. 2015, 18, 1059–1074. [Google Scholar] [CrossRef]
- Evangelho, J.A.D.; Vanier, N.L.; Pinto, V.Z.; Berrios, J.J.D.; Dias, A.R.G.; Zavareze, E.D.R. Black bean (Phaseolus vulgaris L.) protein hydrolysates: Physicochemical and functional properties. Food Chem. 2017, 214, 460–467. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, J.; Li, J.; Sun, H.; Liu, Y. Physicochemical and antioxidative characteristics of black bean protein hydrolysates obtained from different enzymes. Food Hydrocoll. 2019, 97, 105222. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Zhang, Y.; Ma, H.; Liang, Q.; Qu, W.; He, R.; Zhou, C.; Mahunu, G.K. Effects of ultrasound and ultrasound assisted alkaline pretreatments on the enzymolysis and structural characteristics of rice protein. Ultrason. Sonochem. 2016, 31, 20–28. [Google Scholar] [CrossRef]
- Jiménez, A.; Sánchez-González, L.; Desobry, S.; Chiralt, A.; Tehrany, E.A. Influence of nanoliposomes incorporation on properties of film forming dispersions and films based on corn starch and sodium caseinate. Food Hydrocoll. 2014, 35, 159–169. [Google Scholar] [CrossRef]
- Jain, A.; Subramanian, R.; Manohar, B.; Radha, C. Preparation, characterization and functional properties of Moringa oleifera seed protein isolate. J. Food Sci. Technol. 2019, 56, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Los, F.G.B.; Demiate, I.M.; Dornelles, R.C.P.; Lamsal, B. Enzymatic hydrolysis of Carioca bean (Phaseolus vulgaris L.) protein as an alternative to commercially rejected grains. LWT 2020, 125, 109191. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Zhao, X.; Yang, Y.; Li, M.; Yu, L. Effect of Limited Enzymatic Hydrolysis on Structural and Functional Properties of Elaeagnus mollis Oil Meal Protein. Foods 2022, 11, 3393. https://doi.org/10.3390/foods11213393
Guo C, Zhao X, Yang Y, Li M, Yu L. Effect of Limited Enzymatic Hydrolysis on Structural and Functional Properties of Elaeagnus mollis Oil Meal Protein. Foods. 2022; 11(21):3393. https://doi.org/10.3390/foods11213393
Chicago/Turabian StyleGuo, Caixia, Xiaoyu Zhao, Yukun Yang, Meiping Li, and Ligang Yu. 2022. "Effect of Limited Enzymatic Hydrolysis on Structural and Functional Properties of Elaeagnus mollis Oil Meal Protein" Foods 11, no. 21: 3393. https://doi.org/10.3390/foods11213393




