Functional and Quality Profile Evaluation of Butters, Spreadable Fats, and Shortenings Available from Czech Market
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Texture Profile Analysis
2.3. Free Fatty Acids and Acid Number
2.4. DSC Analysis
2.5. Rheology
2.6. Fluorescence Spectrometry
2.7. Statistical Analysis
3. Results
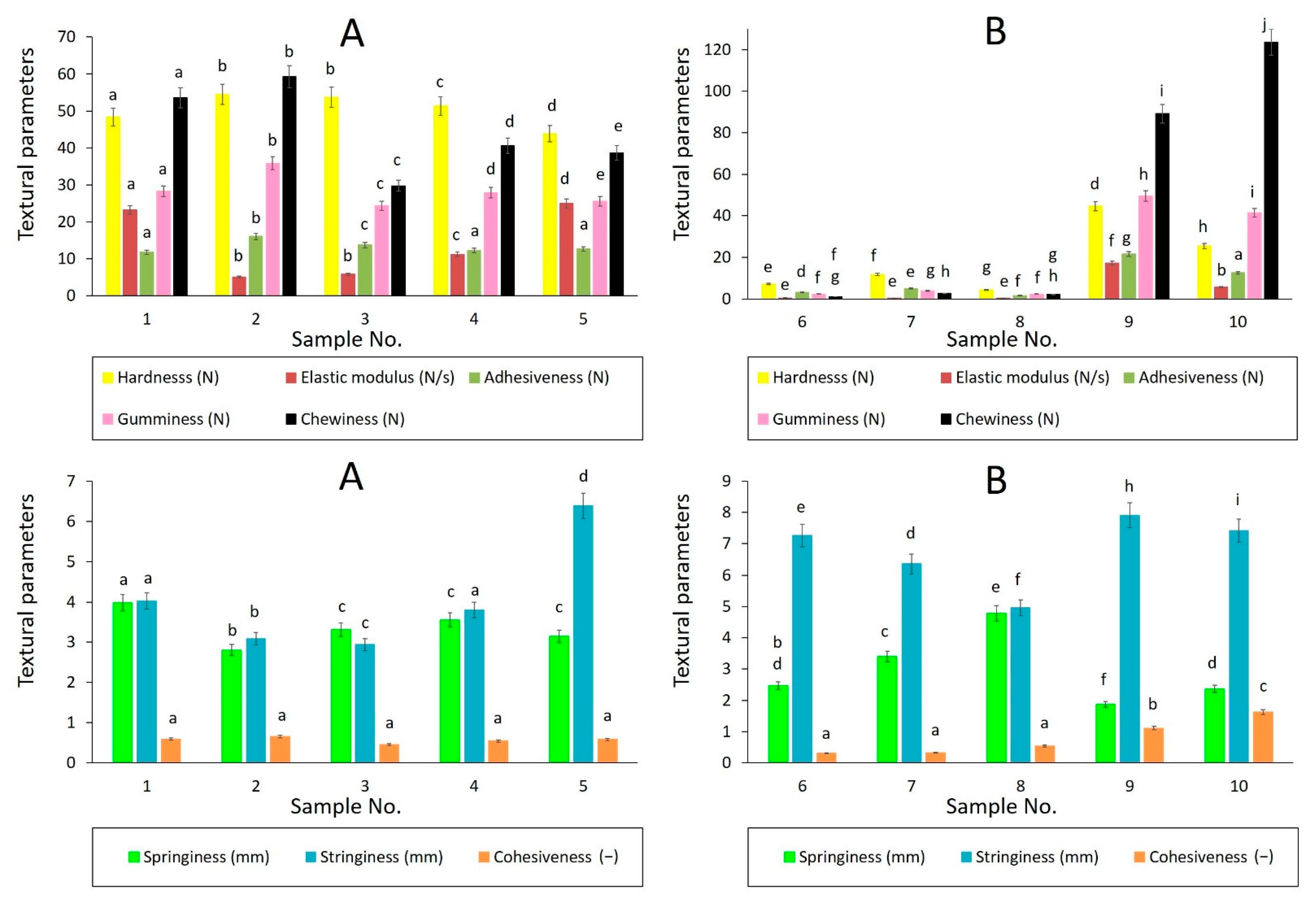
3.1. Texture Profile Analysis
3.2. Free Fatty Acids and Acid Number
3.3. DSC Analysis
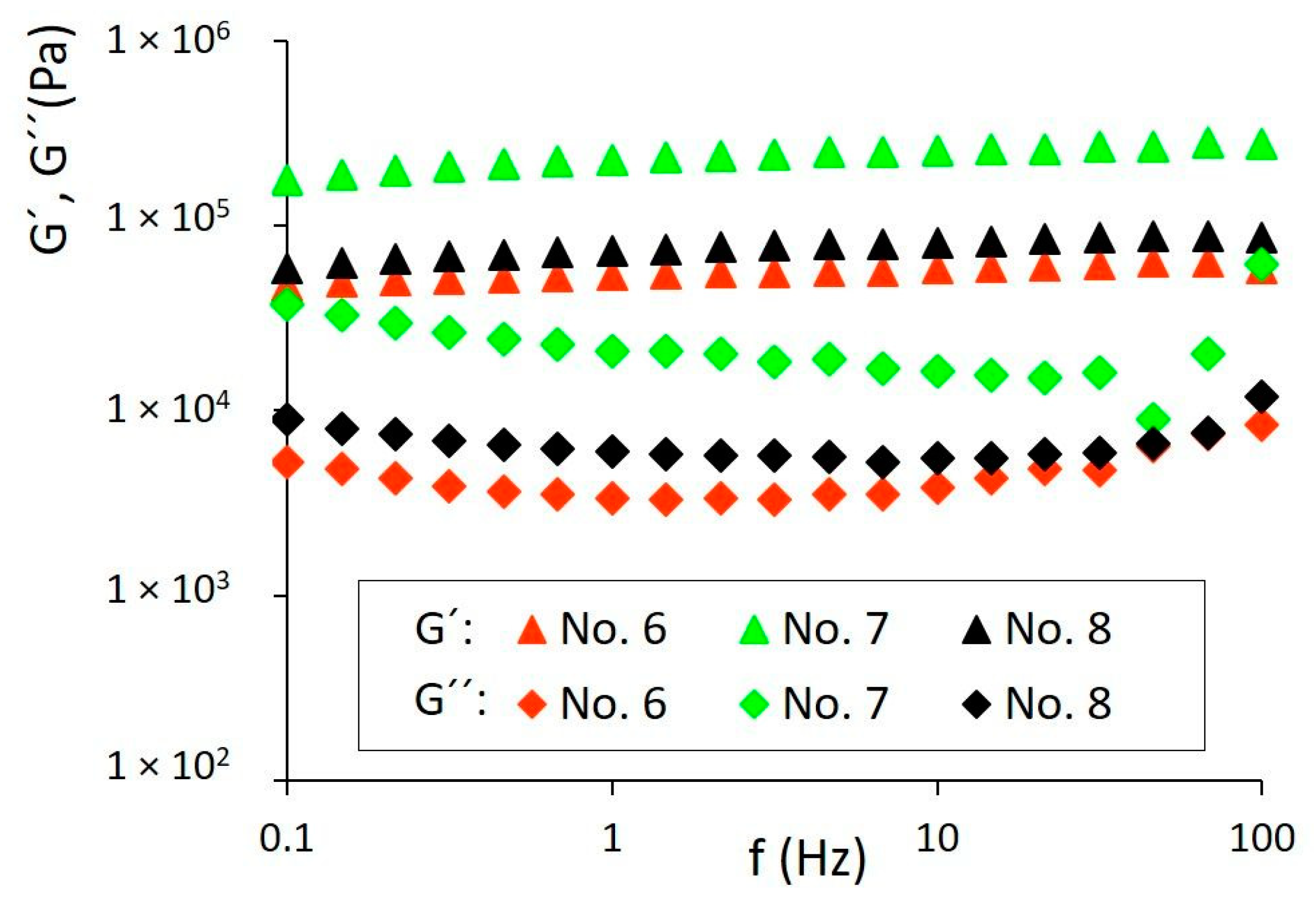
3.4. Rheological Behavior
3.5. Fluorescence Spectrometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez, C. Crystallization and Melting Properties of Milk Fat, 1st ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 205–243. ISBN 978-3-030-41661-4. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Tang, T.-K.; Phuah, E.-T.; Lai, O.-M. Recent Advances in Edible Fats and Oils Technology: Processing, Health Implications, Economic and Environmental Impact, 1st ed.; Springer: Singapore, 2022; p. 492. [Google Scholar] [CrossRef]
- Lee, J.; Martini, S. Modifying the physical properties of butter using high-intensity ultrasound. J. Dairy Sci. 2019, 102, 1918–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panchal, B.; Truong, T.; Prakash, S.; Bansal, N.; Bhandari, B. Influence of fat globule size, emulsifiers, and cream-aging on microstructure and physical properties of butter. Int. Dairy J. 2021, 117, 105003. [Google Scholar] [CrossRef]
- Wiking, L.; De Graef, V.; Rasmussen, M.; Dewettinck, K. Relations between crystallisation mechanisms and microstructure of milk fat. Int. Dairy J. 2009, 19, 424–430. [Google Scholar] [CrossRef]
- Bakry, I.A.; Ali, A.H.; Abdeen, E.-S.M.; Ghazal, A.F.; Wei, W.; Wang, X. Comparative characterisation of fat fractions extracted from Egyptian and Chinese camel milk. Int. Dairy J. 2020, 105, 104691. [Google Scholar] [CrossRef]
- Tomaszewska-Gras, J. Rapid quantitative determination of butter adulteration with palm oil using the DSC technique. Food Control 2016, 60, 629–635. [Google Scholar] [CrossRef]
- Azir, M.; Abbasiliasi, S.; Tengku Ibrahim, T.A.; Manaf, Y.N.A.; Sazili, A.Q.; Mustafa, S. Detection of lard in cocoa butter-its fatty acid composition, triacylglycerol profiles, and thermal characteristics. Foods 2017, 6, 98. [Google Scholar] [CrossRef] [Green Version]
- Rachana, C.R.; Nath, B.S. Crystallization of Milk Fat and its Importance in the Texture of Dairy Products: A Review. Indian J. Dairy Sci. 2008, 61, 408–422. [Google Scholar]
- Declerck, A.; Nelis, V.; Danthine, S.; Dewettinck, K.; Van der Meeren, P. Characterisation of fat crystal polymorphism in cocoa butter by time-domain NMR and DSC deconvolution. Foods 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Sloffer, E.M.; Gaur, S.; Engeseth, N.J.; Andrade, J.E. Development and physico-chemical characterization of a Shea butter-containing lipid nutrition supplement for Sub-Saharan Africa. Foods 2017, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Sert, D.; Mercan, E.; Kara, Ü. Butter production from ozone-treated cream: Effects on characteristics of physicochemical, microbiological, thermal and oxidative stability. LWT 2020, 131, 109722. [Google Scholar] [CrossRef]
- Lee, J.; Martini, S. Effect of cream aging temperature and agitation on butter properties. J. Dairy Sci. 2018, 101, 7724–7735. [Google Scholar] [CrossRef] [PubMed]
- Bourne, M.C. Chapter 4—Principles of Objective Texture Measurement. In Food Texture and Viscosity: Concept and Measurement, 2nd ed.; Bourne, M.C., Ed.; Academic Press: London, UK, 2002; pp. 107–188. ISBN 9780121190620. [Google Scholar] [CrossRef]
- Lapčíková, B.; Lapčík, L.; Valenta, T.; Majar, P.; Ondroušková, K. Impact of particle size on wheat dough and bread characteristics. Food Chem. 2019, 297, 124938. [Google Scholar] [CrossRef] [PubMed]
- McKenna, B.M.; Lyng, J.G. Chapter 6—Introduction to food rheology and its measurement. In Texture in Food, 1st ed.; McKenna, B.M., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2003; Volume 1, pp. 130–160. ISBN 9781855736733. [Google Scholar] [CrossRef]
- Ten Grotenhuis, E.; Van Aken, G.A.; Van Malssen, K.F.; Schenk, H. Polymorphism of milk fat studied by differential scanning calorimetry and real-time X-ray powder diffraction. J. Am. Oil Chem. Soc. 1999, 76, 1031–1039. [Google Scholar] [CrossRef]
- Shi, Y.; Smith, C.M.; Hartel, R.W. Compositional Effects on Milk Fat Crystallization. J. Dairy Sci. 2001, 84, 2392–2401. [Google Scholar] [CrossRef]
- Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Dairy Science and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; p. 808. ISBN 9780429116148. [Google Scholar] [CrossRef]
- Cant, P.A.E.; Palfreyman, K.R.; Boston, G.D.; MacGibbon, A.K.H. Milkfat Products; Dairy Research Institute: Palmerston North, Manawatu-Wanganui, New Zealand, 2017. [Google Scholar]
- Fadzillah, N.A.; Rohman, A.; Salleh, R.A.; Amin, I.; Shuhaimi, M.; Farahwahida, M.Y.; Rashidi, O.; Aizat, J.M.; Khatib, A. Authentication of butter from lard adulteration using high-resolution of nuclear magnetic resonance spectroscopy and high-performance liquid chromatography. Int. J. Food Prop. 2017, 20, 2147–2156. [Google Scholar] [CrossRef] [Green Version]
- Espert, M.; Wiking, L.; Salvador, A.; Sanz, T. Reduced-fat spreads based on anhydrous milk fat and cellulose ethers. Food Hydrocoll. 2020, 99, 105330. [Google Scholar] [CrossRef]
- Karakus, M.S.; Akgul, F.Y.; Korkmaz, A.; Atasoy, A.F. Evaluation of fatty acids, free fatty acids and textural properties of butter and sadeyag (anhydrous butter fat) produced from ovine and bovine cream and yoghurt. Int. Dairy J. 2022, 126, 105229. [Google Scholar] [CrossRef]
- Shahidi-Noghabi, M.; Naji-Tabasi, S.; Mozhdeh Sarraf, M. Effect of emulsifier on rheological, textural and microstructure properties of walnut butter. J. Food Meas. Charact. 2019, 13, 785–792. [Google Scholar] [CrossRef]
- Rush, J.W.E.; Jantzi, P.S.; Dupak, K.; Idziak, S.H.J.; Marangoni, A.G. Acute metabolic responses to butter, margarine, and a monoglyceride gel-structured spread. Food Res. Int. 2009, 42, 1034–1039. [Google Scholar] [CrossRef]
- Dalmazzone, C.; Noïk, C.; Clausse, D. Application of DSC for emulsified system characterization. Oil Gas Sci. Technol. 2009, 64, 543–555. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Gutierrez, J.; Scanlon, M.G. Chapter 5—Rheology and Mechanical Properties of Fats. In Structure-Function Analysis of Edible Fats, 2nd ed.; Marangoni, A.G., Ed.; AOCS Press: Urbana, IL, USA, 2018; pp. 119–168. ISBN 9780128140413. [Google Scholar] [CrossRef]
- Moriya, Y.; Hasome, Y.; Kawai, K. Effect of solid fat content on the viscoelasticity of margarine and impact on the rheological properties of cookie dough and fracture property of cookie at various temperature and water activity conditions. J. Food Meas. Charact. 2020, 14, 2939–2946. [Google Scholar] [CrossRef]
- Dimitrova, T.L.; Eftimov, T.; Kabadzhov, V.G.; Panayotov, P.T.; Boyanova, P.B. Scattering and fluorescence spectra of cow milk. Bulg. Chem. Commun. 2014, 46, 39–43. [Google Scholar]
- Sikorska, E.; Górecki, T.; Khmelinskii, I.V.; Sikorski, M.; Kozioł, J. Classification of edible oils using synchronous scanning fluorescence spectroscopy. Food Chem. 2005, 89, 217–225. [Google Scholar] [CrossRef]
- Ahmad, N.; Saleem, M. Studying heating effects on desi ghee obtained from buffalo milk using fluorescence spectroscopy. PLoS ONE 2018, 13, e0197340. [Google Scholar] [CrossRef] [Green Version]
- Croce, A.C.; Ferrigno, A.; Berardo, C.; Bottiroli, G.; Vairetti, M.; Di Pasqua, L.G. Spectrofluorometric analysis of autofluorescing components of crude serum from a rat liver model of ischemia and reperfusion. Molecules 2020, 25, 1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rønholt, S.; Madsen, A.S.; Kirkensgaard, J.J.K.; Mortensen, K.; Knudsen, J.C. Polymorphism, microstructure and rheology of butter. Effects of cream heat treatment. Food Chem. 2012, 135, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Bobe, G.; Hammond, E.G.; Freeman, A.E.; Lindberg, G.L.; Beitz, D.C. Texture of butter from cows with different milk fatty acid compositions. J. Dairy Sci. 2003, 86, 3122–3127. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Baer, R.J.; Schingoethe, D.J.; Hippen, A.R.; Kasperson, K.M.; Whitlock, L.A. Composition and flavor of milk and butter from cows fed fish oil, extruded soybeans, or their combination. J. Dairy Sci. 2001, 84, 2144–2151. [Google Scholar] [CrossRef]
- Subroto, E.; Indiarto, R.T.; Marta, H.; Wulan, A.S. Physicochemical and sensorial properties of recombined butter produced from milk fat and fish oil blend. Biosci. Res. 2018, 15, 3720–3727. [Google Scholar]
- McSweeney, P.L.H.; Fox, P.F.; O’Mahony, J.A. Advanced Dairy Chemistry, 4th ed.; Springer: Cham, Switzerland, 2020; Volume 2, p. 489. ISBN 978-3-030-48686-0. [Google Scholar] [CrossRef]
- Mannion, D.T.; Furey, A.; Kilcawley, K.N. Free fatty acids quantification in dairy products. Int. J. Dairy Technol. 2016, 69, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Catala, A. Fatty Acids; IntechOpen Ltd.: London, UK, 2017; p. 248. [Google Scholar] [CrossRef]
- McDaniel, M.R.; Sather, L.A.; Lindsay, R.C. Influence of free fatty acids on sweet cream butter flavor. J. Food Sci. 1969, 34, 251–254. [Google Scholar] [CrossRef]
- Pădureţ, S. The Quantification of Fatty Acids, Color, and Textural Properties of Locally Produced Bakery Margarine. Appl. Sci. 2022, 12, 1731. [Google Scholar] [CrossRef]
- Bauman, D.E.; Barbano, D.M.; Dwyer, D.A.; Griinari, J.M. Technical Note: Production of Butter with Enhanced Conjugated Linoleic Acid for Use in Biomedical Studies with Animal Models 1, 2. J. Dairy Sci. 2000, 83, 2422–2425. [Google Scholar] [CrossRef]
- Cunha, C.R.; Grimaldi, R.; Alcântara, M.R.; Viotto, W.H. Effect of the type of fat on rheology, functional properties and sensory acceptance of spreadable cheese analogue. Int. J. Dairy Technol. 2013, 66, 54–62. [Google Scholar] [CrossRef]
- Melo, E.; Michels, F.; Arakaki, D.; Lima, N.; Gonçalves, D.; Cavalheiro, L.; Oliveira, L.; Caires, A.; Hiane, P.; Nascimento, V. First study on the oxidative stability and elemental analysis of Babassu (Attalea speciosa) edible oil produced in Brazil using a domestic extraction machine. Molecules 2019, 24, 4235. [Google Scholar] [CrossRef]
- Mallia, S.; Piccinali, P.; Rehberger, B.; Badertscher, R.; Escher, F.; Schlichtherle-Cerny, H. Determination of storage stability of butter enriched with unsaturated fatty acids/conjugated linoleic acids (UFA/CLA) using instrumental and sensory methods. Int. Dairy J. 2008, 18, 983–993. [Google Scholar] [CrossRef]
- Veberg, A.; Olsen, E.; Nilsen, A.N.; Wold, J.P. Front-face fluorescence measurement of photosensitizers and lipid oxidation products during the photooxidation of butter. J. Dairy Sci. 2007, 90, 2189–2199. [Google Scholar] [CrossRef]
- Wold, J.P.; Bro, R.; Veberg, A.; Lundby, F.; Nilsen, A.N.; Moan, J. Active photosensitizers in butter detected by fluorescence spectroscopy and multivariate curve resolution. J. Agric. Food Chem. 2006, 54, 10197–10204. [Google Scholar] [CrossRef]




| Nutritional Values/Energy Intake per 100 g | Products Name, Producer | ||||
| Rokitnianka Butter (No. 1), Mleczarska Rokitnianka | Olomoucké Butter (No. 2), OLMA, a.s. | Tatra Farmářské Butter (No. 3), Mlékárna Hlinsko, a.s. | Česká Chuť Butter (No. 4), OLMA, a.s. | Dr. Halíř Butter (No. 5), Mlékárna Čejetičky, spol. s r.o. | |
| Energy intake (kJ/kcal) | 3060/744 | 3095/739 | 3134/762 | 3095/739 | 3056/743 |
| Total fat (g) a (of which saturated fatty acids) | 82.1 (57.4) | 83.0 (51.0) | 84.0 (55.0) | 83.0 (51.0) | 82.0 (52.0) |
| Saccharides (g) (of which monosaccharides) | 0.6 (0.6) | 0.8 (0.8) | 0.8 (0.8) | 0.8 (0.8) | 0.7 (0.7) |
| Proteins (g) | 0.7 | 0.6 | 0.7 | 0.6 | 0.6 |
| Salt (g) | 0.02 | 0.02 | 0.10 | 0.02 | 0.02 |
| Vitamin A (µg) | - | - | - | - | - |
| Vitamin D (µg) | - | - | - | - | - |
| Vitamin E (mg) | - | - | - | - | - |
| Perla (No. 6), UNILEVER ČR, spol. s r.o. | RamaClassic(No. 7), UNILEVER ČR, spol. s r.o. | Flora (No. 8), UNILEVER ČR, spol. s r.o. | Hera (No. 9), UNILEVER ČR, spol. s r.o. | Zlatá Haná (No. 10), OLMA, a.s. | |
| Energy intake (kJ/kcal) | 1450/346 | 2241/535 | 1673/405 | 2666/637 | 2762/659 |
| Total fat (g) a (of which saturated fatty acids) | 39.0 (10.0) | 60.0 (24.0) | 45.0 b (10.0) | 72.0 (34.0) | 74.0 c (35.0) |
| Saccharides (g) (of which monosaccharides) | 0 (0) | 0.5 (0.5) | <0.5 (<0.5) | <0.5 (<0.5) | 0.8 (0.8) |
| Proteins (g) | 0 | <0.5 | <0.5 | <0.5 | 0.6 |
| Salt (g) | 0.32 | 0.23 | 0.52 | 0.21 | 0.02 |
| Vitamin A (µg) | 800 (100%) d | 800 (100%) d | 800 (100%) d | 800 (100%) d | - |
| Vitamin D (µg) | 7.5 (150%) d | 7.5 (150%) d | 7.5 (150%) d | 7.5 (150%) d | - |
| Vitamin E (mg) | 18 (150%) d | 7.5 (60%) d | 14 (120%) d | - | - |
| Sample | Crystallization Peak (Cooling) | 1st Melting Peak (Heating) | 2nd/3rd Melting Peak (Heating) | |||
|---|---|---|---|---|---|---|
| Tc (°C) | ∆H (J/g) | Tp (°C) | ∆H (J/g) | Tp (°C) | ∆H (J/g) | |
| No. 1 | −14.09 ± 0.14 a | 32.22 ± 0.47 a | 16.15 ± 0.12 a | 18.13 ± 0.24 a | 34.20 ± 0.21 a | 192.41 ± 0.48 a |
| No. 2 | −19.66 ± 0.18 b | 34.21 ± 0.20 b | 15.10 ± 0.13 b | 30.60 ± 0.35 b | 31.55 ± 0.25 b | 66.79 ± 0.29 b |
| No. 3 | −16.92 ± 0.22 c | 30.61 ± 0.28 c | 15.09 ± 0.10 b | 34.35 ± 0.26 c | 31.24 ± 0.12 b | 40.59 ± 0.37 c |
| No. 4 | −13.32 ± 0.19 d | 38.87 ± 0.30 d | 15.34 ± 0.11 b | 14.21 ± 0.15 d | 31.84 ± 0.27 b,c | 38.54 ± 0.31 d |
| No. 5 | −9.16 ± 0.15 e | 35.20 ± 0.28 e | 15.49 ± 0.22 b | 14.54 ± 0.17 d | 34.38 ± 0.19 a | 148.99 ± 0.52 e |
| No. 6 | −15.83 ± 0.10 f | 42.61 ± 0.31 f | - | - | - 44.57 ± 0.23 d | - 335.97 ± 0.40 f |
| No. 7 | −16.91 ± 0.18 c | 74.34 ± 0.29 g | - | - | 32.30 ± 0.19 c 51.11 ± 0.17 e | 417.88 ± 0.55 g 8.50 ± 0.12 h |
| No. 8 | −14.46 ± 0.09 a | 42.90 ± 0.15 f | 24.57 ± 0.16 c | 83.69 ± 0.35 e | - 50.83 ± 0.14 e | - 57.29 ± 0.20 i |
| No. 9 | −16.71 ± 0.12 c | 58.72 ± 0.26 h | - | - | 37.57 ± 0.28 f 50.01 ± 0.19 g | 316.11 ± 0.48 j 26.56 ± 0.11 k |
| No. 10 | −21.60 ± 0.28 g | 39.01 ± 0.15 d | 8.48 ± 0.20 d | 22.18 ± 0.14 f | 34.12 ± 0.10 a | 43.35 ± 0.19 l |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapčíková, B.; Lapčík, L.; Valenta, T.; Kučerová, T. Functional and Quality Profile Evaluation of Butters, Spreadable Fats, and Shortenings Available from Czech Market. Foods 2022, 11, 3437. https://doi.org/10.3390/foods11213437
Lapčíková B, Lapčík L, Valenta T, Kučerová T. Functional and Quality Profile Evaluation of Butters, Spreadable Fats, and Shortenings Available from Czech Market. Foods. 2022; 11(21):3437. https://doi.org/10.3390/foods11213437
Chicago/Turabian StyleLapčíková, Barbora, Lubomír Lapčík, Tomáš Valenta, and Tereza Kučerová. 2022. "Functional and Quality Profile Evaluation of Butters, Spreadable Fats, and Shortenings Available from Czech Market" Foods 11, no. 21: 3437. https://doi.org/10.3390/foods11213437
APA StyleLapčíková, B., Lapčík, L., Valenta, T., & Kučerová, T. (2022). Functional and Quality Profile Evaluation of Butters, Spreadable Fats, and Shortenings Available from Czech Market. Foods, 11(21), 3437. https://doi.org/10.3390/foods11213437





