Fusion of 2DGC-MS, HPLC-MS and Sensory Data to Assist Decision-Making in the Marketing of International Monovarietal Chardonnay and Sauvignon Blanc Wines
Abstract
:1. Introduction
2. Material and Methods
2.1. Chardonnay and Sauvignon Blanc Wines
2.2. Climate of the Geographical Area
2.3. Sensory Analysis
2.4. Method Optimization for HS-SPME
2.5. HS-SPME-GC × GC-ToF/MS Analysis of the Volatile Profiles
2.6. UHPLC-MS Analysis of the Phenolic Profiles
2.7. Statistical Analysis
3. Results
3.1. Analysis of Variance
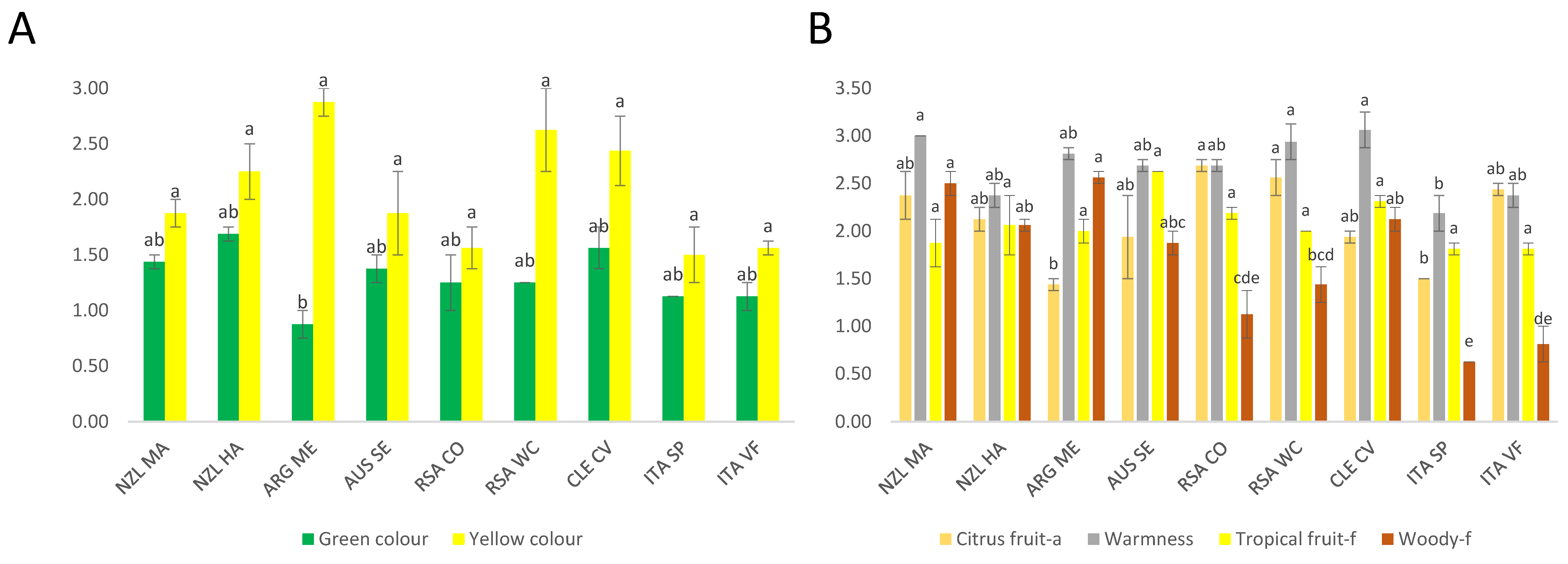
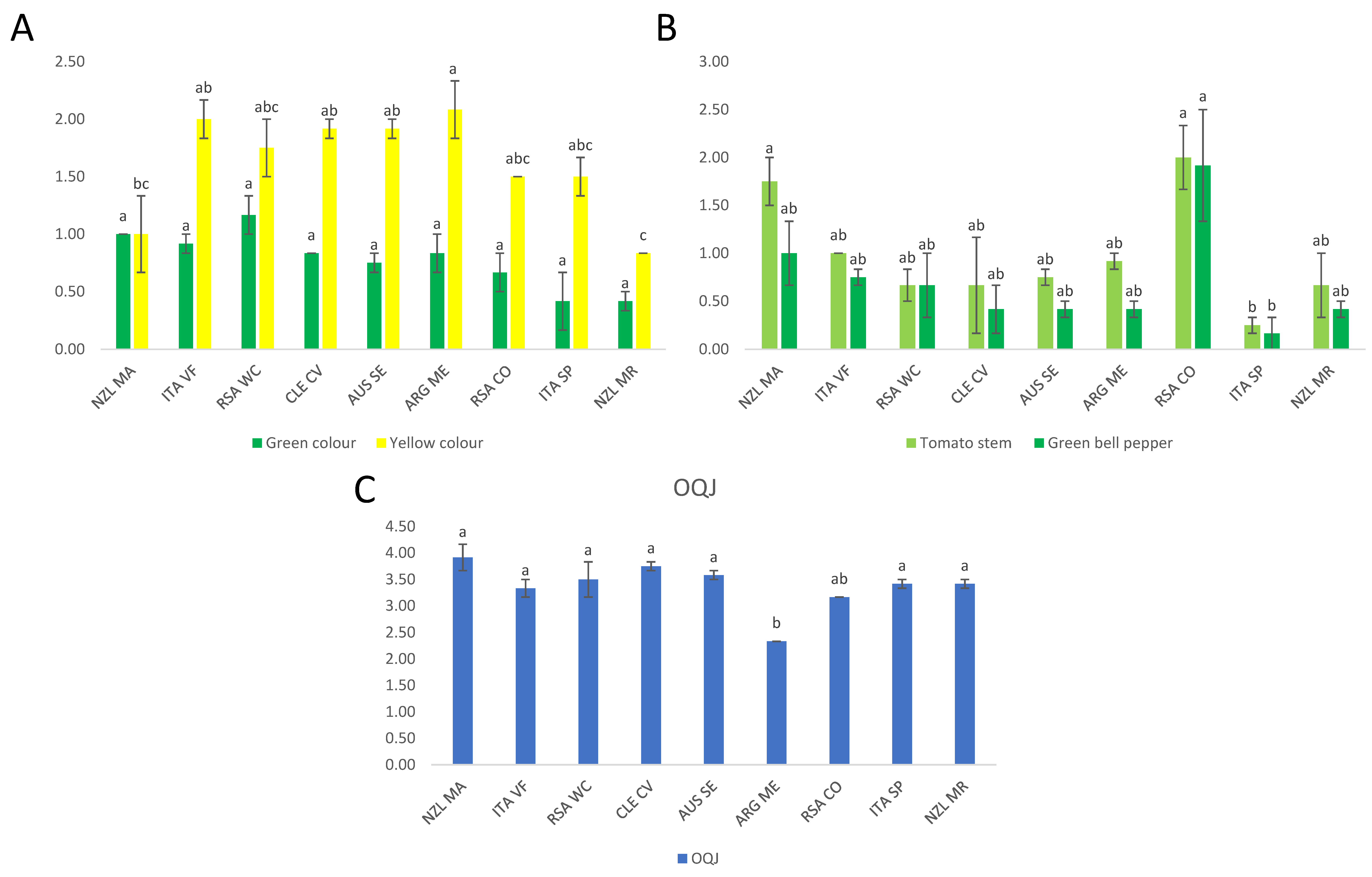
3.1.1. Chardonnay Sensory Data
3.1.2. Sauvignon Blanc Sensory Data
Analysis of Variance
Agglomerative Hierarchical Cluster Analysis (AHC)
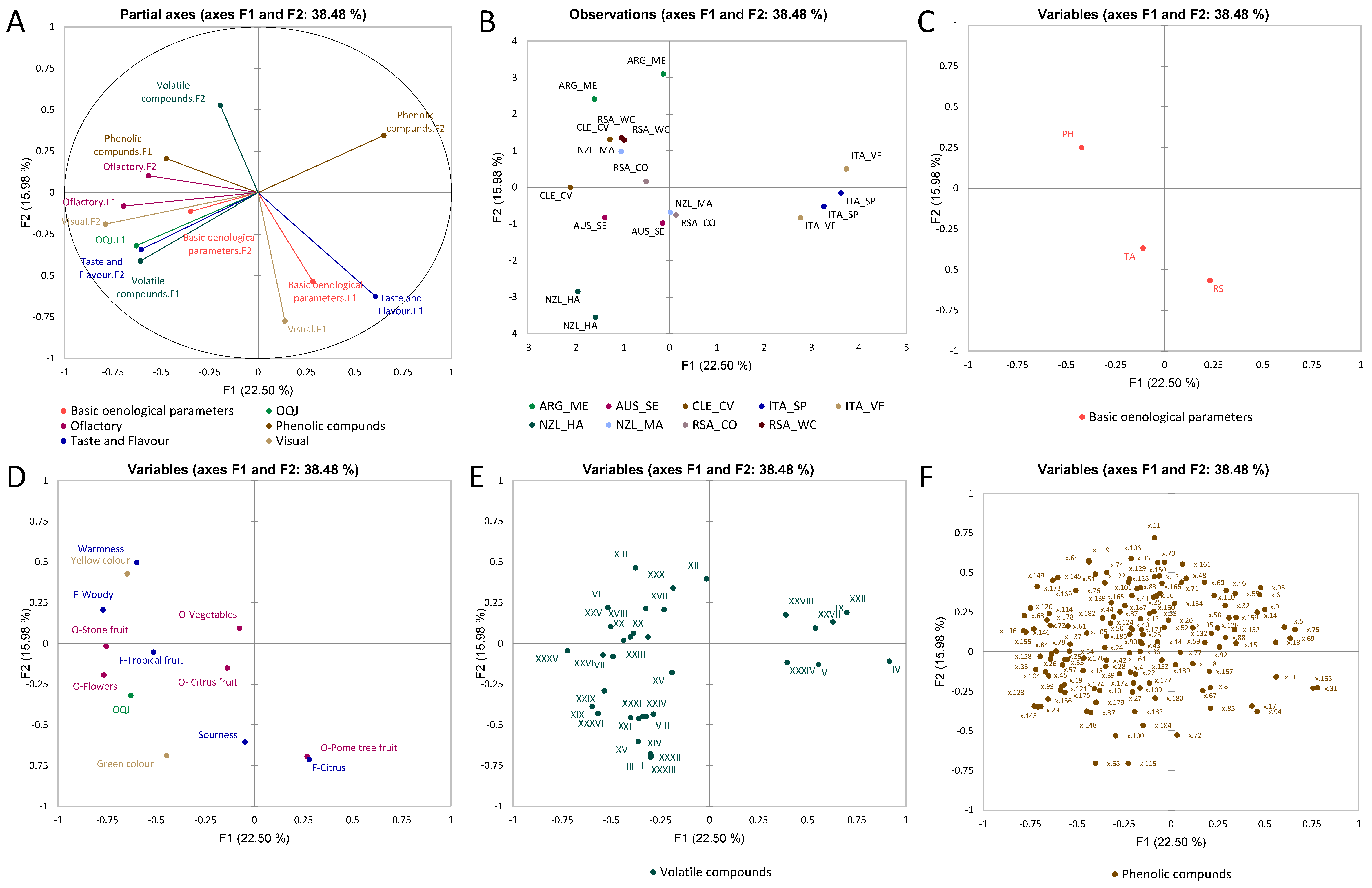
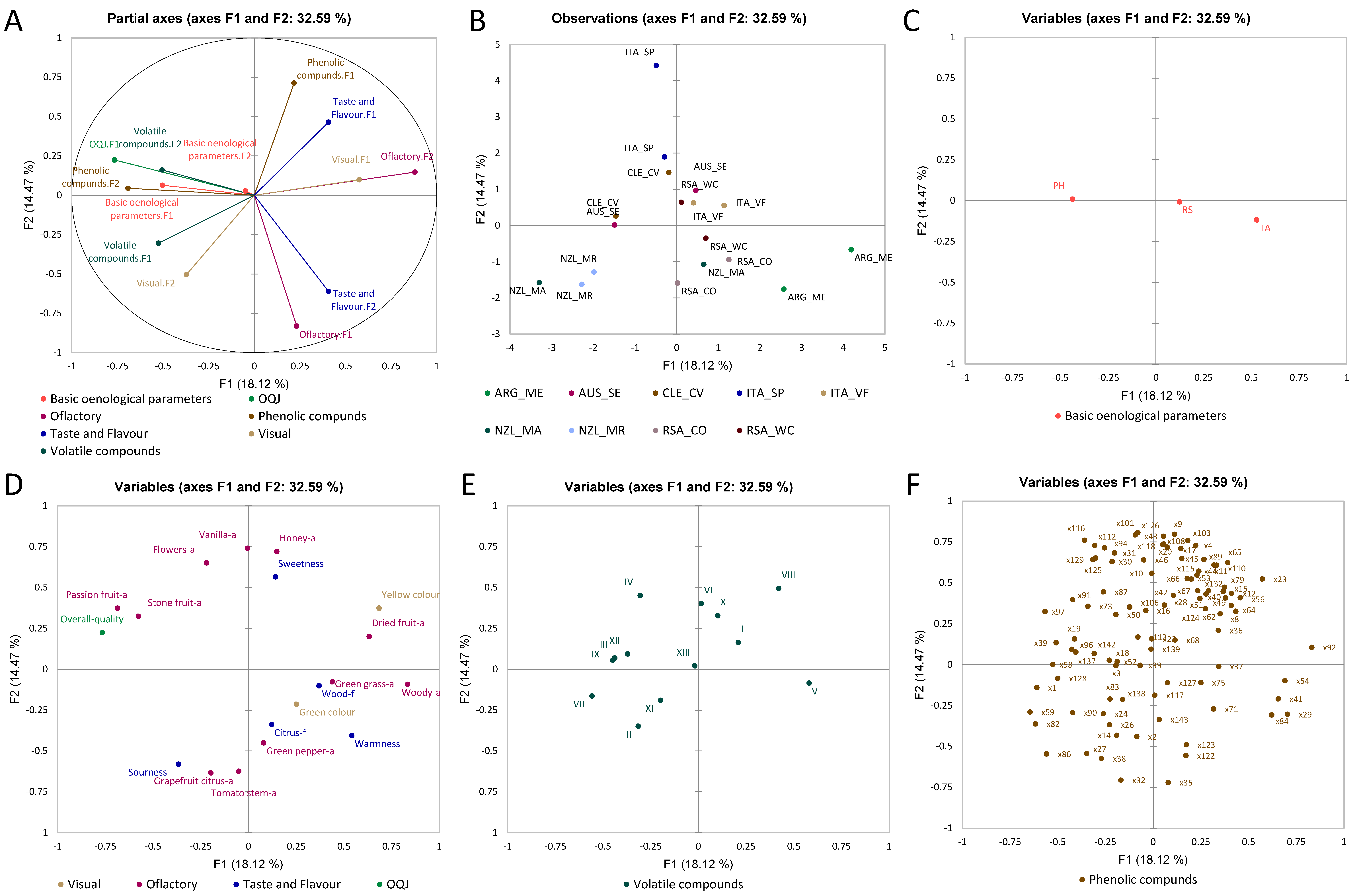
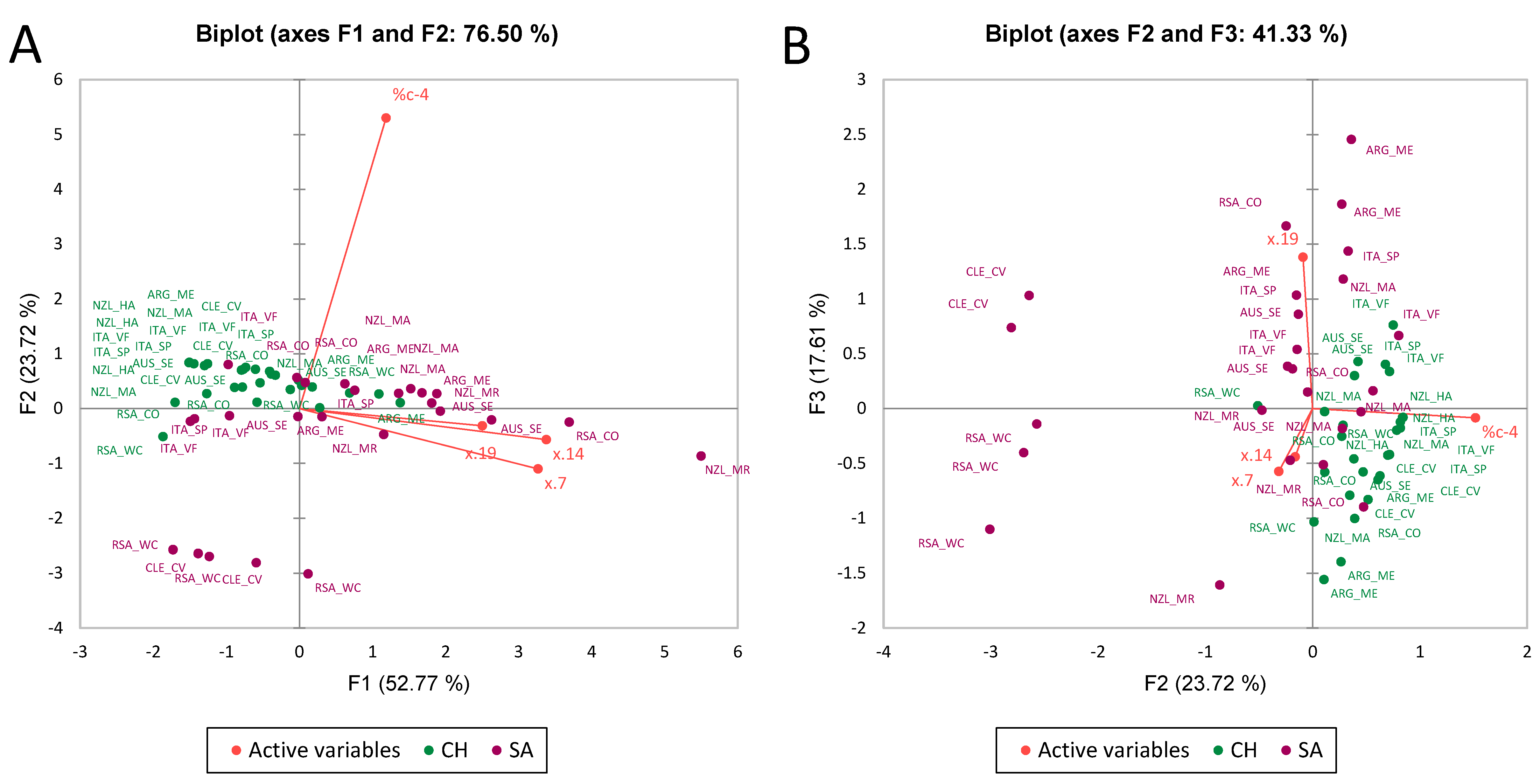
3.2. Multiple Factor Analysis
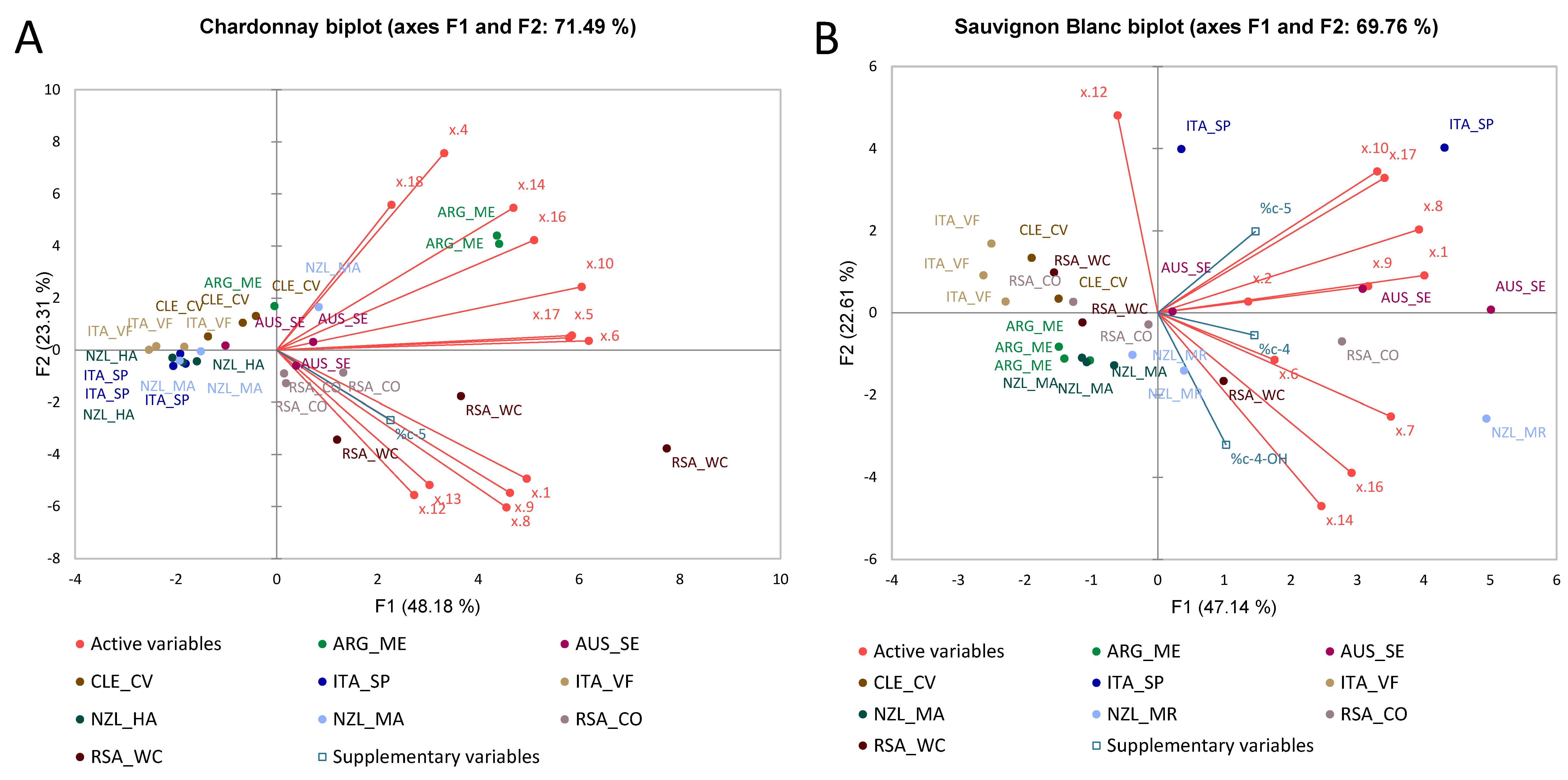
3.2.1. Chardonnay Wines
3.2.2. Sauvignon Blanc Wines
3.3. Regression Models
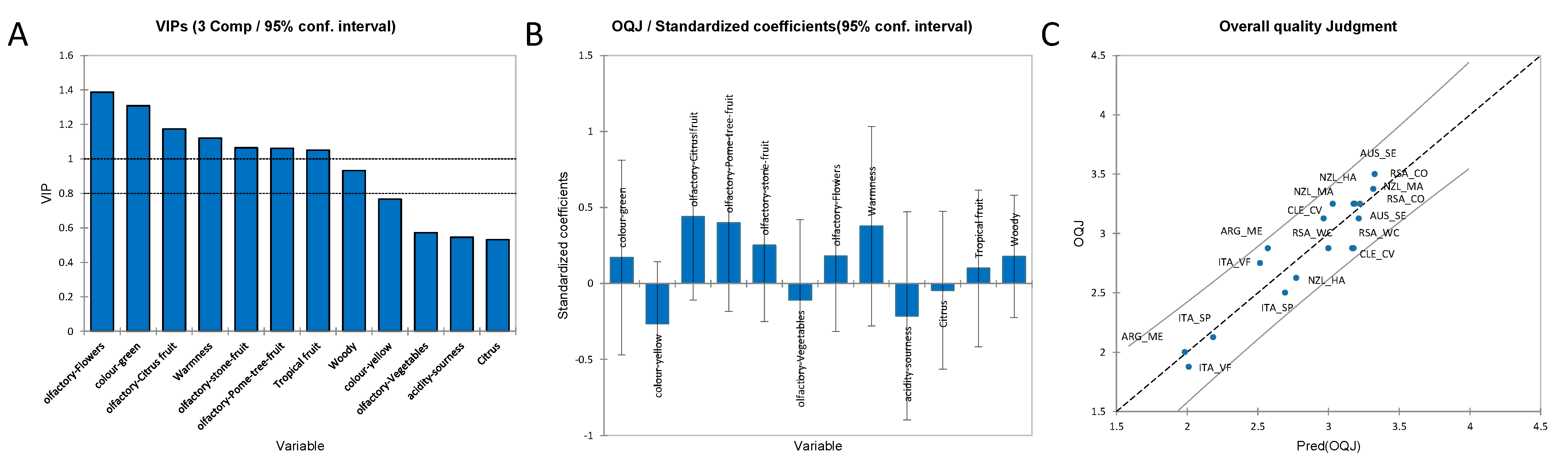
3.3.1. Partial Least Squares Regression (PLS-R) for the OQJ Score of Chardonnay Wines
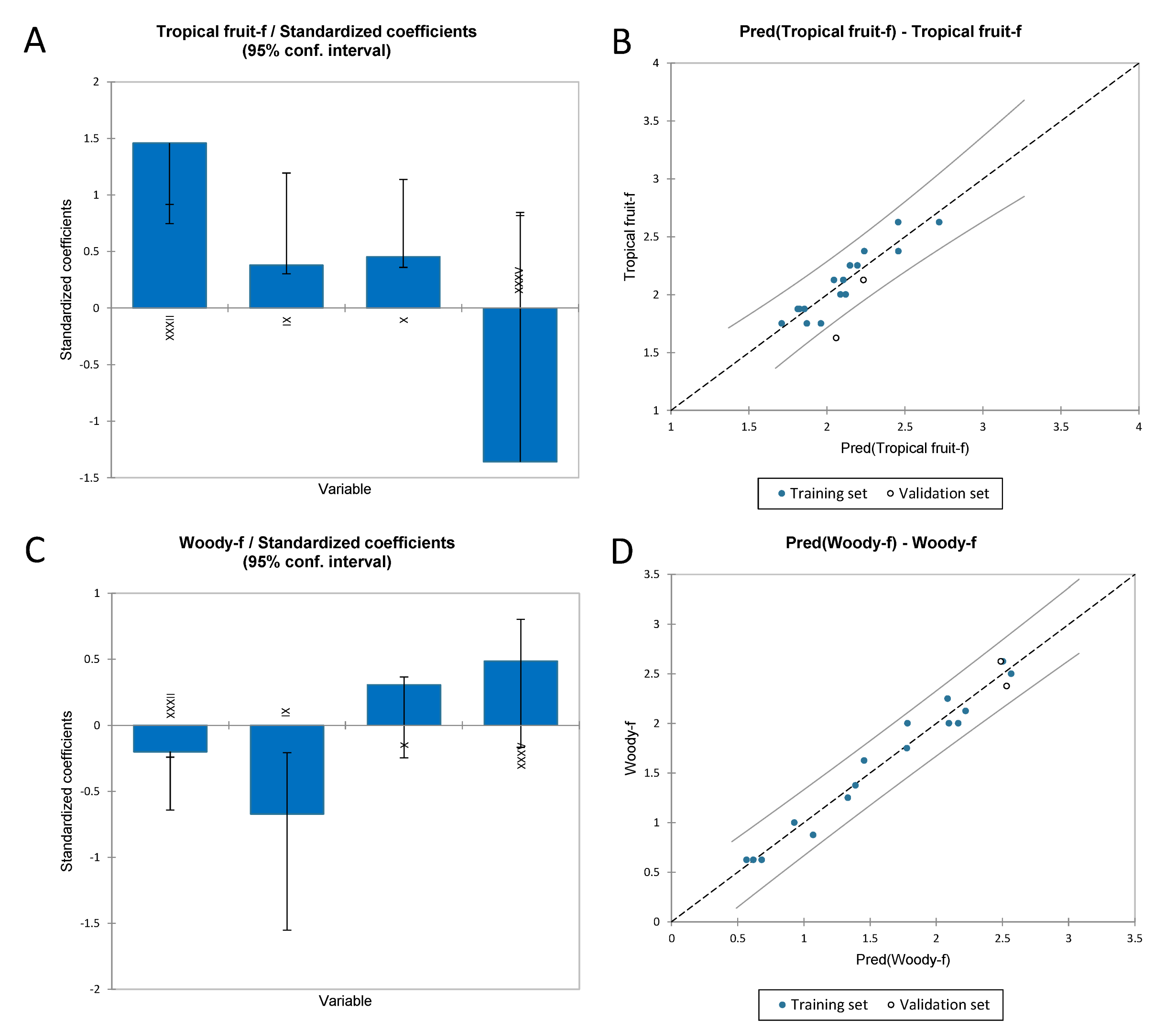
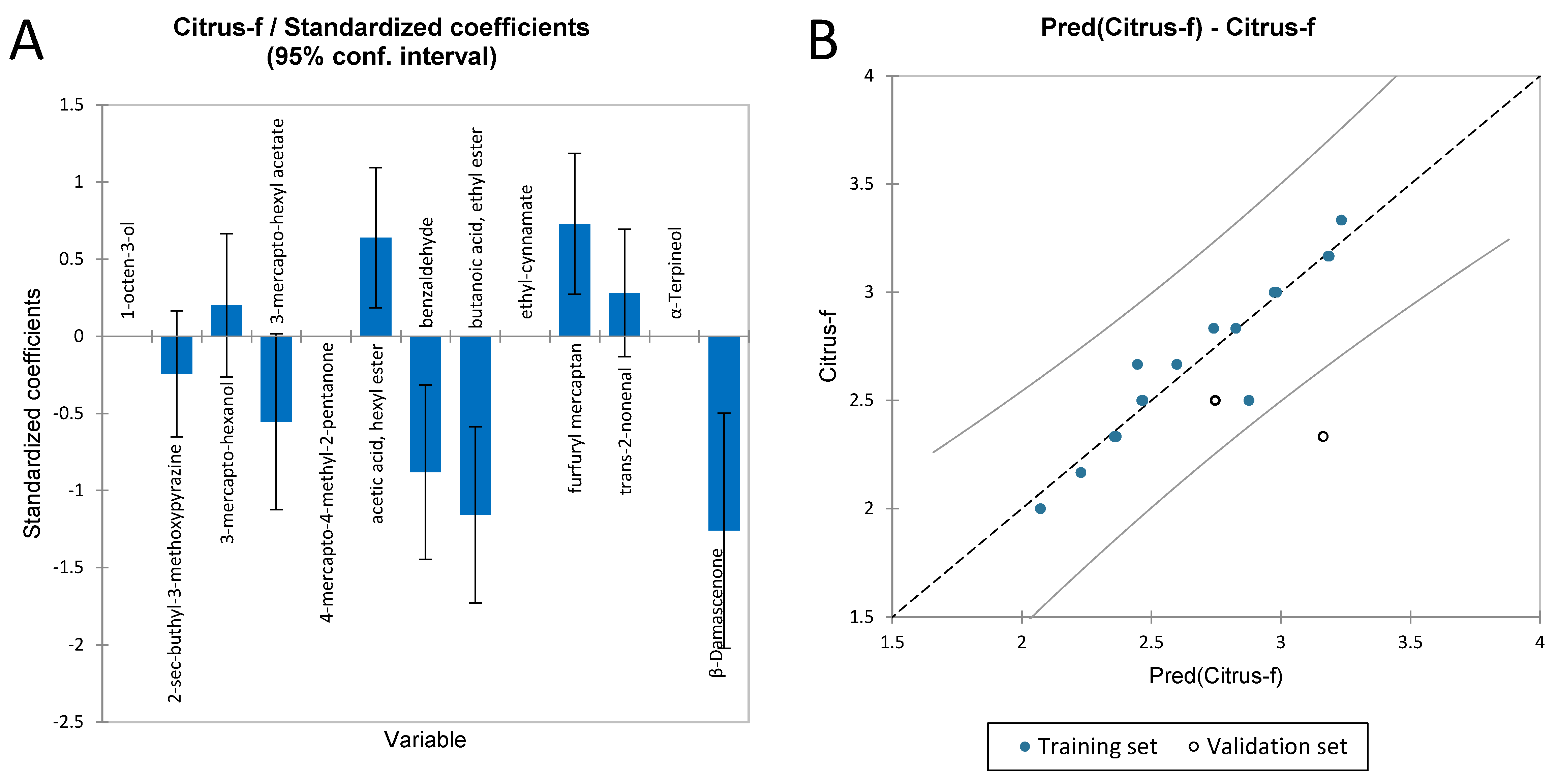
3.3.2. Multiple Linear Regression to Identify the Volatile Compounds Responsible for the Flavour Sensory Attributes of Chardonnay Wines
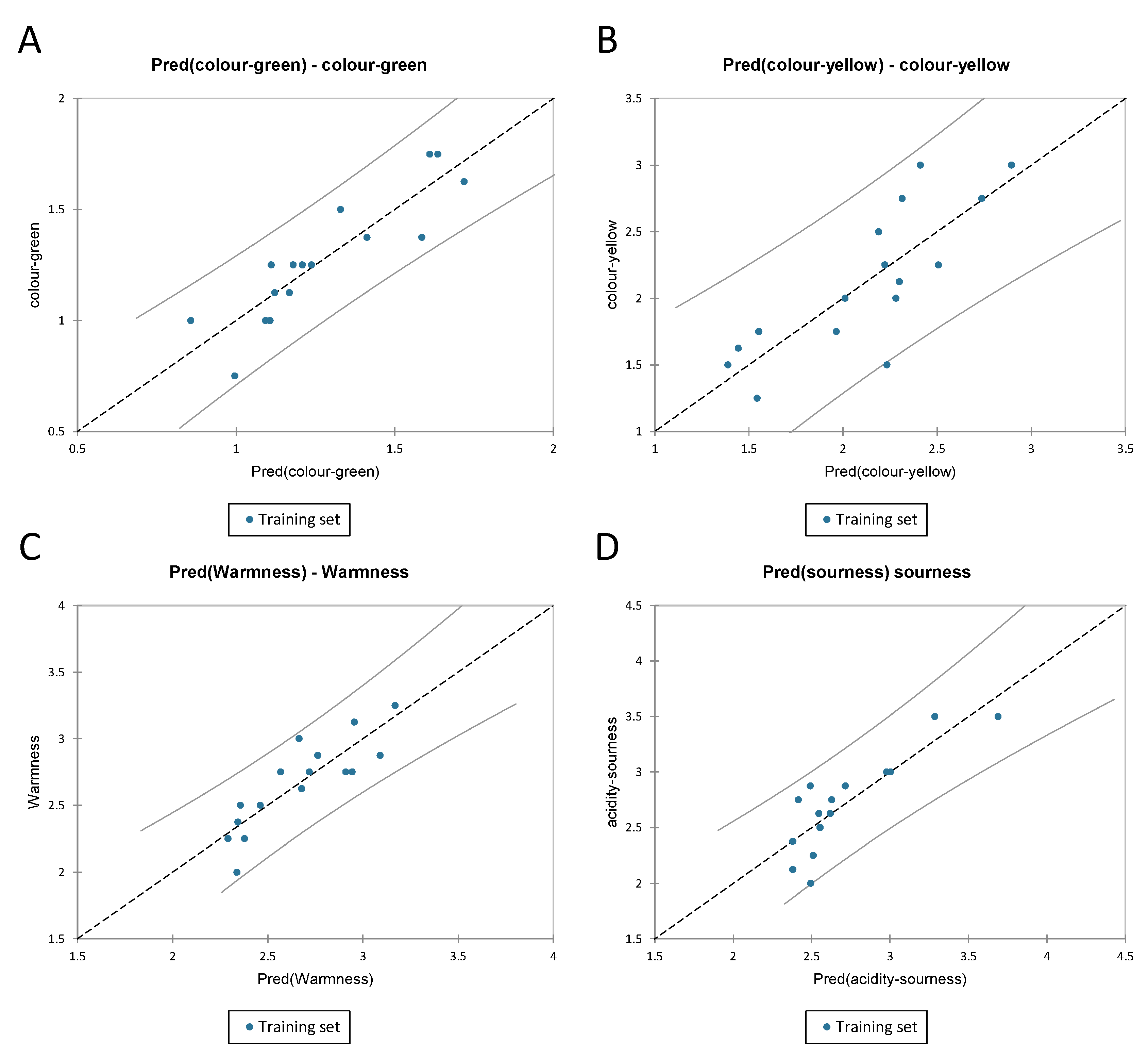
3.3.3. PLS-R for Visual and Gustatory Data for Chardonnay Wines
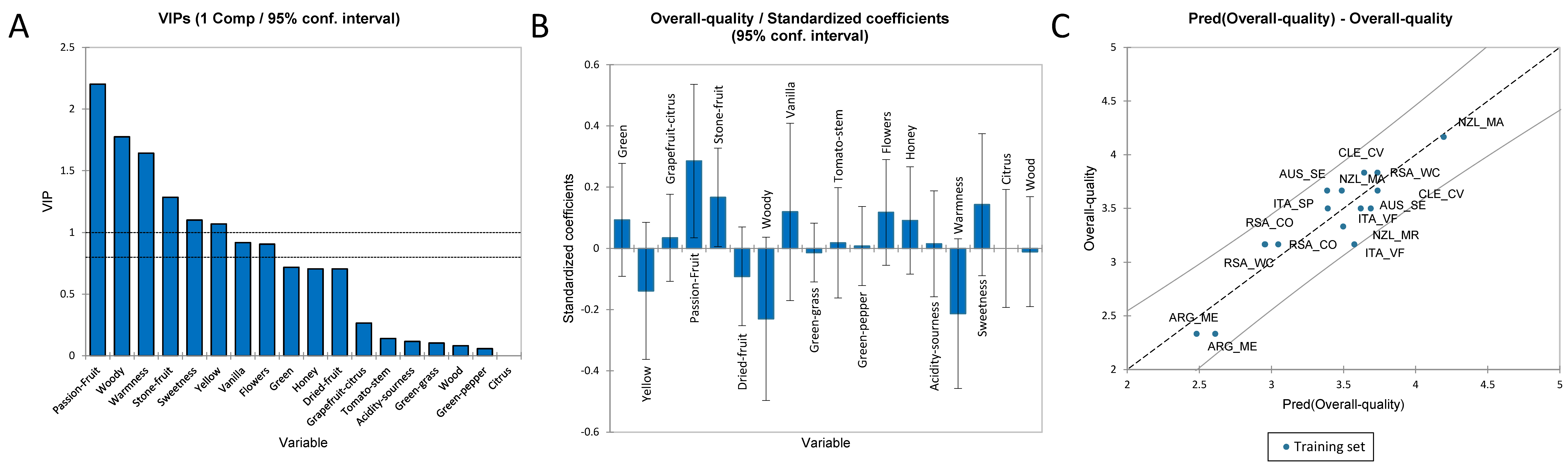
3.3.4. PLS-R for the Overall Quality Score of Sauvignon Blanc Wines
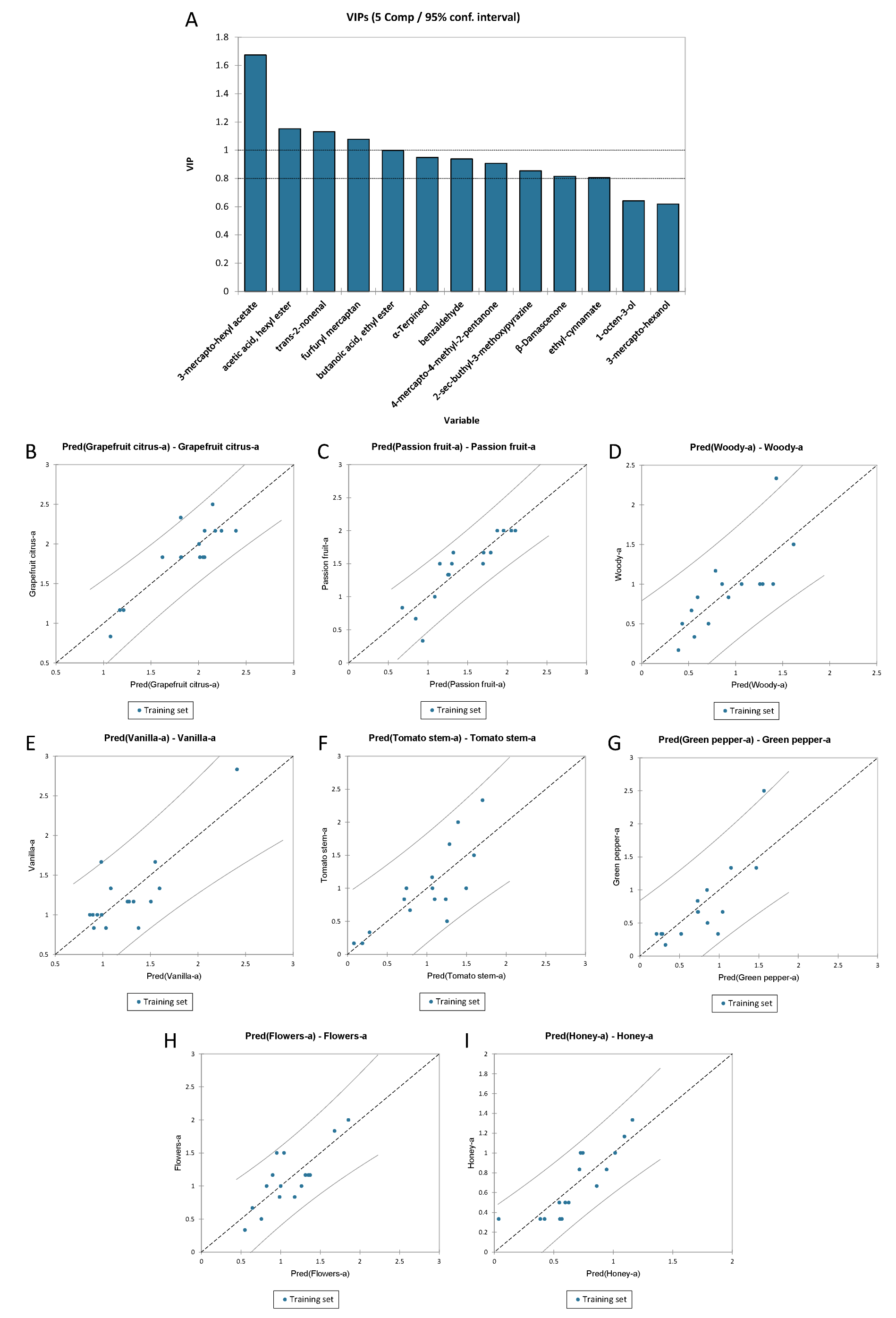
3.3.5. PLS-R for the Olfactory Profile of Sauvignon Blanc Wines
3.3.6. Multiple Linear Regression for the Flavours of Sauvignon Blanc Wines
3.4. Proanthocyanidins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Charters, S.; Pettigrew, S. Conceptualizing product quality: The case of wine. Mark. Theory 2006, 6, 467–483. [Google Scholar] [CrossRef]
- Moskowitz, H.R. Food quality: Conceptual and sensory aspects. Food Qual. Prefer. 1995, 6, 157–162. [Google Scholar] [CrossRef]
- Lawless, H.T. Sensory Evaluation of Food: Principles and Practices; Chapman & Hall/International Thomson Publishing: New York, NY, USA, 1998. [Google Scholar]
- Guld, Z.; Nyitrainé Sárdy, D.; Gere, A.; Rácz, A. Comparison of sensory evaluation techniques for Hungarian wines. J. Chemom. 2020, 34, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Xu, Y.; Tang, K. Aroma of Icewine: A Review on How Environmental, Viticultural, and Oenological Factors Affect the Aroma of Icewine. J. Agric. Food Chem. 2021, 69, 6943–6957. [Google Scholar] [CrossRef] [PubMed]
- Poggesi, S.; Dupas de Matos, A.; Longo, E.; Chiotti, D.; Pedri, U.; Eisenstecken, D.; Boselli, E. Chemosensory Profile of South Tyrolean Pinot Blanc Wines: A Multivariate Regression Approach. Molecules 2021, 26, 6245. [Google Scholar] [CrossRef]
- Gambetta, J.M.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. Factors influencing the aroma composition of chardonnay wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine (OIV). Distribution of the World’s Grapevine Varieties. 2017. Available online: www.oiv.int (accessed on 24 February 2022).
- Calò, A.; Scienza, A.; Costacurta, A. Vitigni d’Italia; Calderini Edagricole: Bologna, Italy, 2001; pp. 696–697. [Google Scholar]
- Benkwitz, F.; Nicolau, L.; Lund, C.; Beresford, M.; Wohlers, M.; Kilmartin, P.A. Evaluation of key odorants in sauvignon blanc wines using three different methodologies. J. Agric. Food Chem. 2012, 60, 6293–6302. [Google Scholar] [CrossRef]
- Longo, E.; Rossetti, F.; Jouin, A.; Teissedre, P.L.; Jourdes, M.; Boselli, E. Distribution of crown hexameric procyanidin and its tetrameric and pentameric congeners in red and white wines. Food Chem. 2019, 299, 125125. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine (OIV). Compendium of International Methods of Wine and Must Analysis; OIV: Paris, France, 2022; Volume 2. [Google Scholar]
- Climate Marlborough: Temperature, Climate Graph, Climate Table for Marlborough. Available online: https://en.climate-data.org/oceania/new-zealand/marlborough-2458/ (accessed on 31 May 2022).
- Climate Hawke’s Bay: Temperature, Climate Graph, Climate Table for Hawke’s Bay. Available online: https://en.climate-data.org/oceania/new-zealand/hawke-s-bay-1985/ (accessed on 31 May 2022).
- Martinborough Climate: Average Temperature, Weather by Month, Martinborough Weather Averages. Available online: https://en.climate-data.org/oceania/new-zealand/wellington/martinborough-32426/ (accessed on 31 May 2022).
- Mendoza Climate, Weather by Month, Average Temperature (Argentina). Available online: https://en.climate-data.org/south-america/argentina/mendoza/mendoza-1893/ (accessed on 31 May 2022).
- South Eastern Australian Climate Initiative (SEACI). Available online: http://www.seaci.org/ (accessed on 31 May 2022).
- Geography and Climate|South African Government. Available online: www.gov.za (accessed on 31 May 2022).
- Central Valley|Valley, Chile|Britannica. Available online: https://www.britannica.com/place/Central-Valley-Chile (accessed on 31 May 2022).
- The Sweet Sicilian Climate|Visit Sicily Official Page. Available online: https://www.visitsicily.info/dolce-clima-siciliano/ (accessed on 31 May 2022).
- Puglia, Italy. Live Weather Update. Holiday Weather. Available online: www.holiday-weather.com (accessed on 31 May 2022).
- Climate in Veneto. Available online: https://www.venetoinside.com (accessed on 31 May 2022).
- Il Clima Della Regione Friuli-Venezia Giulia|Climatologia | Il Tempo in Media e Agli Estremi. Available online: http://www.centrometeo.com (accessed on 31 May 2022).
- Nishida, M.; Lestringant, P.; Cantu, A.; Heymann, H. Comparing classical descriptive analysis with modified descriptive analysis, modified rate-all-that-apply, and modified check-all-that-apply. J. Sens. Stud. 2021, 36, e12684. [Google Scholar] [CrossRef]
- Noble, A.C.; Arnold, R.A.; Buechsenstein, J.; Leach, E.J.; Schmidt, J.O.; Stern, P.M. Modification of a Standardized System of Wine Aroma Terminology. Am. J. Enol. Vitic. 1987, 38, 143–146. [Google Scholar]
- Waehrens, S.S.; Zhang, S.; Hedelund, P.I.; Petersen, M.A.; Byrne, D.V. Application of the Fast Sensory Method ‘Rate-All-That-Apply’ in Chocolate Quality Control Compared with DHS-GC-MS. Int. J. Food Sci. Technol. 2016, 51, 1877–1887. [Google Scholar] [CrossRef]
- Coetzee, C.; du Toit, W.J. A comprehensive review on Sauvignon blanc aroma with a focus on certain positive volatile thiols. Food Res. Int. 2012, 45, 287–298. [Google Scholar] [CrossRef]
- Lacey, M.J.; Allen, M.S.; Harris, R.L.; Brown, W.V. Methoxypyrazines in Sauvignon blanc grapes and wines. Am. J. Enol. Vitic. 1991, 42, 103–108. [Google Scholar]
- Dupas de Matos, A.; Longo, E.; Chiotti, D.; Pedri, U.; Eisenstecken, D.; Sanoll, C.; Boselli, E. Pinot blanc: Impact of the winemaking variables on the evolution of the phenolic, volatile and sensory profiles. Foods 2020, 9, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagès, J. Multiple Factor Analysis: Main Features and Application to Sensory. Rev. Colomb. De Estad. 2004, 27, 1–26. [Google Scholar]
- Aluja, T.; Morineau, A.; Sanchez, G. Principal Component Analysis for Data Science (pca4ds). 2018. Available online: https://pca4ds.github.io/ (accessed on 25 April 2022).
- Liberatore, M.T.; Pati, S.; Del Nobile, M.A.; La Notte, E. Aroma quality improvement of Chardonnay white wine by fermentation and ageing in barrique on lees. Food Res. Int. 2010, 43, 996–1002. [Google Scholar] [CrossRef]
- Jordan, M.J.; Margaria, C.A.; Shaw, P.E.; Goodner, K.L. Aroma active components in aqueous kiwi fruit essence and kiwi fruit puree by GC-MS and multidimensional GC/GC-O. J. Agric. Food Chem. 2002, 50, 5386–5390. [Google Scholar] [CrossRef]
- Adedeji, J.; Hartman, T.G.; Rosen, R.T.; Ho, C.T. Free and glycosidically bound aroma compounds in hog plum (Spondias mombins L.). J. Agric. Food Chem. 1991, 39, 1494–1497. [Google Scholar] [CrossRef]
- Liu, C.; Wahefu, A.; Lu, X.; Abdulla, R.; Dou, J.; Zhao, H.; Aisa, H.A.; Xin, X.; Liu, Y. Chemical Profiling of Kaliziri Injection and Quantification of Six Caffeoyl Quinic Acids in Beagle Plasma by LC-MS/MS. Pharmaceuticals 2022, 15, 663. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Yang, T.; Slovin, J.; Chen, P. Profiling polyphenols of two diploid strawberry (Fragaria vesca) inbred lines using UHPLC-HRMSn. Food Chem. 2014, 146, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.J.; Inbaraj, B.S.; Chen, B.H. Determination of phenolic acids and flavonoids in Taraxacum formosanum Kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. Int. J. Mol. Sci. 2011, 13, 260–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vosseller, K.; Trinidad, J.C.; Chalkley, R.J.; Specht, C.G.; Thalhammer, A.; Lynn, A.J.; Snedecor, J.O.; Guan, S.; Medzihradszky, K.F.; Maltby, D.A.; et al. O-linked N-acetylglucosamine proteomics of postsynaptic density preparations using lectin weak affinity chromatography and mass spectrometry. Mol. Cell. Proteom. 2006, 5, 923–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekam, P.N.; Martini, S.; Nguefack, J.; Tagliazucchi, D.; Stefani, E. Phenolic compounds profile of water and ethanol extracts of Euphorbia hirta L. leaves showing antioxidant and antifungal properties. S. Afr. J. Bot. 2019, 127, 319–332. [Google Scholar] [CrossRef]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic profiles of leaves, grapes and wine of grapevine variety vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef] [Green Version]
- Gonzales, G.B. Mass Spectrometric Characterization of Flavonoids and In vitro Intestinal Transport and Bioactivity. Ph.D. Dissertation, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium, 2016. [Google Scholar]
- MassBank Record: MSBNK-RIKEN-PR100641. Available online: https://massbank.eu/MassBank/RecordDisplay?id=PR100641 (accessed on 9 September 2022).
- Visan, V.L.; Tamba-Berehoiu, R.M.; Popa, C.N.; Danaila-Guidea, S.M. Identification of the main volatile compound responsible for the aroma of Sauvignon Blanc wines. Sci. Pap. Manag. Econ. Eng. Agric. Rural. Dev. 2017, 17, 357–365. [Google Scholar]
- Parish, K.J.; Herbst-Johnstone, M.; Bouda, F.; Klaere, S.; Fedrizzi, B. Industrial scale fining influences the aroma and sensory profile of Sauvignon blanc. LWT 2017, 80, 423–429. [Google Scholar] [CrossRef]
- Fuchs, C.; Bakuradze, T.; Steinke, R.; Grewal, R.; Eckert, G.P.; Richling, E. Polyphenolic composition of extracts from winery by-products and effects on cellular cytotoxicity and mitochondrial functions in HepG2 cells. J. Funct. Foods 2020, 70, 103988. [Google Scholar] [CrossRef]
- Baenas, N.; Salar, F.J.; Domínguez-Perles, R.; García-Viguera, C. New UHPLC-QqQ-MS/MS method for the rapid and sensitive analysis of ascorbic and dehydroascorbic acids in plant foods. Molecules 2019, 24, 1632. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.H.; Guo, H.; Xu, W.B.; Ge, J.; Li, X.; Alimu, M.; He, D.J. Rapid identification of flavonoid constituents directly from PTP1B inhibitive extract of raspberry (Rubus idaeus L.) leaves by HPLC–ESI–QTOF–MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef] [Green Version]
- Schütz, K.; Kammerer, D.; Carle, R.; Schieber, A. Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 179–186. [Google Scholar] [CrossRef]
- Razgonova, M.; Zakharenko, A.; Pikula, K.; Manakov, Y.; Ercisli, S.; Derbush, I.; Kislin, E.; Seryodkin, I.; Sabitov, A.; Kalenik, T.; et al. LC-MS/MS Screening of Phenolic Compounds in Wild and Cultivated Grapes Vitis amurensis Rupr. Molecules 2021, 26, 3650. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Yang, W.Z.; Liu, K.D.; Qiao, X.; Li, B.J.; Cheng, J.; Feng, J.; Guo, D.-A.; Zhao, Y.Y. Characterization of flavonoids in Millettia nitida var. hirsutissima by HPLC/DAD/ESI-MSn. J. Pharm. Anal. 2012, 2, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliva, E.; Fanti, F.; Palmieri, S.; Viteritti, E.; Eugelio, F.; Pepe, A.; Compagnone, D.; Sergi, M. Predictive Multi Experiment Approach for the Determination of Conjugated Phenolic Compounds in Vegetal Matrices by Means of LC-MS/MS. Molecules 2022, 27, 3089. [Google Scholar] [CrossRef]
- Farid, M.M.; Emam, M.; Mohammed, R.S.; Hussein, S.R.; Marzouk, M.M. Green silver nanoparticles based on the chemical constituents of Glinus lotoides L.: In vitro anticancer and antiviral evaluation. Trop. J. Nat. Prod. Res. 2020, 4, 714–721. [Google Scholar] [CrossRef]
- Ricciutelli, M.; Moretti, S.; Galarini, R.; Sagratini, G.; Mari, M.; Lucarini, S.; Vittori, S.; Caprioli, G. Identification and quantification of new isomers of isopropyl-malic acid in wine by LC-IT and LC-Q-Orbitrap. Food Chem. 2019, 294, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Sheng, F.; Hu, B.; Jin, Q.; Wang, J.; Wu, C.; Luo, Z. The analysis of phenolic compounds in walnut husk and pellicle by uplc-q-orbitrap hrms and hplc. Molecules 2021, 26, 3013. [Google Scholar] [CrossRef]
- Hameed, A.; Liu, Z.; Wu, H.; Zhong, B.; Ciborowski, M.; Suleria, H.A.R. A Comparative and Comprehensive Characterization of Polyphenols of Selected Fruits from the Rosaceae Family. Metabolites 2022, 12, 271. [Google Scholar] [CrossRef]
- Longo, E.; Rossetti, F.; Merkyte, V.; Boselli, E. Disambiguation of Isomeric Procyanidins with Cyclic B-Type and Non-cyclic A-Type Structures from Wine and Peanut Skin with HPLC-HDX-HRMS/MS. J. Am. Soc. Mass Spectrom. 2018, 29, 2268–2277. [Google Scholar] [CrossRef]
- Huang, R.T.; Lu, J.F.; Inbaraj, B.S.; Chen, B.H. Determination of phenolic acids and flavonoids in Rhinacanthus nasutus (L.) kurz by high-performance-liquid-chromatography with photodiode-array detection and tandem mass spectrometry. J. Funct. Foods 2015, 12, 498–508. [Google Scholar] [CrossRef]
- Friščić, M.; Bucar, F.; Hazler Pilepić, K. LC-PDA-ESI-MSn analysis of phenolic and iridoid compounds from Globularia spp. J. Mass Spectrom. 2016, 51, 1211–1236. [Google Scholar] [CrossRef]
- Ben Said, R.; Hamed, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative Characterization of Polyphenolic Compounds in the Male Flowers of Phoenix dactylifera by Liquid Chromatography Coupled with Mass Spectrometry and DFT. Int. J. Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Pons-Mercadé, P.; Richard, T.; Krisa, S.; Teissèdre, P.L.; Jourdes, M. Crown procyanidin tetramer: A procyanidin with an unusual cyclic skeleton with a potent protective effect against amyloid-β-induced toxicity. Molecules 2019, 24, 1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oczkowski, E. The preferences and prejudices of Australian wine critics. J. Wine Res. 2017, 28, 56–67. [Google Scholar] [CrossRef]
| Code | Indication of Origin | Country of Origin | Alcohol (% Vol) | RS (g/L) | TA (g/L Tartaric Acid) | pH | Time and Temperature of Fermentation |
|---|---|---|---|---|---|---|---|
| CH_NZL_MA | Marlborough (South Island) | New Zealand | 14.0 | 3.2 | 5 | 3.5 | 21 days, 16 °C |
| CH_NZL_HA | Hawkes Bay (Nord Island) | New Zealand | 12.5 | 3.6 | 6.7 | 3.4 | 14 days, 12–14 °C |
| CH_ARG_ME | Mendoza | Argentina | 14.4 | 2.3 | 5.9 | 3.4 | 12 days, 13 °C |
| CH_AUS_SE | South-Eastern Australia | Australia | 13.3 | 4.5 | 6.1 | 3.3 | 25–32 days, 12–18 °C |
| CH_RSA_CO | Coastal (dry land) | South Africa | 13.5 | 2.1 | 5.1 | 3.6 | 13 °C |
| CH_RSA_WC | Western Cape (irrigated area) | South Africa | 13.2 | 1.9 | 5.7 | 3.8 | |
| CH_CLE_CV | Central Valley | Chile | 12.9 | 1.6 | 5.6 | 3.3 | 10–12 °C |
| CH_ITA_SP | Sicilia/Puglia | Italy | 11.9 | 3.7 | 5.2 | 3.2 | 8 days, 14–16 °C |
| CH_ITA_VF | Veneto/Friuli | Italy | 12.3 | 2.8 | 6.4 | 3.3 | 18–20 °C |
| Code | Indication of Origin | Country of Origin | Alcohol (% Vol) | RS (g/L) | TA (g/L Tartaric Acid) | pH | Time and Temperature of Fermentation |
|---|---|---|---|---|---|---|---|
| SA_NZL_MA | Marlborough (South Island) | New Zealand | 13.0 | 3.8 | 7.4 | 3.1 | 14–21 days, 12 °C |
| SA_NZL_MR | Martinborough (Nord Island) | New Zealand | 12.5 | 2.5 | 7.7 | 2.9 | 14 days, 12–14 °C |
| SA_ARG_ME | Mendoza | Argentina | 13.0 | 2.3 | 6.5 | 3.3 | 12 days, 15 °C |
| SA_AUS_SE | South-Eastern Australia | Australia | 11.7 | 4.41 | 6.4 | 3.2 | 17–21 days, 12–18 °C |
| SA_RSA_CO | Coastal (dry land) | South Africa | 13.5 | 2.1 | 5.6 | 3.4 | 13 °C |
| SA_RSA_WC | Western Cape (irrigated area) | South Africa | 13.3 | 1.95 | 5.43 | 3.5 | |
| SA_CLE_CV | Central Valley | Chile | 13.0 | 1.7 | 6.9 | 3.1 | 8–10 °C |
| SA_ITA_SP | Sicilia/Puglia | Italy | 12.1 | 1.1 | 5.4 | 3.3 | 8 days, 14–16 °C |
| SA_ITA_VF | Veneto/Friuli | Italy | 12.9 | 4.2 | 6.3 | 3.3 | 18–20 °C |
| Origins | Zone | Climate |
|---|---|---|
| New Zealand | Marlborough (South Island) | Warm and temperate. Heavy rainfall even in driest months. Oceanic climate [13]. |
| Hawkes Bay (North Island) | Mild, generally warm, and temperate with a significant amount of rainfall during the year [14]. | |
| Martinborough (North Island) | Mild, generally warm, and temperate. Significant amount of rainfall during the year [15]. | |
| Argentina | Mendoza | Hot and clear summer, cold and cloudy winter, and dry all year round [16]. |
| Australia | South-Eastern Australia | Cool, humid eastern uplands, temperate southeast mallee, inland subtropical northern areas, and hot, dry, arid, and semi-arid country in the far west [17]. |
| South Africa | Coastal (dry land) | Dry, summer-rainfall, relatively warm in winter [18]. |
| Western Cape (irrigated area) | Mediterranean with warm and dry summers. Mild and moist winters. Low summer rainfall [18]. | |
| Chile | Central Valley | Mediterranean climate with cool, dry summers. Mild rainy winters [19]. |
| Italy | Sicilia/Puglia | Mediterranean with hot summers and short mild winters [20]. Mediterranean climate with hot sunny summers and mild winters, dry [21]. |
| Veneto/Friuli | Moderately continental hill and plain areas; alpine region characterised by cool summers and cold winters with frequent snowfalls [22]. The climate in Friuli Venezia Giulia ranges from the sub-Mediterranean climate of the coastal areas to the more humid temperate climate of the plains, to the Alpine climate in the mountains [23]. |
| Wine | Attributes | Descriptors | Description |
|---|---|---|---|
| CHARDONNAY | VISUAL | ||
| GREENISH | From pale grass to an intense green | ||
| YELLOWISH | From a pale straw to a rich yellow | ||
| OLFACTORY | |||
| CITRUS FRUIT-a | Grapefruit, lemon | Aromas from citrus fruit (zest of lemon), particularly grapefruit | |
| POME TREE FRUIT-a | Green apple, pear | Aromas from pome tree fruit (green apple, pear) | |
| STONE FRUIT-a | Apricot, peach | Aromas from yellow tree fruit (apricot, peach) | |
| VEGETATIVE-a | Green bell pepper | Aromas from green bell pepper when cut | |
| FLORAL-a | Green tea, rose | The fresh scent of wildflowers, jasmine, green tea (infused), and rose water | |
| CITRUS FRUIT-a | Grapefruit, lemon | Aromas from citrus fruit (zest of lemon), particularly grapefruit | |
| POME TREE FRUIT-a | Green apple, Pear | Aromas from pome tree fruit (green apple, pear) | |
| STONE FRUIT-a | Apricot, peach | Aromas from yellow tree fruit (apricot, peach) | |
| GUSTATORY | |||
| WARMNESS | Alcohol | Warm/burning sensation in the mouth | |
| ACIDITY-SOURNESS | Acid (citric, tartaric, malic, lactic) | Acid taste resembling that of vinegar, lemon juice etc. | |
| BITTERNESS | Caffeine | The bitter taste of caffeine (coffee) | |
| FLAVOUR | |||
| CITRUS FRUIT-f | Grapefruit, lemon | The flavour of citrus fruit: grapefruit, orange, lemon zests | |
| TROPICAL FRUIT-f | Ananas | The flavour of tropical fruit: ananas | |
| WOODY-f | Wood, oak | The flavour of fresh and toasted wood (oak, resinous aromas) | |
| CITRUS FRUIT-f | Grapefruit, lemon | The flavour of citrus fruit: grapefruit, orange, lemon zests | |
| SAUVIGNON BLANC | VISUAL | ||
| GREENISH | From pale grass to an intense green | ||
| YELLOWISH | From a pale straw to a rich yellow | ||
| OLFACTORY | |||
| GRAPEFRUIT CITRUS-a | Grapefruit, citrus zest | Aromas from citrus fruit (zest of lemon), particularly grapefruit | |
| TROPICAL FRUIT-a | Passion fruit, tropical fruit | Tropical fruit aromas, passion fruit (mango, maracuja) | |
| STONE FRUIT-a | Apricot, peach | Aromas from three yellow fruit (apricot, peach) | |
| DRIED FRUIT-a | Nutty, almond | Aromas from dried fruit (nutty, almond, hazelnut, walnut) | |
| WOODY-a | Wood, oak | The aroma of fresh and toasted wood (oak, resinous aromas) | |
| VANILLA-a | Vanilla, vanilla sugar | The aroma of vanilla (vanilla sugar, aromas like cake) | |
| GREEN GRASS | Fresh cut grass | Cut grass aromas or hay | |
| TOMATO STEM-a | Tomato stem | The aroma of tomato stem | |
| GREEN BELL PEPPER-a | Green bell pepper | The aroma of green bell pepper when cut | |
| FLOWERS-a | Wildflowers, jasmine | The fresh scent of wildflowers and jasmine | |
| HONEY-a | Honey | Aromas of wildflower-honey | |
| GUSTATORY | |||
| WARMNESS | Alcohol | Warm/burning sensation in the mouth | |
| SOURNESS | Acid (citric, tartaric, malic) | The acid taste resembles that of vinegar, lemon juice, etc. | |
| SWEETNESS | Sucrose. glucose, fructose | Sensation typical of sweet food/beverages | |
| FLAVOUR | |||
| CITRUS-f | Orange, grapefruit, lemon and their zest | The flavour of citrus fruit: grapefruit, orange, lemon, and zest. | |
| WOODY-f | Wood, oak | The flavour of fresh and toasted wood (oak, resinous aromas) | |
| OVERALL QUALITY JUDGEMENT (OQJ) | An objective answer by the panel on the quality of the product | ||
| Extraction Temperature (°C) | Incubation Time (min) | Extraction Time (min) | Fiber Composition | |
|---|---|---|---|---|
| Type | Quantitative | Quantitative | Quantitative | Qualitative |
| Min level | 30 | 5 | 10 | PDMS Polyacrylate CAR-PDMS DVB-CAR-PDMS |
| Max level | 60 | 40 | 60 |
| Statistic | Tropical Fruit | Woody |
|---|---|---|
| R2 | 0.866 | 0.968 |
| Adjusted R2 | 0.817 | 0.956 |
| MSE | 0.016 | 0.021 |
| RMSE | 0.125 | 0.143 |
| Press | 0.593 | 0.662 |
| Q2 | 0.539 | 0.905 |
| Colour Green | Colour Yellow | Warmness | Sourness | |
|---|---|---|---|---|
| R2 | 0.795 | 0.657 | 0.730 | 0.719 |
| Std. deviation | 0.149 | 0.378 | 0.205 | 0.265 |
| MSE | 0.015 | 0.098 | 0.029 | 0.048 |
| RMSE | 0.124 | 0.314 | 0.170 | 0.220 |
| Grapefruit Citrus | Passion Fruit | Woody | Vanilla | Tomato Stem | Green Pepper | Flowers | Honey | |
|---|---|---|---|---|---|---|---|---|
| R2 | 0.771 | 0.790 | 0.663 | 0.640 | 0.638 | 0.664 | 0.639 | 0.727 |
| Std. deviation | 0.264 | 0.283 | 0.392 | 0.363 | 0.457 | 0.435 | 0.329 | 0.216 |
| MSE | 0.044 | 0.050 | 0.096 | 0.083 | 0.131 | 0.118 | 0.067 | 0.029 |
| RMSE | 0.209 | 0.224 | 0.310 | 0.287 | 0.362 | 0.344 | 0.260 | 0.171 |
| Citrus Fruit F | |
|---|---|
| R2 | 0.897 |
| Adjusted R2 | 0.743 |
| MSE | 0.038 |
| RMSE | 0.195 |
| Press | 1.697 |
| Q2 | 0.234 |
| Dimers 2-OH (PD) x.7 | Tetramers c-OH x.14 | Pentamers x.20 | %c-4 | |
|---|---|---|---|---|
| SA | 18,298.216 b | 4153.848 b | 84.447 b | 73.348 a |
| CH | 11,328.794 a | 3158.361 a | 12.081 a | 94.812 b |
| Pr > F(Model) | 0.025 | 0.013 | <0.0001 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poggesi, S.; Darnal, A.; Ceci, A.T.; Longo, E.; Vanzo, L.; Mimmo, T.; Boselli, E. Fusion of 2DGC-MS, HPLC-MS and Sensory Data to Assist Decision-Making in the Marketing of International Monovarietal Chardonnay and Sauvignon Blanc Wines. Foods 2022, 11, 3458. https://doi.org/10.3390/foods11213458
Poggesi S, Darnal A, Ceci AT, Longo E, Vanzo L, Mimmo T, Boselli E. Fusion of 2DGC-MS, HPLC-MS and Sensory Data to Assist Decision-Making in the Marketing of International Monovarietal Chardonnay and Sauvignon Blanc Wines. Foods. 2022; 11(21):3458. https://doi.org/10.3390/foods11213458
Chicago/Turabian StylePoggesi, Simone, Aakriti Darnal, Adriana Teresa Ceci, Edoardo Longo, Leonardo Vanzo, Tanja Mimmo, and Emanuele Boselli. 2022. "Fusion of 2DGC-MS, HPLC-MS and Sensory Data to Assist Decision-Making in the Marketing of International Monovarietal Chardonnay and Sauvignon Blanc Wines" Foods 11, no. 21: 3458. https://doi.org/10.3390/foods11213458






