Propionate and Butyrate Inhibit Biofilm Formation of Salmonella Typhimurium Grown in Laboratory Media and Food Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bacterial Strains and Culture Conditions
2.3. Preparation of Milk and Chicken Broth for Biofilm Growth
2.4. Minimum Inhibitory Concentrations (MICs) and Sub-Inhibitory Concentration (SICs) Assay
2.5. Specific Biofilm Formation Inhibition Assay
2.6. Scanning Electron Microscopy (SEM)
2.7. XTT Reduction Assay
2.8. Fluorescence Microscopic Analysis
2.9. Quantitative QS Inhibition Assay
2.10. Swimming Motility Assay
2.11. Adhesion to and Invasion of Caco-2 Cells
2.12. Quantitative Real-Time PCR
2.13. Statistical Analysis
3. Results
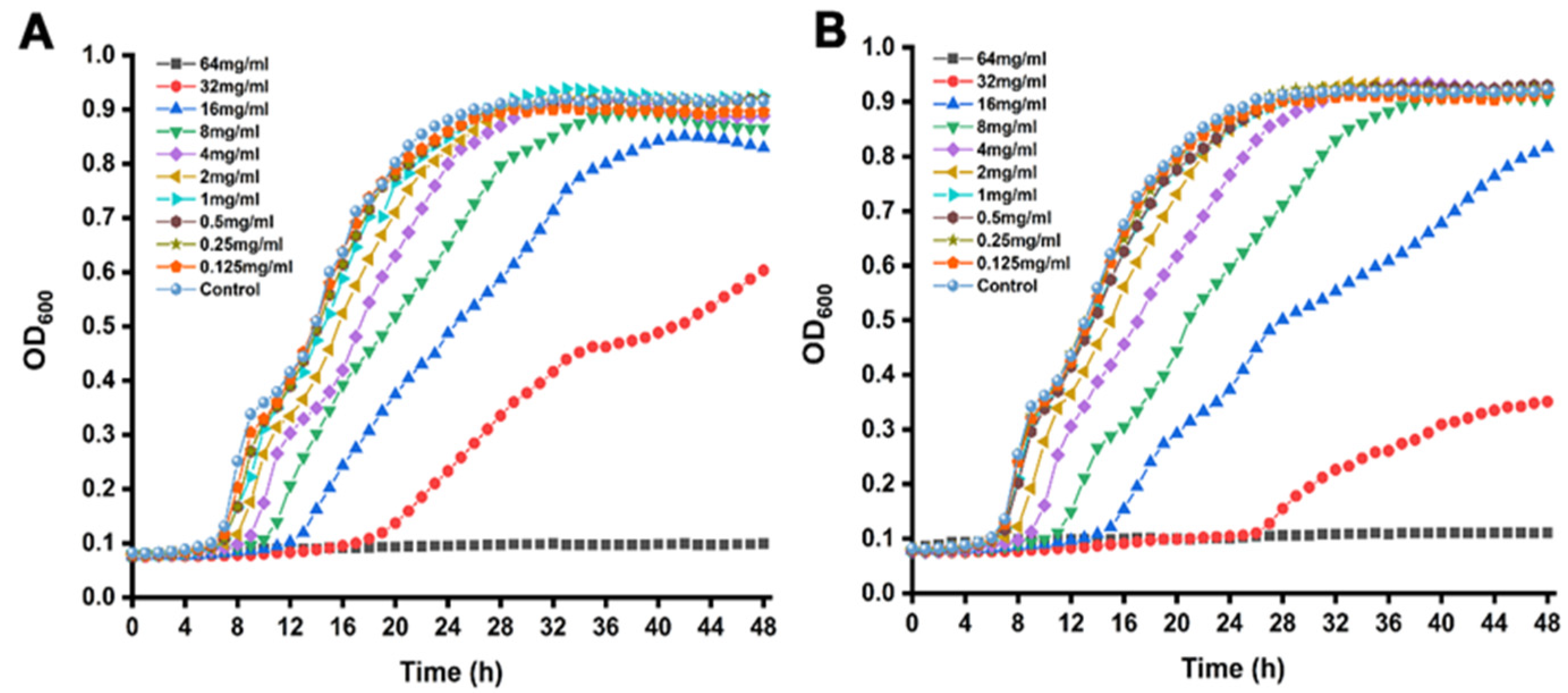
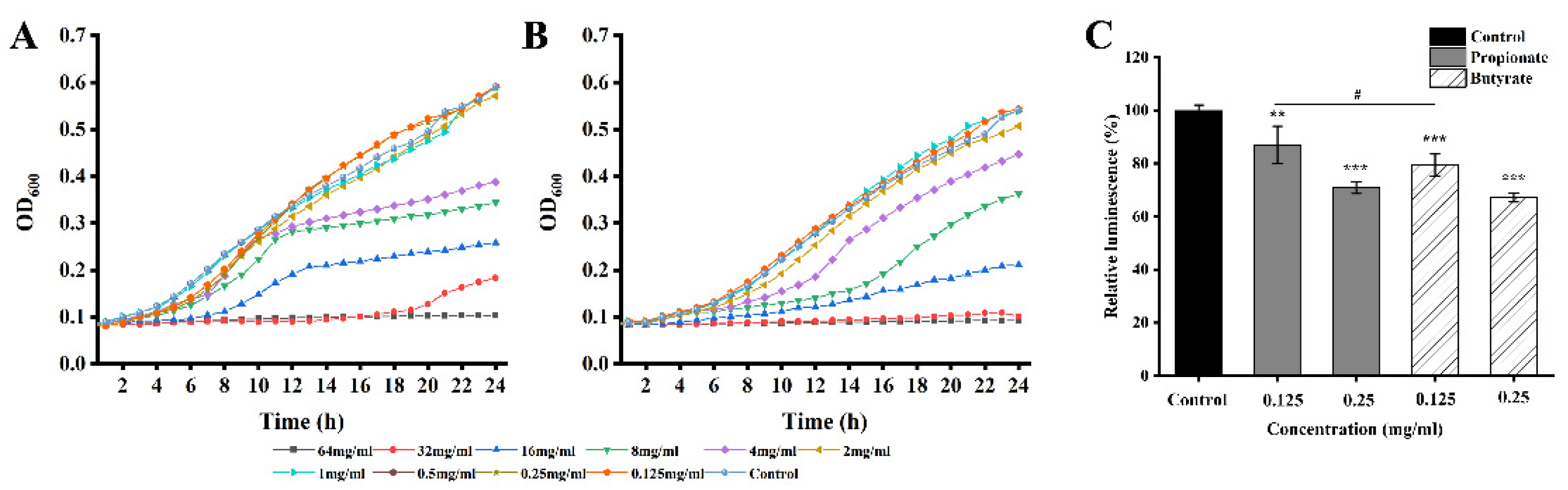
3.1. MICs and SICs
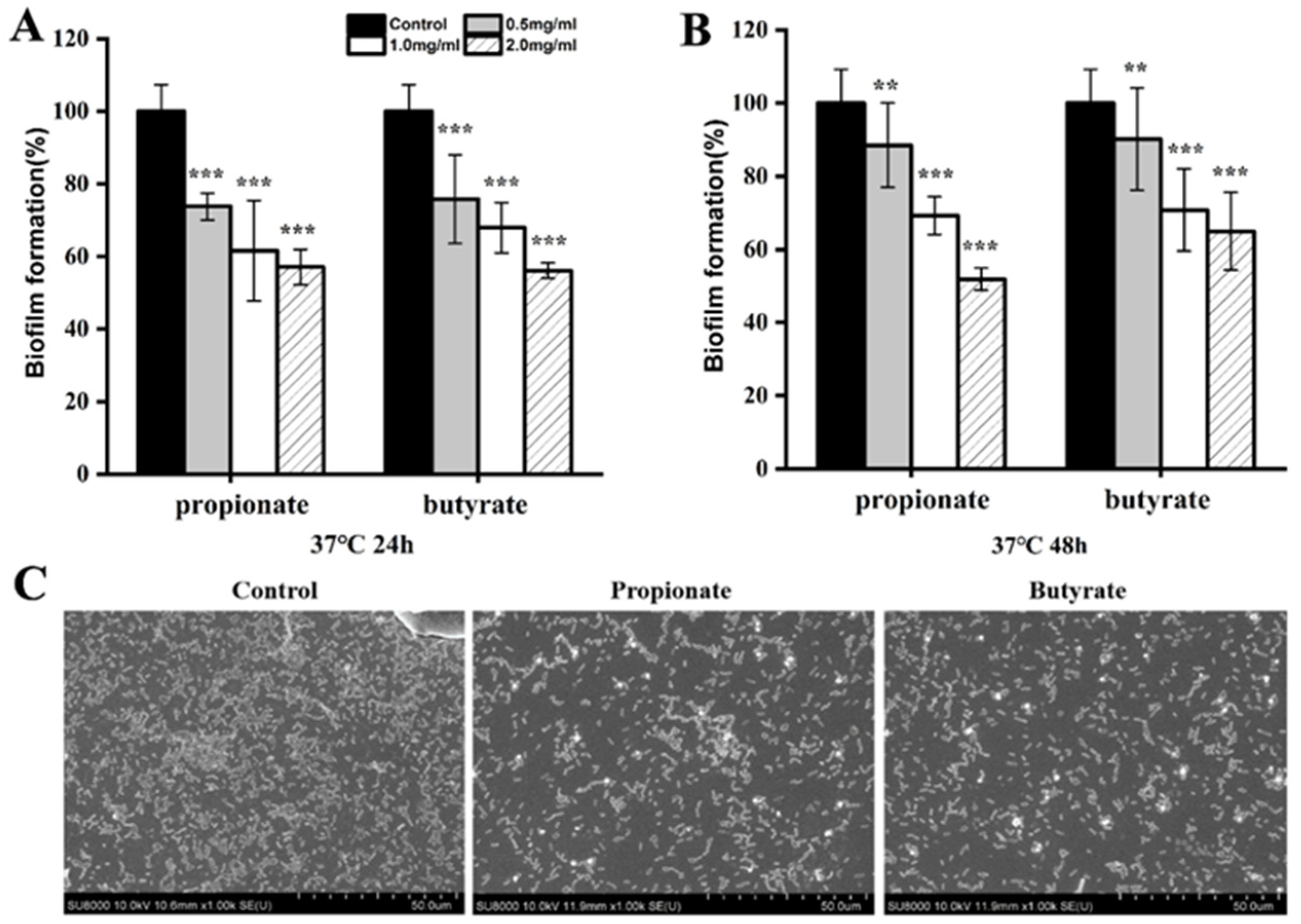
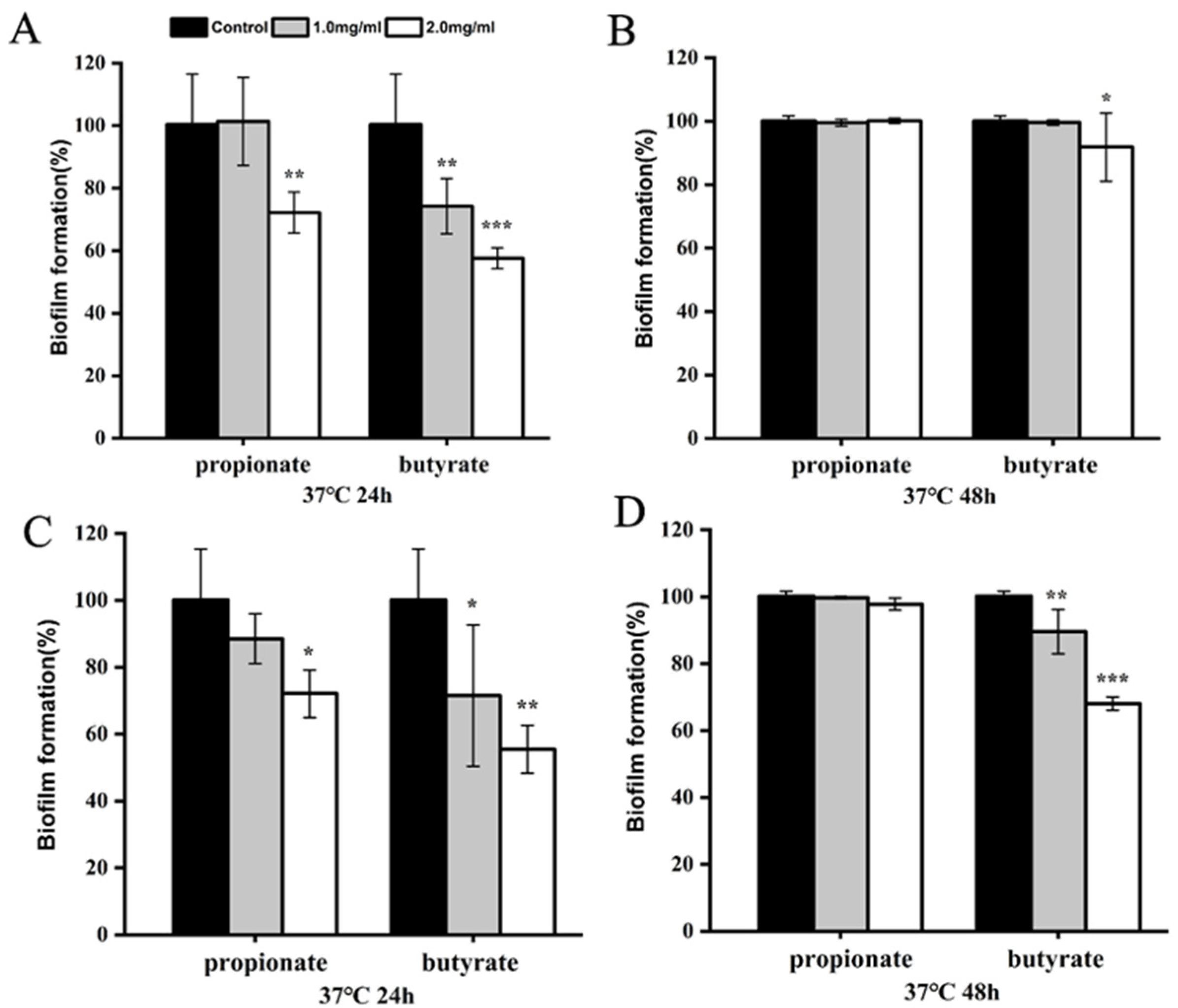
3.2. Biofilm Reduction
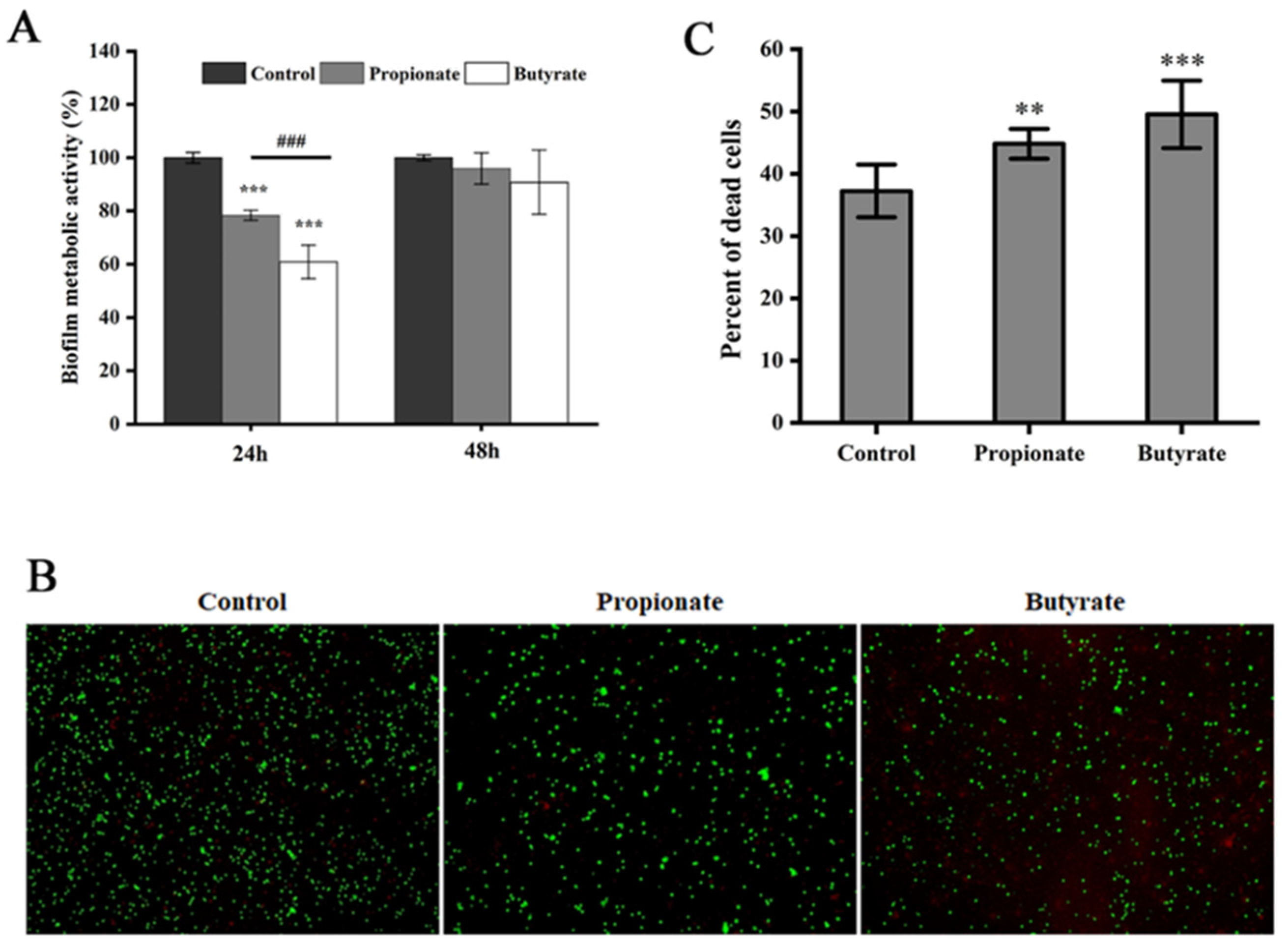
3.3. Biofilm Metabolic Activity
3.4. AI-2 Quorum Sensing
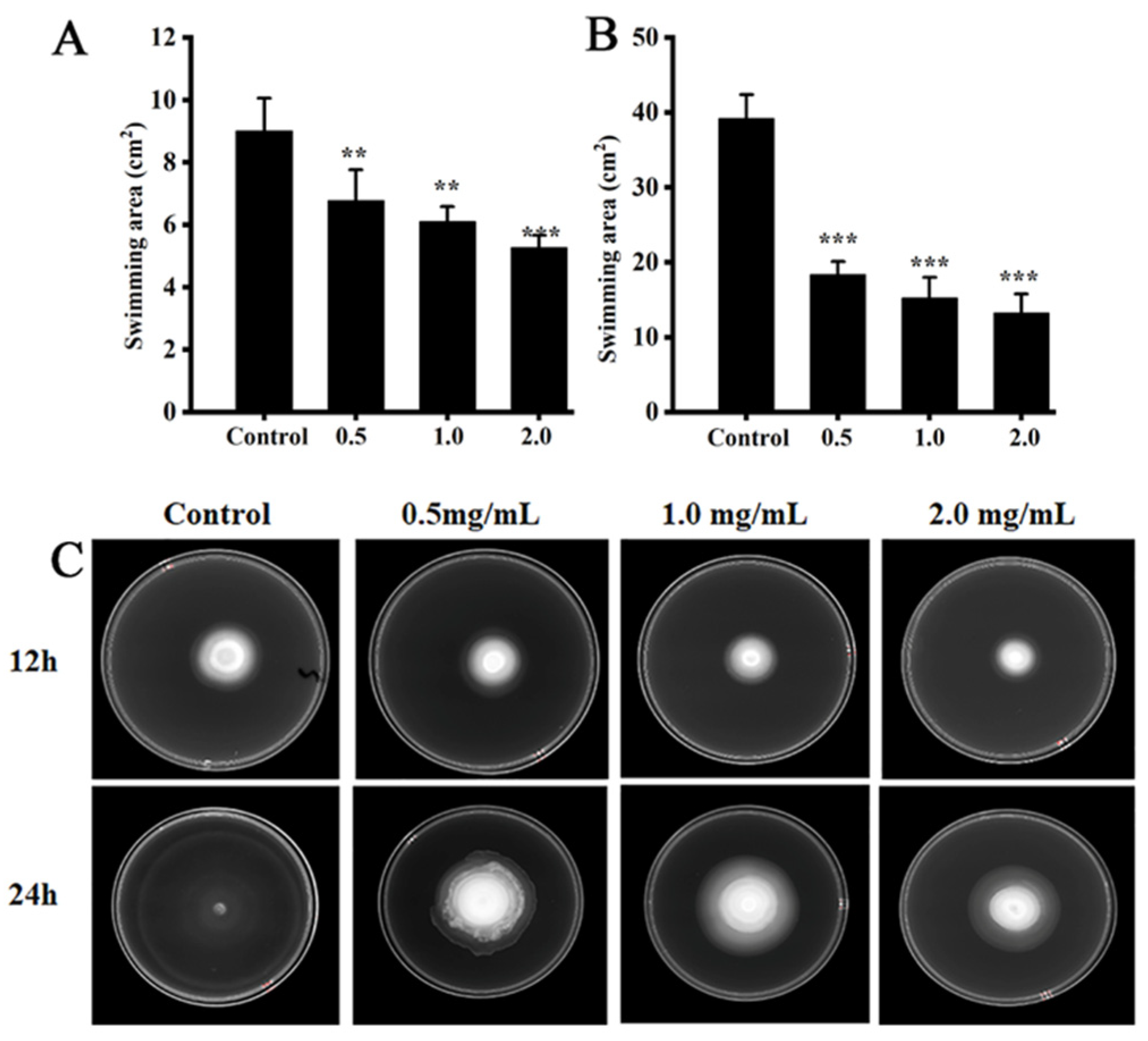
3.5. Swimming Motility
3.6. Bacterial Adhesion and Invasion
3.7. Genes Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- EFSA. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017, 15, 5077. [Google Scholar]
- Sharan, M.; Vijay, D.; Dhaka, P.; Bedi, J.S.; Gill, J.P.S. Biofilms as a microbial hazard in the food industry: A scoping review. J. Appl. Microbiol. 2022, 133, 2210–2234.e4. [Google Scholar] [CrossRef] [PubMed]
- Bronner, D.N.; Faber, F.; Olsan, E.E.; Byndloss, M.X.; Sayed, N.A.; Xu, G.; Yoo, W.; Kim, D.; Ryu, S.; Lebrilla, C.B.; et al. Genetic Ablation of Butyrate Utilization Attenuates Gastrointestinal Salmonella Disease. Cell Host Microbe. 2018, 23, 266–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Gao, J.; Liu, H.; Liu, J.; Jin, T.; Qin, N.; Ren, X.; Xia, X. Antibiofilm effect of sodium butyrate against Vibrio parahaemolyticus. Food Control 2022, 131, 108422. [Google Scholar] [CrossRef]
- Kim, N.N.; Kim, W.J.; Kang, S.S. Anti-biofilm effect of crude bacteriocin derived from Lactobacillus brevis DF01 on Escherichia coli and Salmonella typhimurium. Food Control 2019, 98, 274–280. [Google Scholar] [CrossRef]
- Luppens, S.B.I.; Reij, M.W.; van der Heijden, R.W.L.; Rombouts, F.M.; Abee, T. Development of a Standard Test To Assess the Resistance of Staphylococcus aureus Biofilm Cells to Disinfectants. Appl. Environ. Microbiol. 2002, 68, 4194. [Google Scholar] [CrossRef] [Green Version]
- Desai, S.K.; Winardhi, R.S.; Periasamy, S.; Dykas, M.M.; Jie, Y.; Kenney, L.J. The horizontally-acquired response regulator SsrB drives a Salmonella lifestyle switch by relieving biofilm silencing. eLife 2016, 5, e10747. [Google Scholar] [CrossRef]
- Desai, S.K.; Padmanabhan, A.; Harshe, S.; Zaidel-Bar, R.; Kenney, L.J. Salmonella biofilms program innate immunity for persistence in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2019, 116, 12462–12467. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Dong, Y.; Wang, G.; Xu, X.; Zhou, G. Effect of growth media on gene expression levels in Salmonella Typhimurium biofilm formed on stainless steel surface. Food Control 2016, 59, 546–552. [Google Scholar] [CrossRef]
- Shatila, F.; Yaşa, İ.; Yalçın, H.T. Yalçın Biofilm Formation by Salmonella enterica Strains. Curr. Microbiol. 2021, 78, 1150–1158. [Google Scholar] [CrossRef]
- Mizan, M.; Jahid, I.K.; Kim, M.; Lee, K.H.; Kim, T.J.; Ha, S.D. Variability in biofilm formation correlates with hydrophobicity and quorum sensing among Vibrio parahaemolyticus isolates from food contact surfaces and the distribution of the genes involved in biofilm formation. Biofouling 2016, 32, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Manson, M.D. Bacterial motility and chemotaxis. Adv. Microb. Physiol. 1992, 33, 277–304. [Google Scholar] [PubMed]
- Roy, P.K.; Song, M.G.; Park, S.Y. Park Impact of Quercetin against Salmonella Typhimurium Biofilm Formation on Food-Contact Surfaces and Molecular Mechanism Pattern. Foods 2022, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.e7. [Google Scholar] [CrossRef] [Green Version]
- Chassaing, B.; Cascales, E. Antibacterial Weapons: Targeted Destruction in the Microbiota. Trends Microbiol. 2018, 26, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Brown, H.L.; Reuter, M.; Salt, L.J.; Cross, K.L.; Betts, R.P.; van Vliet, A.H.M. Chicken Juice Enhances Surface Attachment and Biofilm Formation of Campylobacter jejuni. Appl. Environ. Microbiol. 2014, 80, 7053–7060. [Google Scholar] [CrossRef] [Green Version]
- Lamas, A.; Regal, P.; Vázquez, B.; Cepeda, A.; Franco, C.M. Short chain fatty acids commonly produced by gut microbiota influence Salmonella enterica motility, biofilm formation, and gene expression. Antibiotics 2019, 8, 265. [Google Scholar] [CrossRef] [Green Version]
- Lamas, A.; Regal, P.; Vázquez, B.; Miranda, J.M.; Cepeda, A.; Franco, C.M. Influence of milk, chicken residues and oxygen levels on biofilm formation on stainless steel, gene expression and small RNAs in Salmonella enterica. Food Control 2018, 90, 1–9. [Google Scholar] [CrossRef]
- Barnes, L.M.; Lo, M.F.; Adams, M.R.; Chamberlain, A. Effect of Milk Proteins on Adhesion of Bacteria to Stainless Steel Surfaces. Appl. Environ. Microbiol. 1999, 65, 4543–4548. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q.; Sun, L.; Fang, T.; Wang, Y.; Li, Z.; Wang, X.; Wu, M.; Zhang, H. Biofilm Formation of Listeria monocytogenes and Pseudomonas aeruginosa in a Simulated Chicken Processing Environment. Foods 2022, 11, 1917. [Google Scholar] [CrossRef]
- Lee, D.U.; Park, Y.J.; Yu, H.H.; Jung, S.C.; Park, J.H.; Lee, D.H.; Lee, N.K.; Paik, H.D. Antimicrobial and Antibiofilm Effect of ε-Polylysine against Salmonella Enteritidis, Listeria monocytogenes, and Escherichia coli in Tryptic Soy Broth and Chicken Juice. Foods 2021, 10, 2211. [Google Scholar] [CrossRef] [PubMed]
- Parlapani, F.F.; Mallouchos, A.; Haroutounian, S.A.; Boziaris, I.S. Volatile organic compounds of microbial and non-microbial origin produced on model fish substrate un-inoculated and inoculated with gilt-head sea bream spoilage bacteria. LWT-Food Sci. Technol. 2017, 78, 54–62. [Google Scholar] [CrossRef]
- CLSI (Clinical and Laboratory Standards Institute). Antimicrobial Susceptibility Testing Standards; Clinical and Laboratory Standards Institute: Atlanta, GA, USA, 2009; p. 12. [Google Scholar]
- Li, G.; Yan, C.; Xu, Y.; Feng, Y.; Wu, Q.; Lv, X.; Yang, B.; Wang, X.; Xia, X. Punicalagin inhibits Salmonella virulence factors and has anti-quorum-sensing potential. Appl. Environ. Microbiol. 2014, 80, 6204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Guo, D.; Hua, Z.; Sun, H.; Zheng, Z.; Xia, X.; Shi, C. Attenuation of Multiple Vibrio parahaemolyticus Virulence Factors by Citral. Front. Microbiol. 2019, 10, 894. [Google Scholar] [CrossRef] [Green Version]
- Amalaradjou, M.A.R.; Kim, K.S.; Venkitanarayanan, K. Venkitanarayanan Sub-Inhibitory Concentrations of Trans-Cinnamaldehyde Attenuate Virulence in Cronobacter sakazakii In Vitro. Int. J. Mol. Sci. 2014, 15, 8639–8655. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.; Kim, J.; Kim, W.J. Isolation and characterization of bacteriocin-producing Pediococcus acidilactici HW01 from malt and its potential to control beer spoilage lactic acid bacteria. Food Control 2017, 80, 59–66. [Google Scholar] [CrossRef]
- Sorbara, M.; Dubin, K.; Littmann, E.; Moody, T.; Fontana, E.; Seok, R.; Leiner, I.; Taur, Y.; Peled, J.; van den Brink, M.; et al. Inhibiting antibiotic-resistant Enterobacteriaceae by microbiota-mediated intracellular acidification. J. Exp. Med. 2019, 216, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Osbelt, L.; Thiemann, S.; Smit, N.; Lesker, T.R.; Strowig, T. Variations in microbiota composition of laboratory mice influence Citrobacter rodentium infection via variable short-chain fatty acid production. PLoS Pathog. 2020, 16, e1008448. [Google Scholar] [CrossRef] [Green Version]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Immerseel, F.V. Butyrate Specifically Down-Regulates Salmonella Pathogenicity Island 1 Gene Expression. Appl. Environ. Microbiol. 2006, 72, 946–949. [Google Scholar] [CrossRef] [Green Version]
- Merino, L.; Procura, F.; Trejo, F.M.; Bueno, D.J.; Golowczyc, M.A. Biofilm formation by Salmonella sp. in the poultry industry: Detection, control and eradication strategies. Food Res Int. 2019, 119, 530–540. [Google Scholar] [CrossRef] [Green Version]
- Brownlie, E.J.E.; Chaharlangi, D.; Wong, E.O.; Kim, D.; Navarre, W.W. Acids produced by Lactobacilli inhibit the growth of commensal Lachnospiraceae and S24-7 bacteria. Gut. Microbes. 2022, 14, 2046452. [Google Scholar] [CrossRef]
- Lories, B.; Belpaire, T.; Yssel, A.; Ramon, H.; Steenackers, H.P. Agaric acid reduces Salmonella biofilm formation by inhibiting flagellar motility. Biofilm 2020, 2, 100022. [Google Scholar] [CrossRef]
- Sun, J.; Sun, Z.; Wang, D.; Liu, F.; Wang, D. Contribution of ultrasound in combination with chlorogenic acid against Salmonella enteritidis under biofilm and planktonic condition. Microb. Pathog. 2022, 165, 105489. [Google Scholar] [CrossRef]
- Xu, J.G.; Hu, H.X.; Chen, J.Y.; Xue, Y.S.; Kodirkhonov, B.; Han, B.Z. Comparative study on inhibitory effects of ferulic acid and p-coumaric acid on Salmonella Enteritidis biofilm formation. World J. Microbiol. Biotechnol. 2022, 38, 136. [Google Scholar] [CrossRef]
- Borges, A.; Saavedra, M.J.; Simões, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef]
- Chen, K.; Peng, C.; Chi, F.; Yu, C.; Yang, Q.; Li, Z. Antibacterial and Antibiofilm Activities of Chlorogenic Acid Against Yersinia enterocolitica. Front. Microbiol. 2022, 13, 885092. [Google Scholar] [CrossRef]
- Sun, J.; Wang, D.; Sun, Z.; Liu, F.; Du, L.; Wang, D. The combination of ultrasound and chlorogenic acid to inactivate Staphylococcus aureus under planktonic, biofilm, and food systems. Ultrason Sonochem. 2021, 80, 105801. [Google Scholar] [CrossRef]
- Li, J.; Feng, J.; Ma, L.; Núñez, C.d.; Gölz, G.; Lu, X. Effects of meat juice on biofilm formation of Campylobacter and Salmonella. Int. J. Food Microbiol. 2017, 253, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Huber, B.; Riedel, K.; Köthe, M.; Molin, S.; Eberl, L. Genetic analysis of functions involved in the late stages of biofilm development in Burkholderia cepacia H111. Mol. Microbiol. 2002, 46, 411–426. [Google Scholar] [CrossRef]
- Lemon, K.P.; Higgins, D.E.; Kolter, R. Flagellar motility is critical for Listeria monocytogenes biofilm formation. J. Bacteriol. 2007, 189, 4418–4424. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Kami-Ike, N.; Yokota, J.; Kudo, S.; Minamino, T.; Namba, K. Effect of Intracellular pH on the Torque–Speed Relationship of Bacterial Proton-Driven Flagellar Motor. J. Mol. Biol. 2009, 386, 332–338. [Google Scholar] [CrossRef]
- Monteiro, C.; Papenfort, K.; Hentrich, K.; Ahmad, I.; Le Guyon, S.; Reimann, R.; Grantcharova, N.; Römling, U. Hfq and Hfq-dependent small RNAs are major contributors to multicellular development in Salmonella enterica serovar Typhimurium. RNA Biol. 2012, 9, 489–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, T.; Wang, W.; Xia, M.; Liang, S.; Hu, G.; Ye, H.; Cao, Q.; Dong, Z.; Zhang, C.; Feng, D.; et al. Involvement of the Heat Shock Protein HtpG of Salmonella Typhimurium in Infection and Proliferation in Hosts. Front. Cell Infect Microbiol. 2021, 11, 758898. [Google Scholar] [CrossRef] [PubMed]
- Defoirdt, T.; Brackman, G.; Coenye, T. Quorum sensing inhibitors: How strong is the evidence? Trends Microbiol. 2013, 21, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Muciño, E.; Arenas-Hernández, M.M.P.; Luna-Guevara, M.L. Mechanisms of Inhibition of Quorum Sensing as an Alternative for the Control of E. coli and Salmonella. Microorganisms 2022, 10, 884. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wang, Y.; Lin, Z.; Wang, D.; Sun, H. Resistance risk induced by quorum sensing inhibitors and their combined use with antibiotics: Mechanism and its relationship with toxicity. Chemosphere 2021, 265, 129153. [Google Scholar] [CrossRef]
- Cappuyns, A.M.; Bernaerts, K.; Vanderleyden, J.; Impe, J.F.V. A dynamic model for diauxic growth, overflow metabolism, and AI-2-mediated cell–cell communication of Salmonella Typhimurium based on systems biology concepts. Biotechnol. Bioeng. 2010, 102, 280–293. [Google Scholar] [CrossRef]
- Ryan, D.; Mukherjee, M.; Suar, M. The expanding targetome of small RNAs in Salmonella Typhimurium. Biochimie 2017, 137, 69–77. [Google Scholar] [CrossRef]
- Kader, A.; Simm, R.; Gerstel, U.; Morr, M.; Römling, U. Hierarchical involvement of various GGDEF domain proteins in rdar morphotype development of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2010, 60, 602–616. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, L.; Yan, Y.; Yang, K.; Zhu, L. Lactic Acid and Peroxyacetic Acid Inhibit Biofilm of Escherichia coli O157:H7 Formed in Beef Extract. Foodborne Pathog. Dis. 2021, 41, 744–751. [Google Scholar] [CrossRef]


| NO. | Gene | Primers |
|---|---|---|
| 1 | SipA | CGCTGTCAGGGGAAATTAAA |
| ATTATCGCTTTCTTACCGGC | ||
| 2 | SipB | GCCGATGAAATTGTGAAGGC |
| CCTAATCCTTCCAGCGCTTT | ||
| 3 | SipC | GAATAAATCCCGCCGCTTAT |
| GGTCACTGACTTTACTGCTG | ||
| 4 | arcZ | ACTGCGCCTTTGACATCATC |
| CGAATACTGCGCCAACACCA | ||
| 5 | csgD | TCCTGGTCTTCAGTAGCGTAA |
| TATGATGGAAGCGGATAAGAA | ||
| 6 | adrA | GAAGCTCGTCGCTGGAAGTC |
| TTCCGCTTAATTTAATGGCCG | ||
| 7 | pipB | GCTCCTGTTAATGATTTCGCTAAAG |
| GCTCAGACTTAACTGACACCAAACTAA | ||
| 8 | HilD | TAACGTGACGCTTGAAGAGG |
| GGTACCGCCATTTTGGTTTG | ||
| 9 | 16s rRNA | AGGCCTTCGGGTTGTAAAGT |
| GTTAGCCGGTGCTTCTTCTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhu, W.; Qin, N.; Ren, X.; Xia, X. Propionate and Butyrate Inhibit Biofilm Formation of Salmonella Typhimurium Grown in Laboratory Media and Food Models. Foods 2022, 11, 3493. https://doi.org/10.3390/foods11213493
Liu J, Zhu W, Qin N, Ren X, Xia X. Propionate and Butyrate Inhibit Biofilm Formation of Salmonella Typhimurium Grown in Laboratory Media and Food Models. Foods. 2022; 11(21):3493. https://doi.org/10.3390/foods11213493
Chicago/Turabian StyleLiu, Jiaxiu, Wenxiu Zhu, Ningbo Qin, Xiaomeng Ren, and Xiaodong Xia. 2022. "Propionate and Butyrate Inhibit Biofilm Formation of Salmonella Typhimurium Grown in Laboratory Media and Food Models" Foods 11, no. 21: 3493. https://doi.org/10.3390/foods11213493
APA StyleLiu, J., Zhu, W., Qin, N., Ren, X., & Xia, X. (2022). Propionate and Butyrate Inhibit Biofilm Formation of Salmonella Typhimurium Grown in Laboratory Media and Food Models. Foods, 11(21), 3493. https://doi.org/10.3390/foods11213493







