Structural Characteristics and Emulsifying Properties of Soy Protein Isolate Glycated with Galacto-Oligosaccharides under High-Pressure Homogenization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SPI
2.3. Preparation of SPI-GOS Conjugates by HPH
- (1)
- Traditional wet heating Maillard reaction group (0.1 MPa): SPI-GOS mixed solution was heated in a water bath at 70 °C for 10 min to obtain SPI-GOS conjugates. This sample was named SPI-GOS-0.1.
- (2)
- HPH treatment groups: SPI-GOS mixed solution was subjected to HPH (ATS Engineering Limited, Suzhou, China) at 70 °C under 80, 100, 120, and 140 MPa for 10 min to obtain SPI-GOS conjugates. The samples were named SPI-GOS-80, SPI-GOS-100, SPI-GOS-120, and SPI-GOS-140.
- (3)
- Control groups: native 2% (w/v) SPI solution (SPI) and a mixture of SPI and GOS (SPI-GOS-M) were employed as controls.
2.4. Measurement of Intermediate Products and Degree of Browning
2.5. Measurement of Free Amino Acids
2.6. SDS-PAGE
2.7. Measurement of Surface Hydrophobicity
2.8. Measurement of Sulfhydryl Content
2.9. Circular Dichroism (CD) Spectroscopy
2.10. Fourier Transform Infrared (FT-IR) Spectroscopy
2.11. Intrinsic Fluorescence Spectroscopy
2.12. Preparation of Emulsions
2.13. Measurement of Emulsion Activity and Stability
2.14. Measurement of Particle Size
2.15. Measurement of Zeta Potential
2.16. Confocal Laser Scanning Microscopy (CLSM)
2.17. Statistical Analysis
3. Results
3.1. Effect of HPH on Conjugation of SPI and GOS
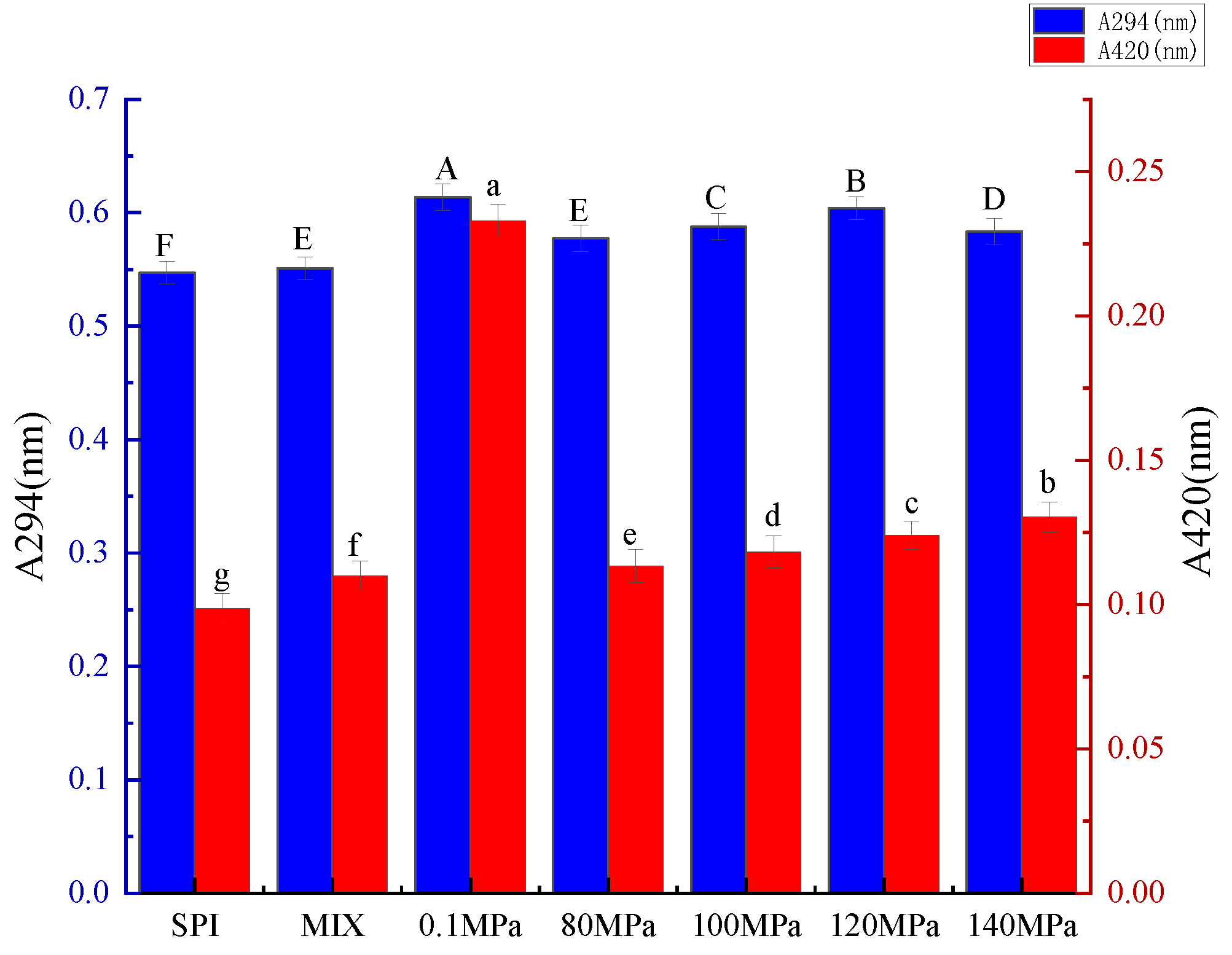
3.1.1. Intermediate MRPs and Browning of SPI-GOS Conjugates
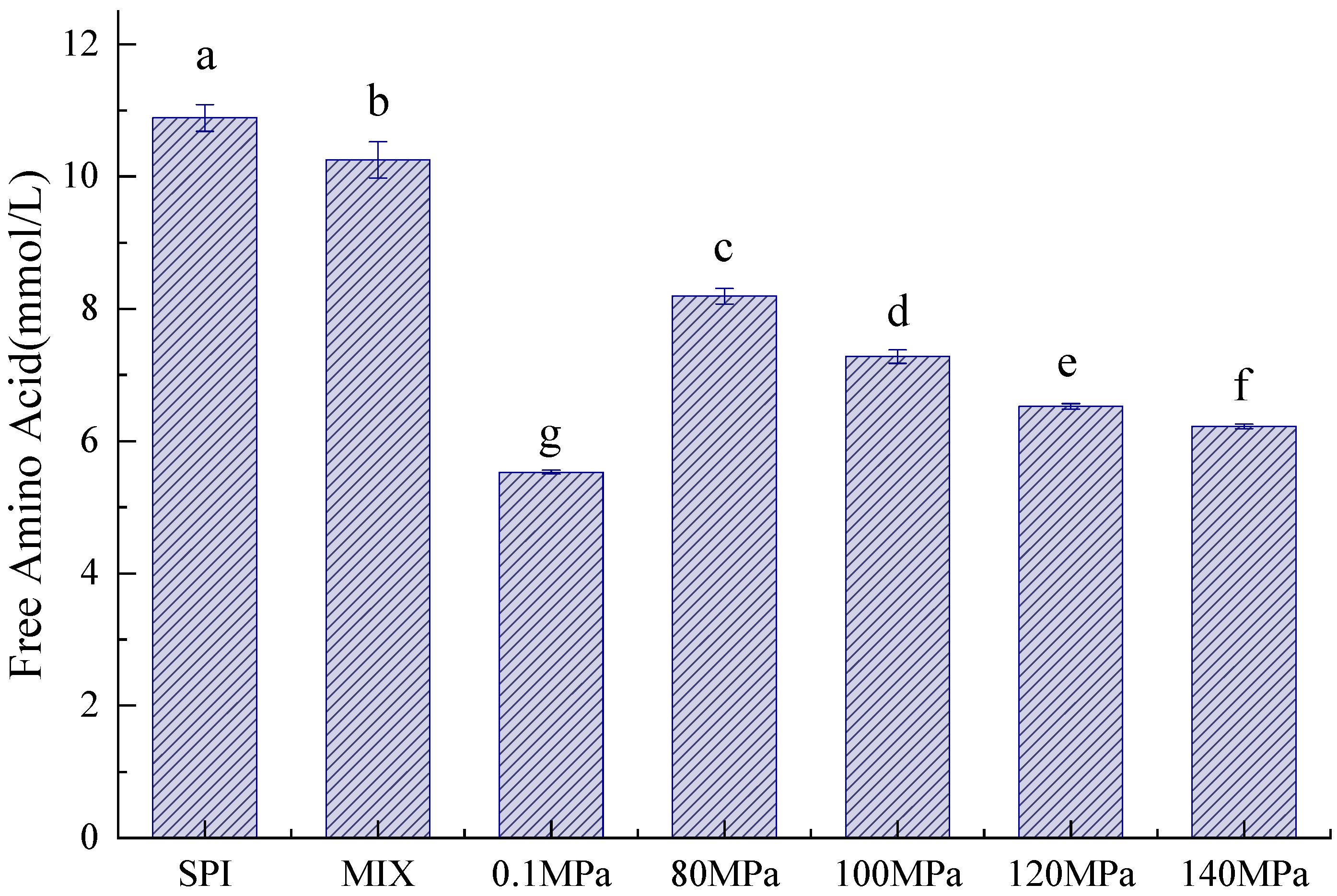
3.1.2. Free Amino Acid Content
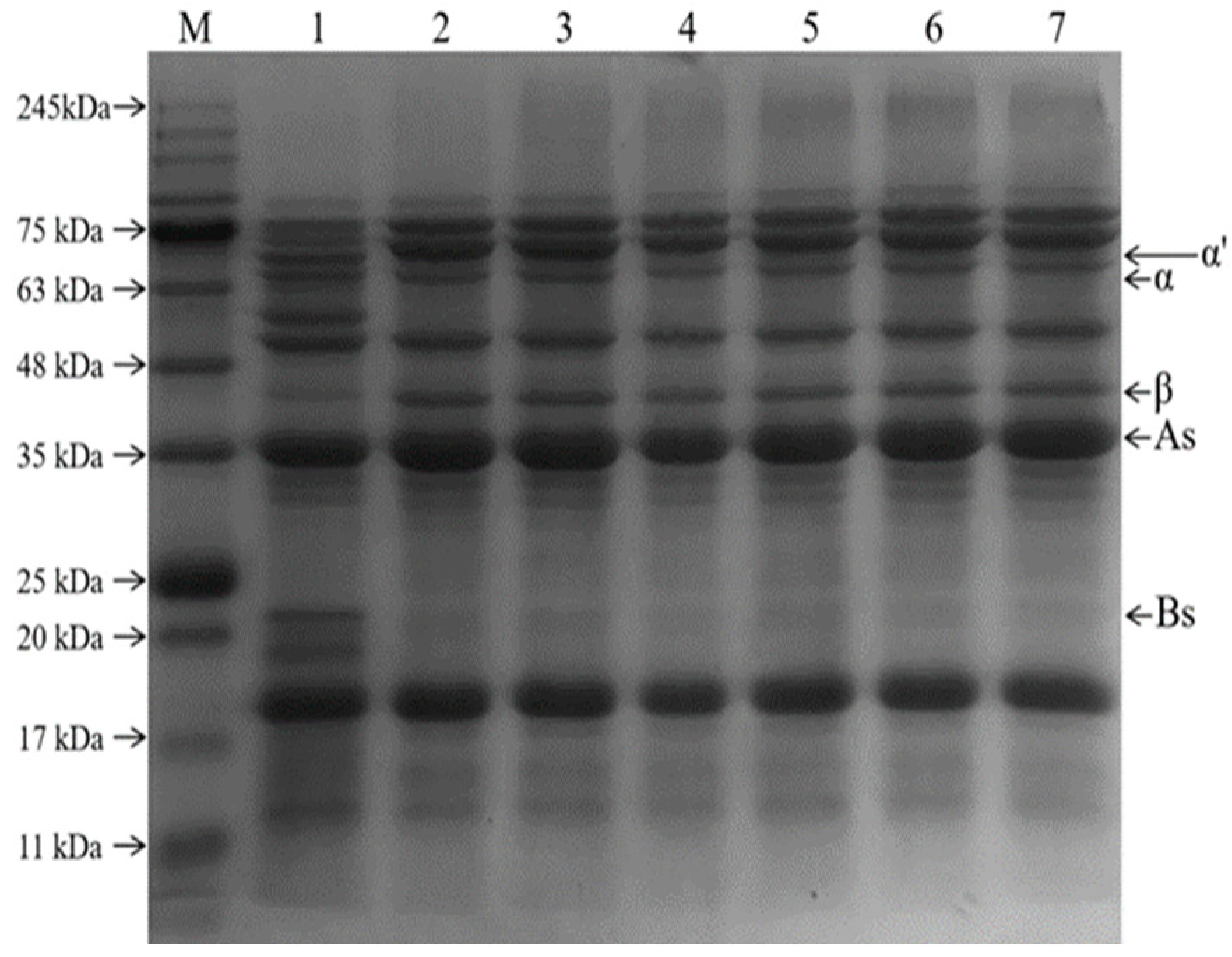
3.1.3. SDS-PAGE Results
3.2. Effect of HPH on Changes in SPI-GOS Conjugate Physicochemical Properties
3.2.1. Surface Hydrophobicity
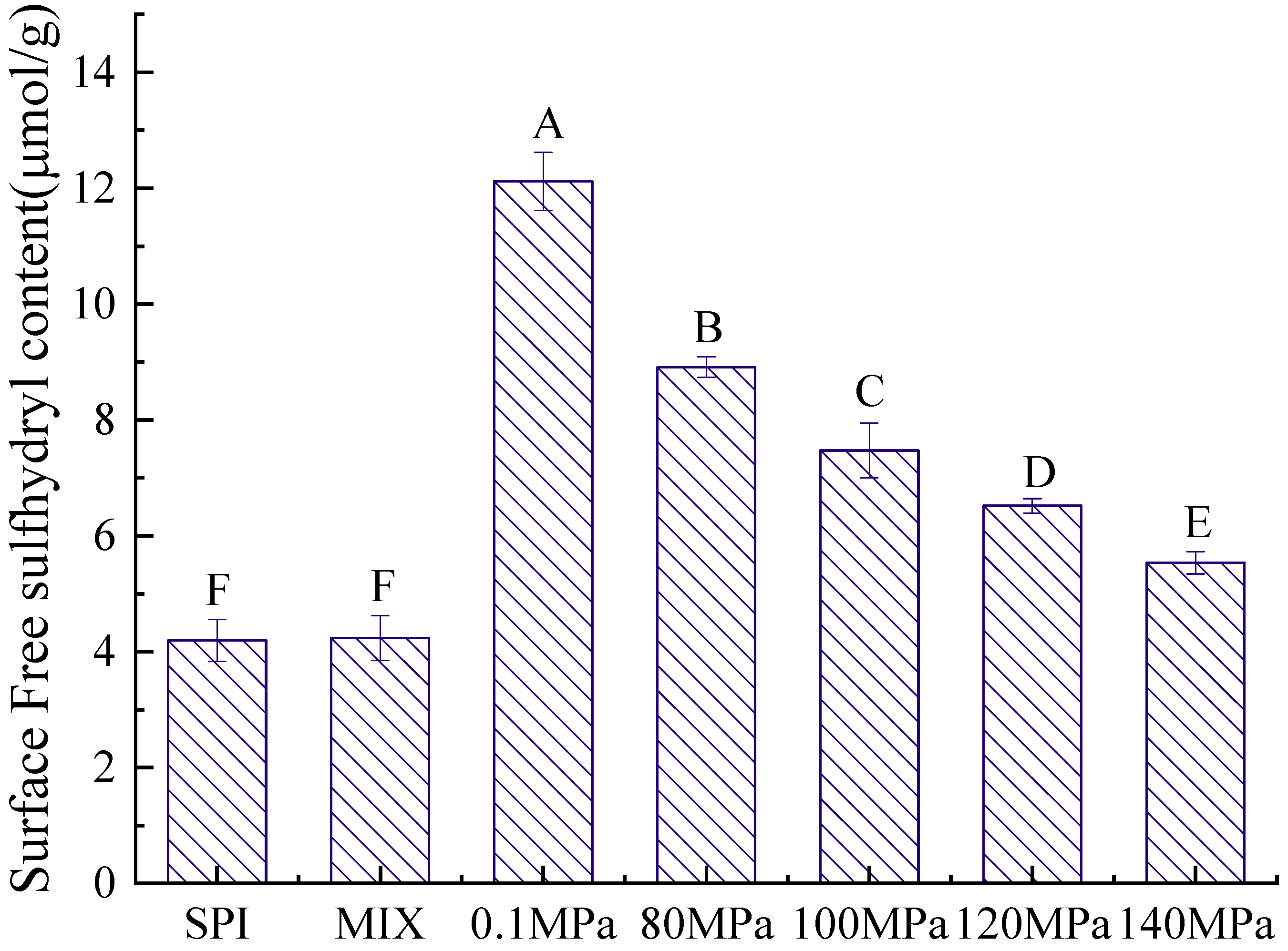
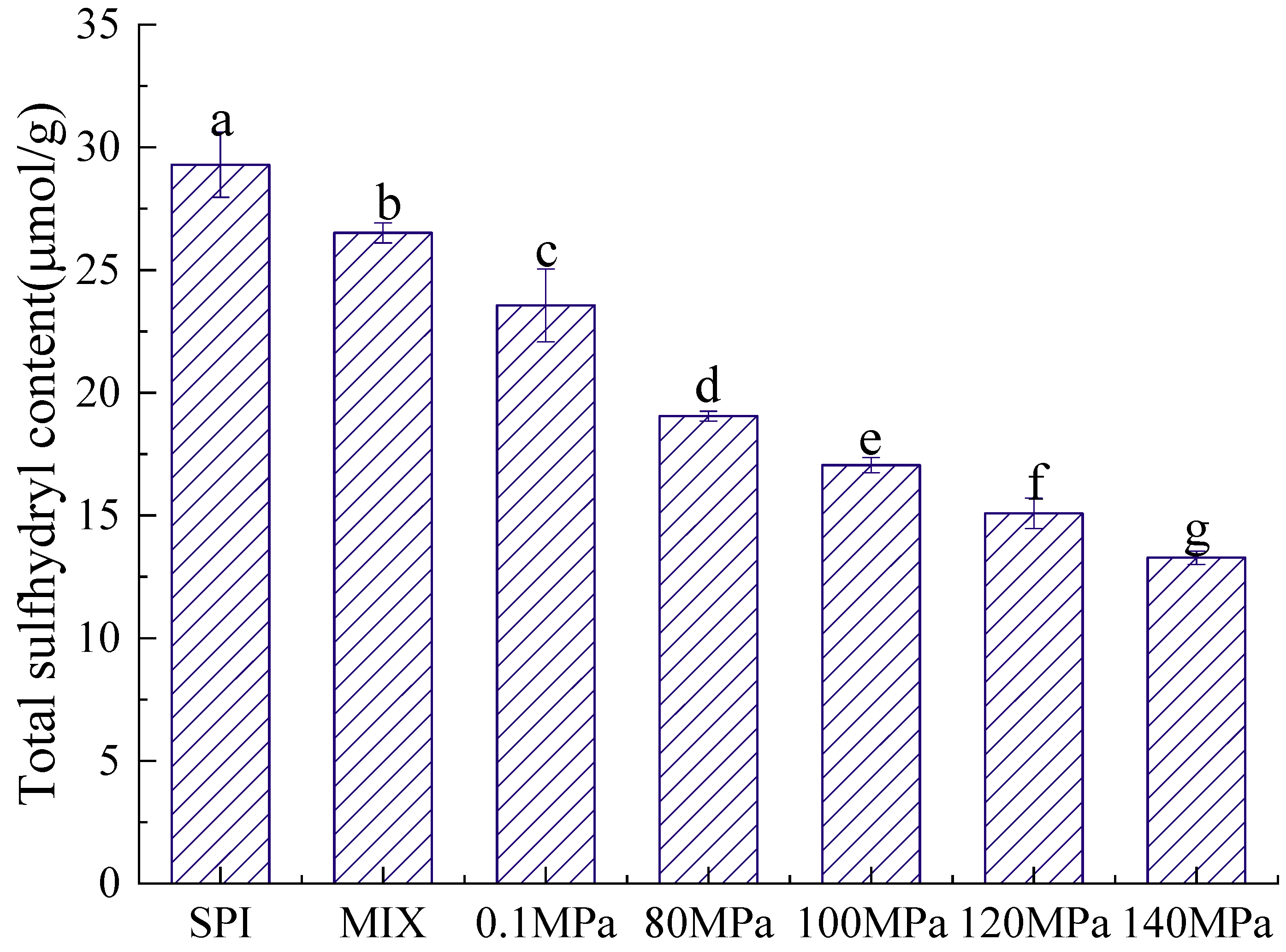
3.2.2. Sulfhydryl Content
3.2.3. CD Analysis of Protein Secondary Structure
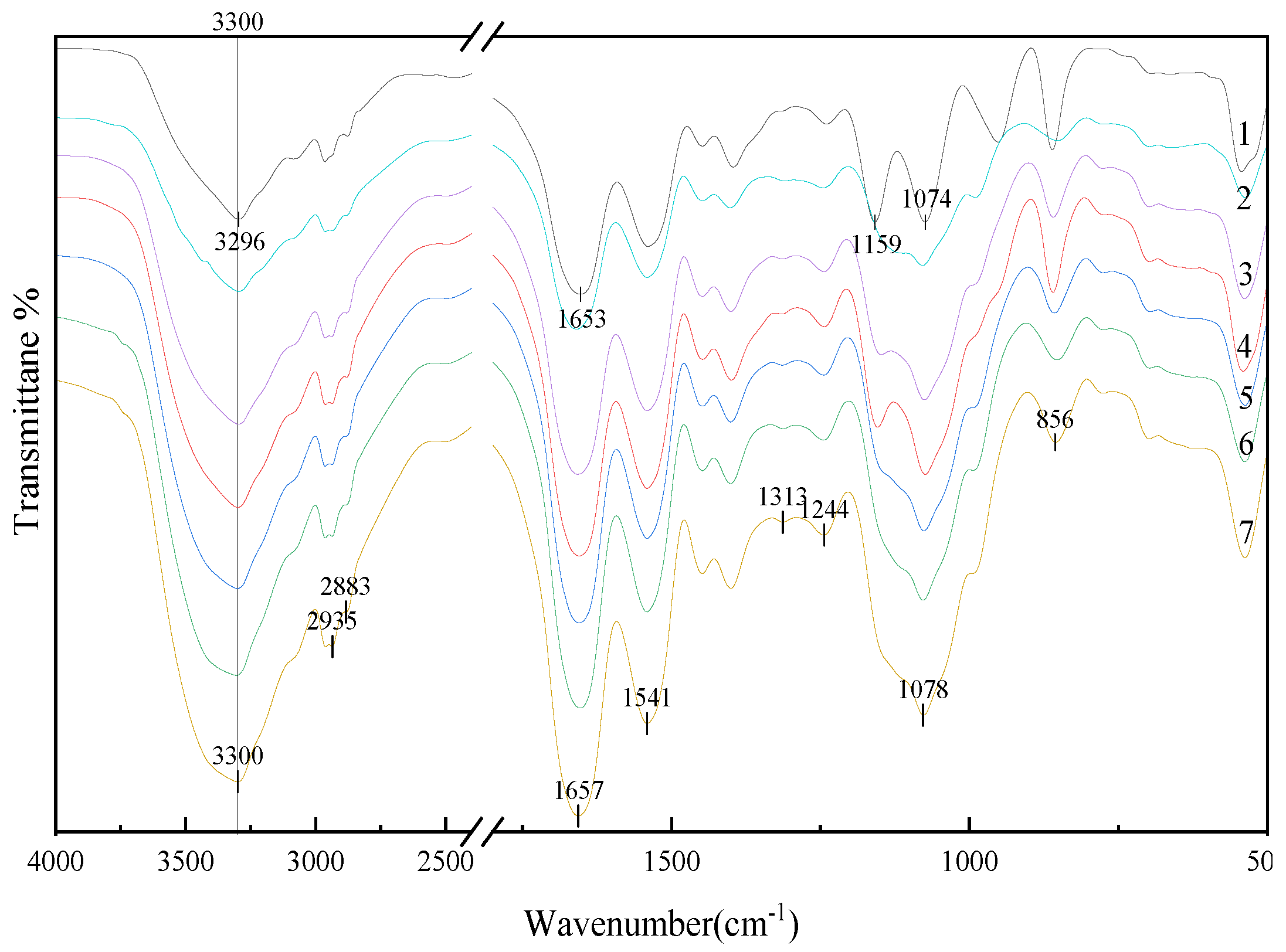
3.2.4. FTIR Analysis of Protein Secondary Structure
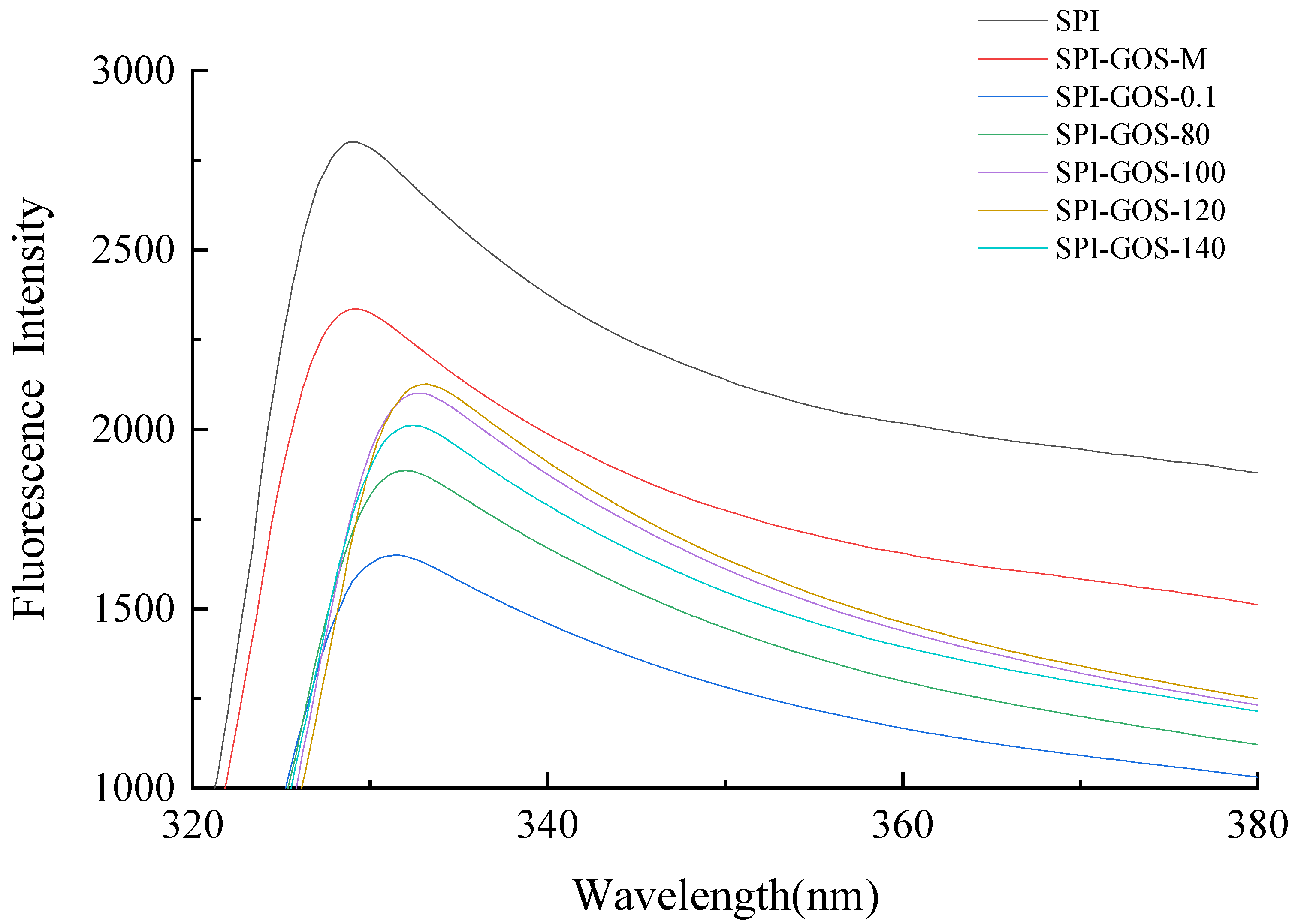
3.2.5. Intrinsic Fluorescence Analysis of Protein Tertiary Structure
3.3. Effect of HPH on SPI Emulsifying Properties
3.3.1. Emulsifying Activity and Stability
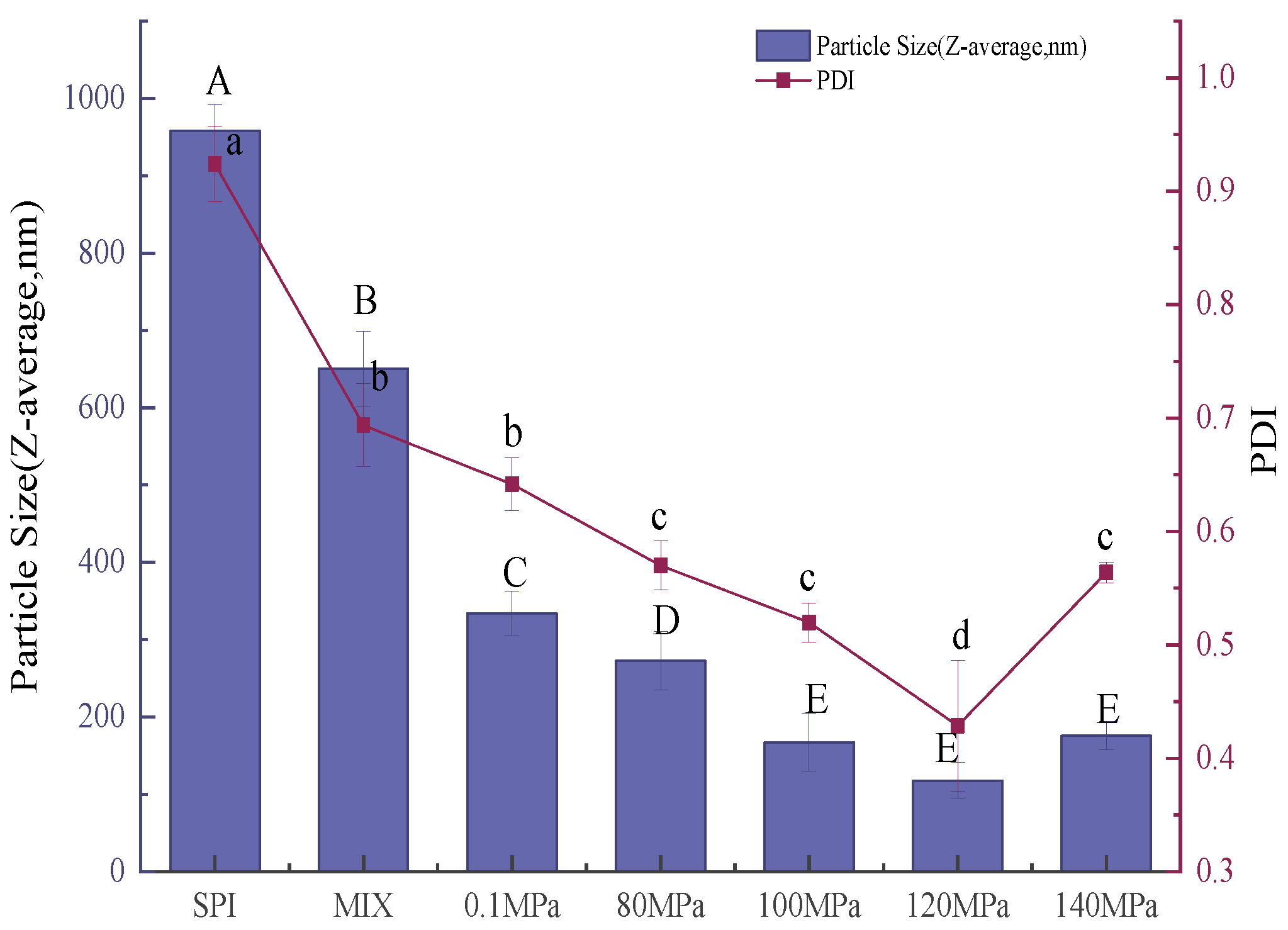
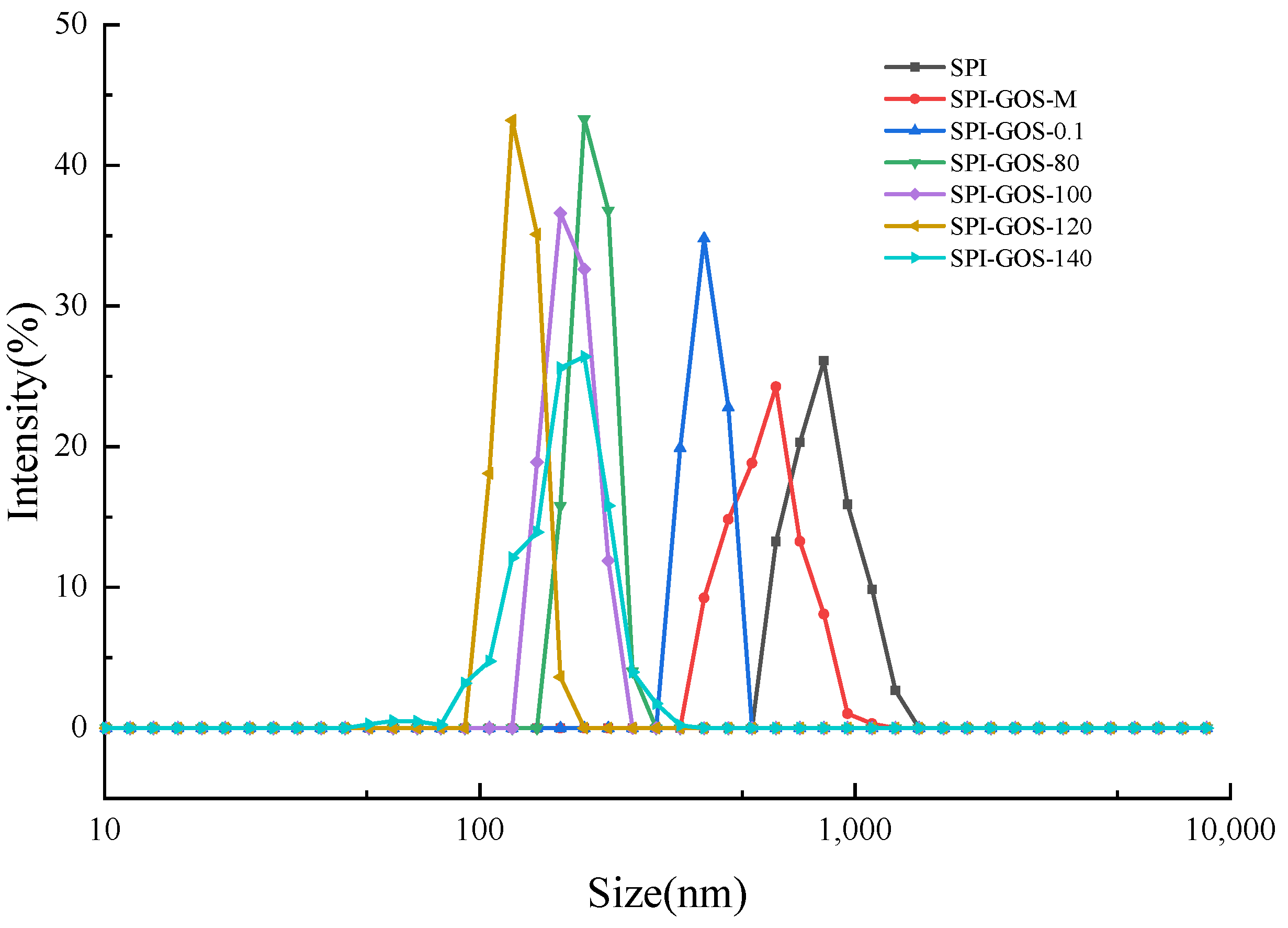
3.3.2. Particle Size of Emulsions
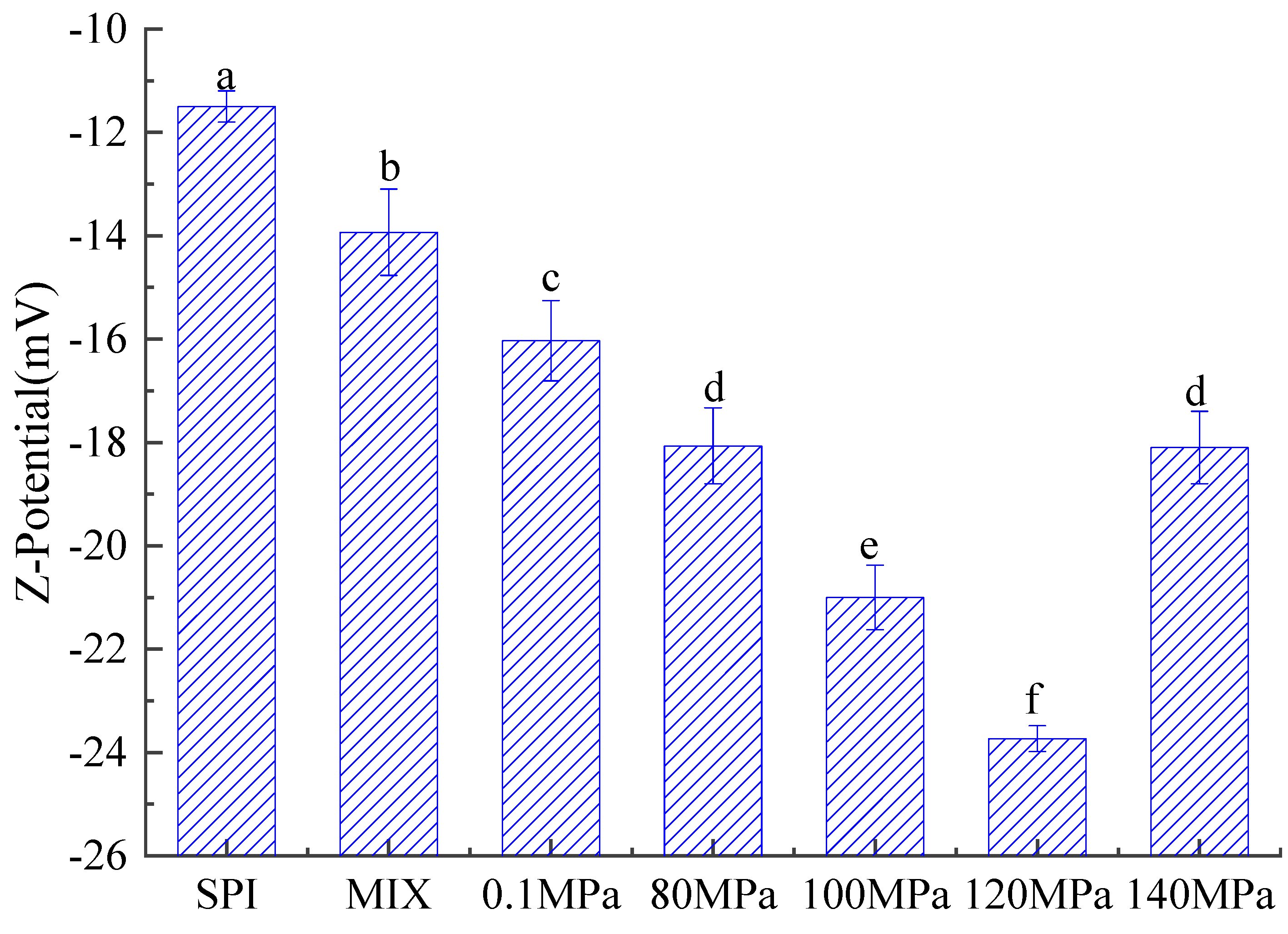
3.3.3. Zeta Potential of Emulsions
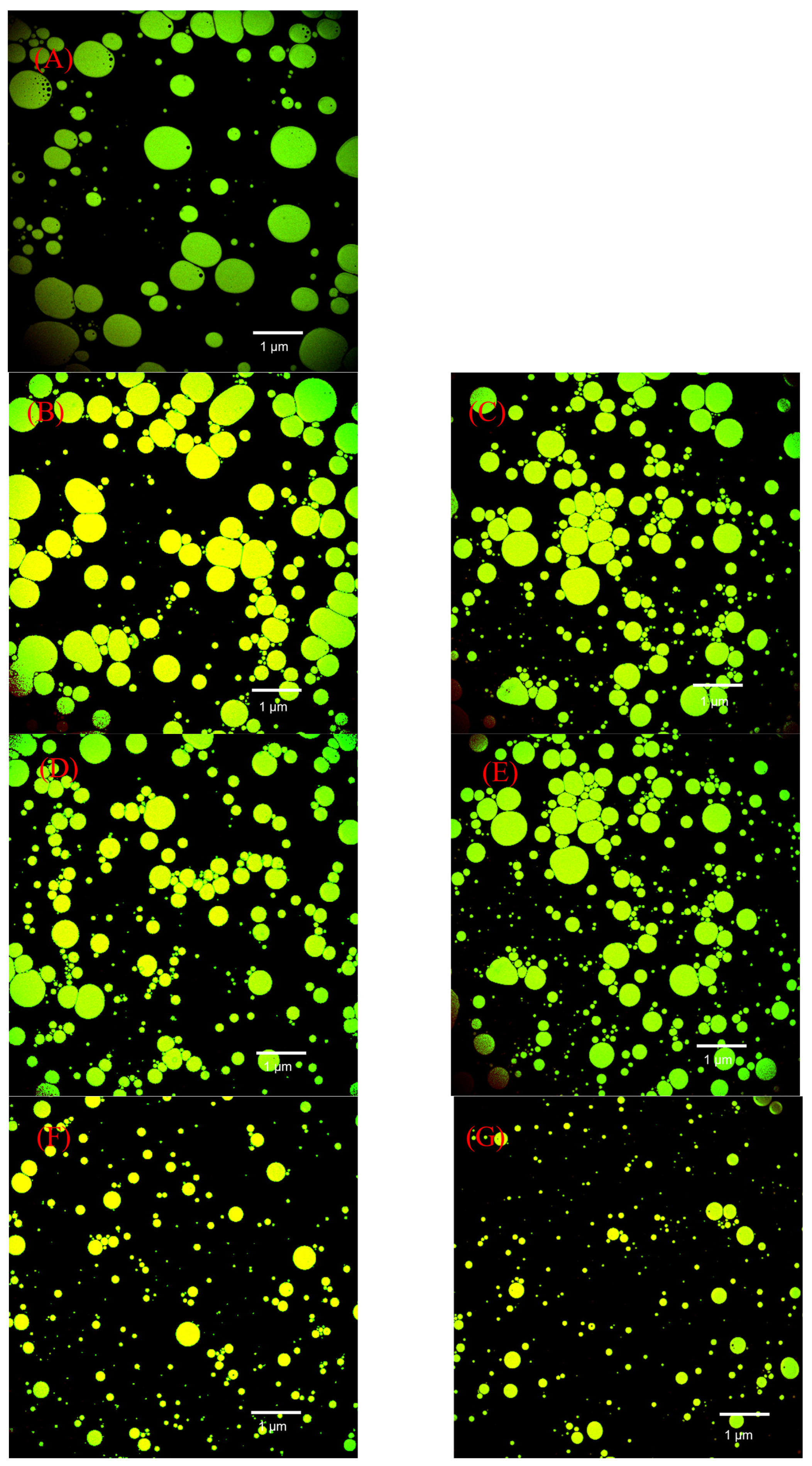
3.3.4. Microscopic Observations of Emulsions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, H.; Diao, X.; Jiang, F.; Han, J.; Kong, B. The enzymatic hydrolysis of soy protein isolate by Corolase PP under high hydrostatic pressure and its effect on bioactivity and characteristics of hydrolysates. Food Chem. 2018, 245, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Domínguez, L.; Cocotle-Ronzón, Y.; Alamilla-Beltrán, L.; Hernandez-Martinez, E.J.F.H. Effect of a cryogenic treatment in the microstructure, functional and flow properties of soy protein isolate. Food Hydrocoll. 2021, 119, 106871. [Google Scholar] [CrossRef]
- Tian, H.; Guo, G.; Fu, X.; Yao, Y.; Yuan, L.; Xiang, A. Fabrication, properties and applications of soy-protein-based materials: A review. Int. J. Biol. Macromol. 2018, 120, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Tang, C.-H. Nanostructured soy proteins: Fabrication and applications as delivery systems for bioactives (a review). Food Hydrocoll. 2019, 91, 92–116. [Google Scholar] [CrossRef]
- Aminlari, M.; Ramezani, R.; Jadidi, F. Effect of Maillard-based conjugation with dextran on the functional properties of lysozyme and casein. J. Sci. Food Agric. 2005, 85, 2617–2624. [Google Scholar] [CrossRef]
- Miralles, B.; Martínez-Rodríguez, A.; Santiago, A.; van de Lagemaat, J.; Heras, A. The occurrence of a Maillard-type protein-polysaccharide reaction between β-lactoglobulin and chitosan. Food Chem. 2007, 100, 1071–1075. [Google Scholar] [CrossRef]
- O’Mahony, J.A.; Drapala, K.P.; Mulcahy, E.M.; Mulvihill, D.M. Whey Protein-Carbohydrate Conjugation; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Chen, S.; Wang, X.; Xu, Y.; Zhang, X.; Wang, X.; Jiang, L. Effect of High Pressure Treatment on Interfacial Properties, Structure and Oxidative Stability of Soy Protein Isolate-Stabilized Emulsions. J. Oleo Sci. 2019, 68, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Tu, Z.; Wang, H.; Zhang, Q.; Hu, Y.; Zhang, L.; Niu, P.; Shi, Y.; Xiao, H. Glycation promoted by dynamic high pressure microfluidisation pretreatment revealed by high resolution mass spectrometry. Food Chem. 2013, 141, 3250–3259. [Google Scholar] [CrossRef]
- Seo, S.; Karboune, S.; L’Hocine, L.; Yaylayan, V. Characterization of glycated lysozyme with galactose, galactooligosaccharides and galactan: Effect of glycation on structural and functional properties of conjugates. LWT–Food Sci. Technol. 2013, 53, 44–53. [Google Scholar] [CrossRef]
- Peled, S.; Livney, Y.D. Oligosaccharide-lactoferrin shell-crosslinked particles for selective targeting of proteins to probiotic bacteria in the colon. Food Hydrocoll. 2021, 120, 106973. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, X.; Ding, J.; Fan, F.; Li, P.; Xia, J.; Luo, X.; Fang, Y. Physicochemical and functional properties of a novel xanthan gum-lysozyme nanoparticle material prepared by high pressure homogenization. LWT 2021, 143, 111136. [Google Scholar] [CrossRef]
- Saricaoglu, F.T. Application of high-pressure homogenization (HPH) to modify functional, structural and rheological properties of lentil (Lens culinaris) proteins. Int. J. Biol. Macromol. 2020, 144, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, G.; Plazzotta, S.; Calligaris, S.; Manzocco, L.; Nicoli, M.C. Impact of high pressure homogenization on physical properties, extraction yield and biopolymer structure of soybean okara. LWT 2019, 113, 108324. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, M.; Yang, B.A.O.; Yang, X.; Shi, J.; Jiang, Y. Effect of High-Pressure Homogenization on the Functional Property of Peanut Protein. J. Food Process Eng. 2011, 34, 2191–2204. [Google Scholar] [CrossRef]
- Shi, X.; Zou, H.; Sun, S.; Lu, Z.; Zhang, T.; Gao, J.; Yu, C. Application of high-pressure homogenization for improving the physicochemical, functional and rheological properties of myofibrillar protein. Int. J. Biol. Macromol. 2019, 138, 425–432. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, L.; Wang, Y.; Li, Z.; Wang, Z.; Han, J. Effect of high hydrostatic pressure on solubility and conformation changes of soybean protein isolate glycated with flaxseed gum. Food Chem. 2020, 333, 127530. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Y.; Sun, Q.; Wang, J.; Zheng, B.; Guo, Z. Structural characteristics and emulsifying properties of myofibrillar protein-dextran conjugates induced by ultrasound Maillard reaction. Ultrason. Sonochemistry 2021, 72, 105458. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, H.; Wang, C.; Qayum, A.; Mu, Z.; Gao, Z.; Jiang, Z. Characterization and comparison of the structure and antioxidant activity of glycosylated whey peptides from two pathways. Food Chem. 2018, 257, 279–288. [Google Scholar] [CrossRef]
- Haskard, C.A.; Li-Chan, E.C. Hydrophobicity of Bovine Serum Albumin and Ovalbumin Determined Using Uncharged (PRODAN) and Anionic (ANS-) Fluorescent Probes. J. Agric. Food Chem. 1998, 46, 2671–2677. [Google Scholar] [CrossRef]
- Liu, H.-H.; Kuo, M.-I. Ultra high pressure homogenization effect on the proteins in soy flour. Food Hydrocoll. 2016, 52, 741–748. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, Y.; McClements, D.J.; Liu, X.; Wang, P.; Liu, F. Improving pea protein functionality by combining high-pressure homogenization with an ultrasound-assisted Maillard reaction. Food Hydrocoll. 2022, 126, 107441. [Google Scholar] [CrossRef]
- Dev, M.J.; Pandit, A.B.; Singhal, R.S. Ultrasound assisted vis-à-vis classical heating for the conjugation of whey protein isolate-gellan gum: Process optimization, structural characterization and physico-functional evaluation. Innov. Food Sci. Emerg. Technol. 2021, 72, 102724. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Qin, W.; Gu, J.; Zhang, H.; Duan, Y.; Ma, H. Structure and functional properties of soy protein isolate-lentinan conjugates obtained in Maillard reaction by slit divergent ultrasonic assisted wet heating and the stability of oil-in-water emulsions. Food Chem. 2020, 331, 127374. [Google Scholar] [CrossRef]
- Pastoriza, S.; Quesada, J.; Rufián-Henares, J.A. Lactose and Oligosaccharides: Maillard Reaction. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Li, W.; Zhao, H.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Modification of soy protein hydrolysates by Maillard reaction: Effects of carbohydrate chain length on structural and interfacial properties. Colloids Surf. B: Biointerfaces 2016, 138, 70–77. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, M.S.; Akhter, F.; Khan, M.S.; Khan, A.; Ashraf, J.M.; Pandey, R.P.; Shahab, U. Glycoxidation of biological macromolecules: A critical approach to halt the menace of glycation. Glycobiology 2014, 24, 979–990. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Guo, Z.; Wu, D.; Fei, X.; Ei-Seedi, H.R.; Wang, C. High-pressure homogenization influences the functional properties of protein from oyster (Crassostrea gigas). LWT 2021, 151, 112107. [Google Scholar] [CrossRef]
- Saito, H.; Sakakibara, Y.; Sakata, A.; Kurashige, R.; Murakami, D.; Kageshima, H.; Saito, A.; Miyazaki, Y. Antibacterial activity of lysozyme-chitosan oligosaccharide conjugates (LYZOX) against Pseudomonas aeruginosa, Acinetobacter baumannii and Methicillin-resistant Staphylococcus aureus. PLoS ONE 2019, 14, e0217504. [Google Scholar] [CrossRef]
- Wang, X.P.; Zhao, X.H. Using an enzymatic galactose assay to detect lactose glycation extents of two proteins caseinate and soy protein isolate via the Maillard reaction. J. Sci. Food Agric. 2017, 97, 2617–2622. [Google Scholar] [CrossRef]
- Ma, X.; Hou, F.; Zhao, H.; Wang, D.; Chen, W.; Miao, S.; Liu, D. Conjugation of soy protein isolate (SPI) with pectin by ultrasound treatment. Food Hydrocoll. 2020, 108, 106056. [Google Scholar] [CrossRef]
- Tirgar, M.; Silcock, P.; Carne, A.; Birch, E.J. Effect of extraction method on functional properties of flaxseed protein concentrates. Food Chem. 2017, 215, 417–424. [Google Scholar] [CrossRef]
- Du, L.; Betti, M. Chicken collagen hydrolysate cryoprotection of natural actomyosin: Mechanism studies during freeze-thaw cycles and simulated digestion. Food Chem. 2016, 211, 791–802. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Thavaraj, P.; Yang, X.; Guo, Y. Effects of thinned young apple polyphenols on the quality of grass carp (Ctenopharyngodon idellus) surimi during cold storage. Food Chem. 2017, 224, 372–381. [Google Scholar] [CrossRef]
- Guan, Y.-G.; Lin, H.; Han, Z.; Wang, J.; Yu, S.-J.; Zeng, X.-A.; Liu, Y.-Y.; Xu, C.-H.; Sun, W.-W. Effects of pulsed electric field treatment on a bovine serum albumin–dextran model system, a means of promoting the Maillard reaction. Food Chem. 2010, 123, 275–280. [Google Scholar] [CrossRef]
- Mao, C.; Wu, J.; Zhang, X.; Ma, F.; Cheng, Y. Improving the Solubility and Digestibility of Potato Protein with an Online Ultrasound-Assisted PH Shifting Treatment at Medium Temperature. Foods 2020, 9, 1908. [Google Scholar] [CrossRef]
- Shen, L.; Tang, C.-H. Microfluidization as a potential technique to modify surface properties of soy protein isolate. Food Res. Int. 2012, 48, 108–118. [Google Scholar] [CrossRef]
- Hoving, S.; Gerrits, B.; Voshol, H.; Müller, D.; Roberts, R.C.; Oostrum, J.V.J.P. Preparative two-dimensional gel electrophoresis at alkaline pH using narrow range immobilized pH gradients. Proteomics 2015, 2, 127–134. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, Z.; Guo, Y.; Li, B.; Yu, W.; Zhou, L.; Jiang, L.; Teng, F.; Wang, Z. Effects of high-pressure homogenization on structural and emulsifying properties of thermally soluble aggregated kidney bean (Phaseolus vulgaris L.) proteins. Food Hydrocoll. 2021, 119, 106835. [Google Scholar] [CrossRef]
- Carullo, D.; Donsì, F.; Ferrari, G. Influence of high-pressure homogenization on structural properties and enzymatic hydrolysis of milk proteins. LWT 2020, 130, 109657. [Google Scholar] [CrossRef]
- Khan, N.M.; Mu, T.H.; Zhang, M.; Chen, J.W. Effects of high hydrostatic pressure on the physicochemical and emulsifying properties of sweet potato protein. Int. J. Food Sci. Technol. 2013, 48, 1260–1268. [Google Scholar] [CrossRef]
- Martinez, K.; Sanchez, C.; Ruizhenestrosa, V.; Rodriguezpatino, J.; Pilosof, A. Effect of limited hydrolysis of soy protein on the interactions with polysaccharides at the air–water interface. Food Hydrocoll. 2007, 21, 813–822. [Google Scholar] [CrossRef]
- He, X.T.; Yuan, D.B.; Wang, J.M.; Yang, X.Q. Thermal aggregation behaviour of soy protein: Characteristics of different polypeptides and sub-units. J. Sci. Food Agric. 2016, 96, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xiong, Z.; Xiong, H.; Chen, L.; Zhang, Z. Effects of dynamic high-pressure microfluidization treatment on the functional and structural properties of potato protein isolate and its complex with chitosan. Food Res. Int. 2021, 140, 109868. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Zhang, X.; Chen, W.; Wang, Z.; He, R.; Ma, H. Effects of ultrasonic and graft treatments on grafting degree, structure, functionality, and digestibility of rapeseed protein isolate-dextran conjugates. Ultrason. Sonochemistry 2018, 42, 250–259. [Google Scholar] [CrossRef]
- Dutta, D.; Das, M.D. Optimization and partial characterization of intracellular anticandidal protein from Aspergillus giganteus MTCC 8408 using taguchi DOE. Bioengineered 2017, 8, 536–548. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Pylypchuk, I.; Johansson, D.P.; Kessler, V.G.; Seisenbaeva, G.A.; Langton, M. Self-assembly of plant protein fibrils interacting with superparamagnetic iron oxide nanoparticles. Sci. Rep. 2019, 9, 8939. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.C.; Tanaka, J.J.B. FT-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials 2002, 23, 4811–4818. [Google Scholar] [CrossRef]
- Gu, F.-L.; Kim, J.M.; Abbas, S.; Zhang, X.-M.; Xia, S.-Q.; Chen, Z.-X. Structure and antioxidant activity of high molecular weight Maillard reaction products from casein–glucose. Food Chem. 2010, 120, 505–511. [Google Scholar] [CrossRef]
- Su, J.-F.; Huang, Z.; Yuan, X.-Y.; Wang, X.-Y.; Li, M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Guan, J.-J.; Qiu, A.-Y.; Liu, X.-Y.; Hua, Y.-F.; Ma, Y.-H. Microwave improvement of soy protein isolate–saccharide graft reactions. Food Chem. 2006, 97, 577–585. [Google Scholar] [CrossRef]
- Tang, C.H.; Sun, X.; Foegeding, E.A. Modulation of physicochemical and conformational properties of kidney bean vicilin (phaseolin) by glycation with glucose: Implications for structure-function relationships of legume vicilins. J. Agric. Food Chem. 2011, 59, 10114–10123. [Google Scholar] [CrossRef]
- Sheng, L.; Tang, G.; Wang, Q.; Zou, J.; Ma, M.; Huang, X. Molecular characteristics and foaming properties of ovalbumin-pullulan conjugates through the Maillard reaction. Food Hydrocoll. 2020, 100, 105384. [Google Scholar] [CrossRef]
- Jiménez-Castano, L.; Villamiel, M.; Hydrocolloids, R.L.-F.J.F. Glycosylation of individual whey proteins by Maillard reaction using dextran of different molecular mass. Food Hydrocoll. 2007, 21, 433–443. [Google Scholar] [CrossRef]
- Xue, F.; Li, C.; Zhu, X.; Wang, L.; Pan, S. Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Res. Int. 2013, 51, 490–495. [Google Scholar] [CrossRef]
- De Maria, S.; Ferrari, G.; Maresca, P. Effects of high hydrostatic pressure on the conformational structure and the functional properties of bovine serum albumin. Innov. Food Sci. Emerg. Technol. 2016, 33, 67–75. [Google Scholar] [CrossRef]
- Dumay, E.; Chevalier-Lucia, D.; Picart-Palmade, L.; Benzaria, A.; Gràcia-Julià, A.; Blayo, C. Technological aspects and potential applications of (ultra) high-pressure homogenisation. Trends Food Sci. Technol. 2013, 31, 13–26. [Google Scholar] [CrossRef]
- Wu, F.; Cha, Y.; Zou, H.; Shi, X.; Zhang, T.; Du, M.; Yu, C. Structure and functionalities changes in high-pressure homogenized clam protein isolate. J. Food Process. Preserv. 2019, 43, e13860. [Google Scholar] [CrossRef]
- Yu, C.; Cha, Y.; Wu, F.; Xu, X.; Qin, Y.; Li, X.; Du, M. Effects of high-pressure homogenisation on structural and functional properties of mussel (Mytilus edulis) protein isolate. Int. J. Food Sci. Technol. 2018, 53, 1157–1165. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, L.; Song, T.; Yu, H.; Wang, X.; Zhao, X.-h. Effects of high pressure homogenization on the structural and emulsifying properties of a vegetable protein: Cyperus esculentus L. LWT 2022, 153, 112542. [Google Scholar] [CrossRef]
- Lloret, S.M.; Andres, J.V.; Legua, C.M.; Falco, P.C. Determination of ammonia and primary amine compounds and Kjeldahl nitrogen in water samples with a modified Roth’s fluorimetric method. Talanta 2005, 65, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.J.; Bridgewater, J.D.; Vachet, R.W.; Waraho, T.; McClements, D.J.; Decker, E.A. Antioxidant mechanisms of enzymatic hydrolysates of beta-lactoglobulin in food lipid dispersions. J. Agric. Food Chem. 2006, 54, 9565–9572. [Google Scholar] [CrossRef] [PubMed]


| Samples | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| SPI | 33.39 ± 0.02 a | 29.34 ± 0.01 a | 10.81 ± 0.02 f | 26.45 ± 0.01 g |
| SPI-GOS-M | 32.08 ± 0.01 b | 28.93 ± 0.01 b | 10.65 ± 0.01 g | 28.34 ± 0.01 f |
| SPI-GOS-0.1 | 19.57 ± 0.02 c | 27.55 ± 0.01 c | 21.35 ± 0.02 a | 31.53 ± 0.01 e |
| SPI-GOS-80 | 16.82 ± 0.02 d | 24.79 ± 0.01 d | 16.26 ± 0.02 e | 42.12 ± 0.02 d |
| SPI-GOS-100 | 16.15 ± 0.02 e | 22.52 ± 0.01 e | 17.57 ± 0.01 d | 43.76 ± 0.01 b |
| SPI-GOS-120 | 16.07 ± 0.01 f | 21.53 ± 0.01 f | 19.12 ± 0.01 c | 43.28 ± 0.01 c |
| SPI-GOS-140 | 15.94 ± 0.02 g | 20.92 ± 0.01 g | 19.32 ± 0.01 b | 43.82 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Liu, R.; Zhang, C.; Liu, D.; Han, J. Structural Characteristics and Emulsifying Properties of Soy Protein Isolate Glycated with Galacto-Oligosaccharides under High-Pressure Homogenization. Foods 2022, 11, 3505. https://doi.org/10.3390/foods11213505
Xie Y, Liu R, Zhang C, Liu D, Han J. Structural Characteristics and Emulsifying Properties of Soy Protein Isolate Glycated with Galacto-Oligosaccharides under High-Pressure Homogenization. Foods. 2022; 11(21):3505. https://doi.org/10.3390/foods11213505
Chicago/Turabian StyleXie, Yitong, Rongxu Liu, Changge Zhang, Danyi Liu, and Jianchun Han. 2022. "Structural Characteristics and Emulsifying Properties of Soy Protein Isolate Glycated with Galacto-Oligosaccharides under High-Pressure Homogenization" Foods 11, no. 21: 3505. https://doi.org/10.3390/foods11213505





