Characterization of Novel Exopolysaccharides from Enterococcus hirae WEHI01 and Its Immunomodulatory Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. The Culture of Strain
2.3. Extraction, Production and Purification of EPS
2.4. Structure Characterization of EPS
2.4.1. Purity and Molecular Weight
2.4.2. FT-IR Spectroscopy Analysis
2.4.3. Analysis of Monosaccharide Composition
2.5. Immunocompetence Assays
2.5.1. Cell Culture and Its Viability Assay
2.5.2. Phagocytosis Assay and Morphology Observation
2.5.3. NO and Cytokines Secretion
2.5.4. Gene Expression Analysis by RT-qPCR
2.6. Statistical Analysis
3. Results and Discussion
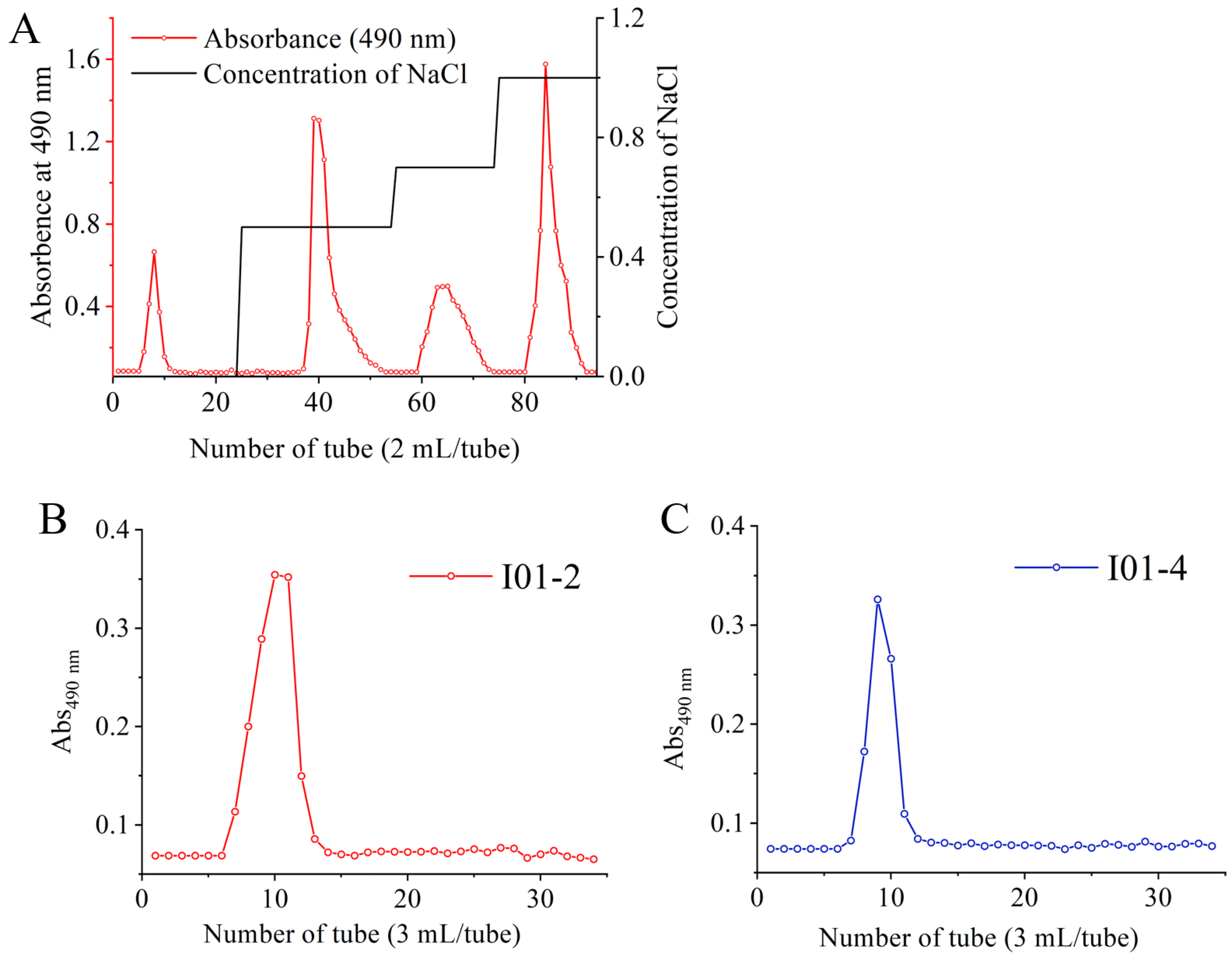
3.1. Extraction and Purification of EPS
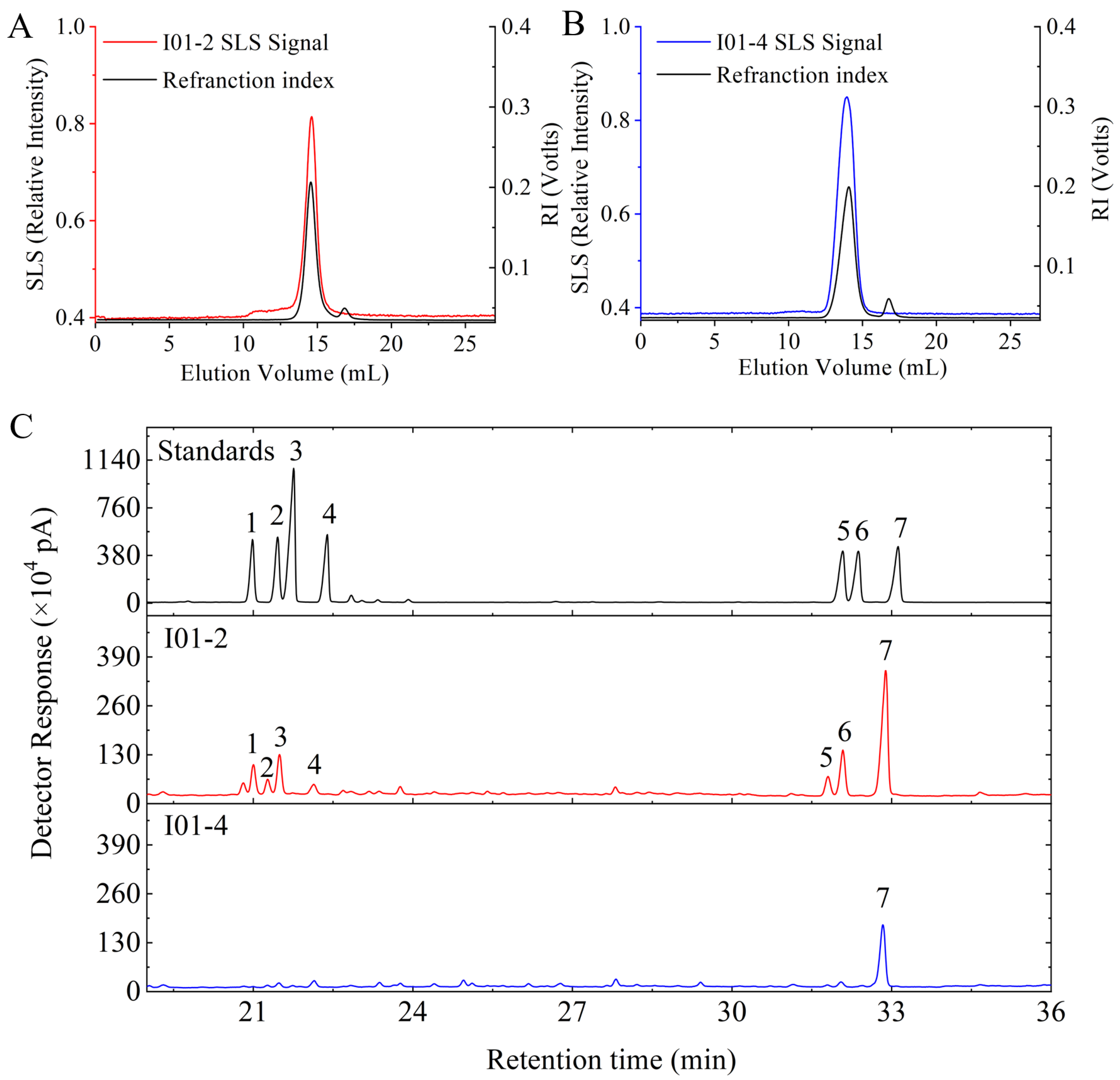
3.2. Mw and Monosaccharides Composition of I01-2 and I01-4
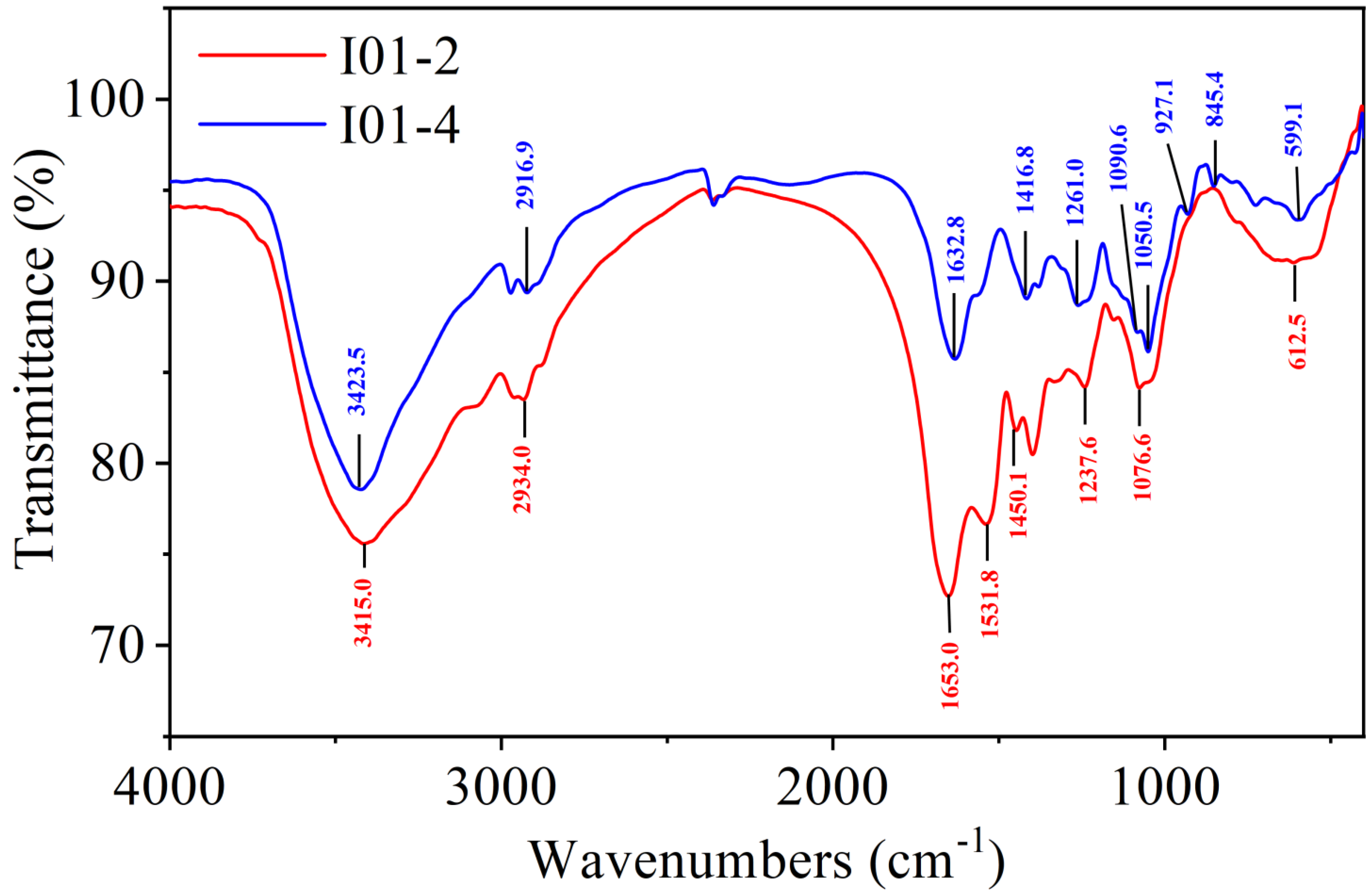
3.3. FT-IR Spectrum Analysis of I01-2 and I01-4
3.4. Immunomodulatory Activities of I01-2 and I01-4 on RAW264.7 Cells
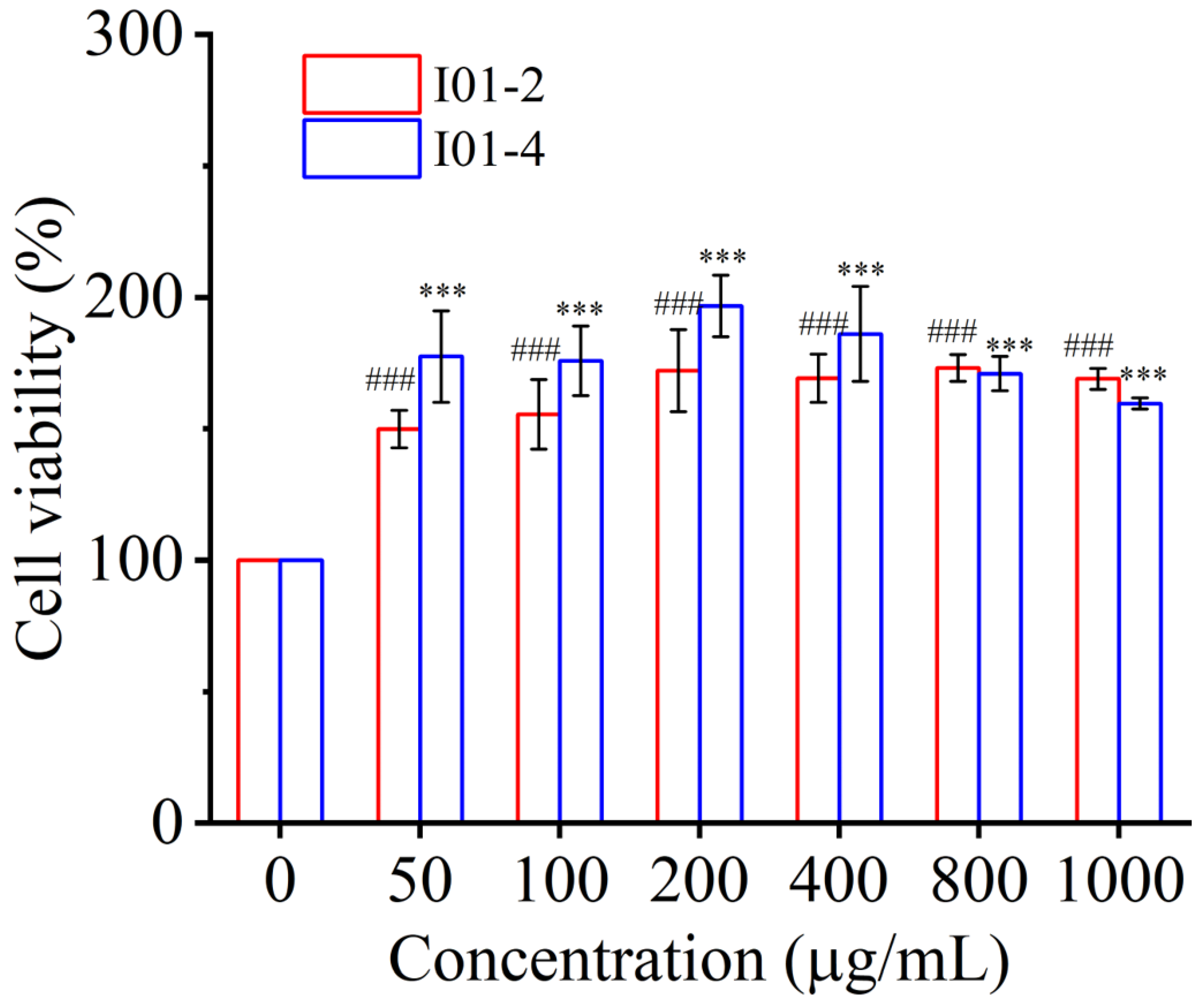
3.4.1. Effect of I01-2 and I01-4 on Cell Viability of RAW264.7
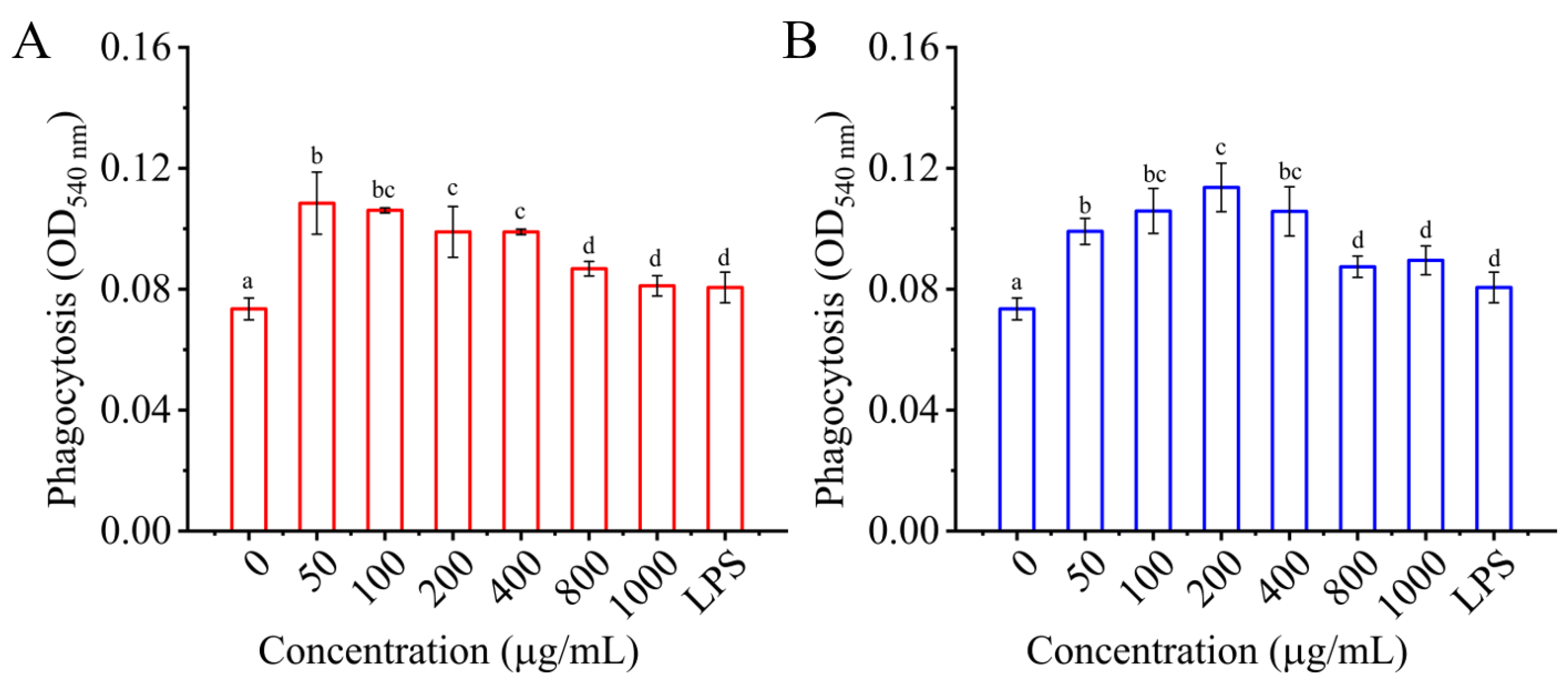
3.4.2. Effects of I01-2 and I01-4 on Phagocytosis of RAW 264.7 Cells
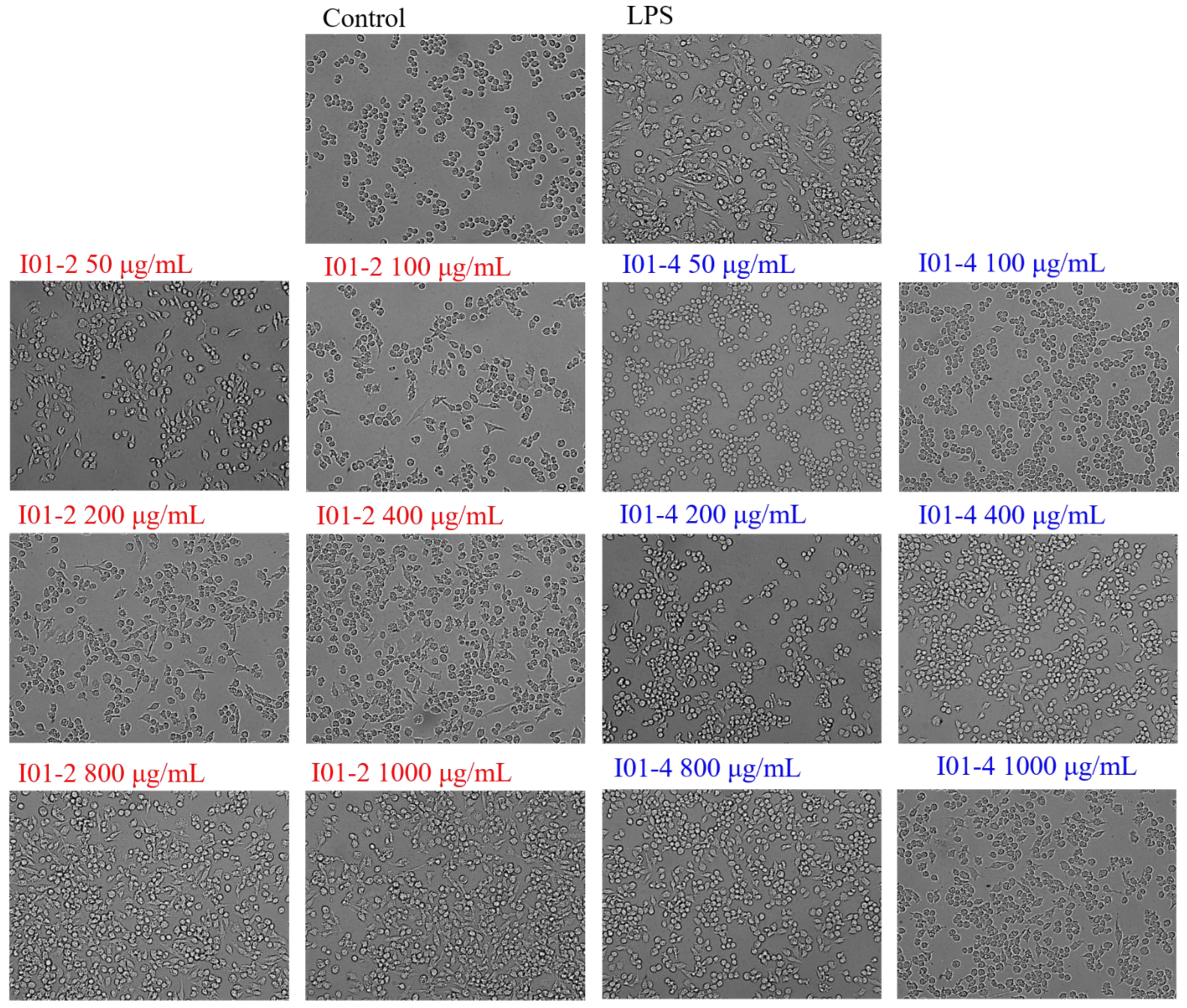
3.4.3. Effects of I01-2 and I01-4 on the RAW264.7 Cells’ Morphology
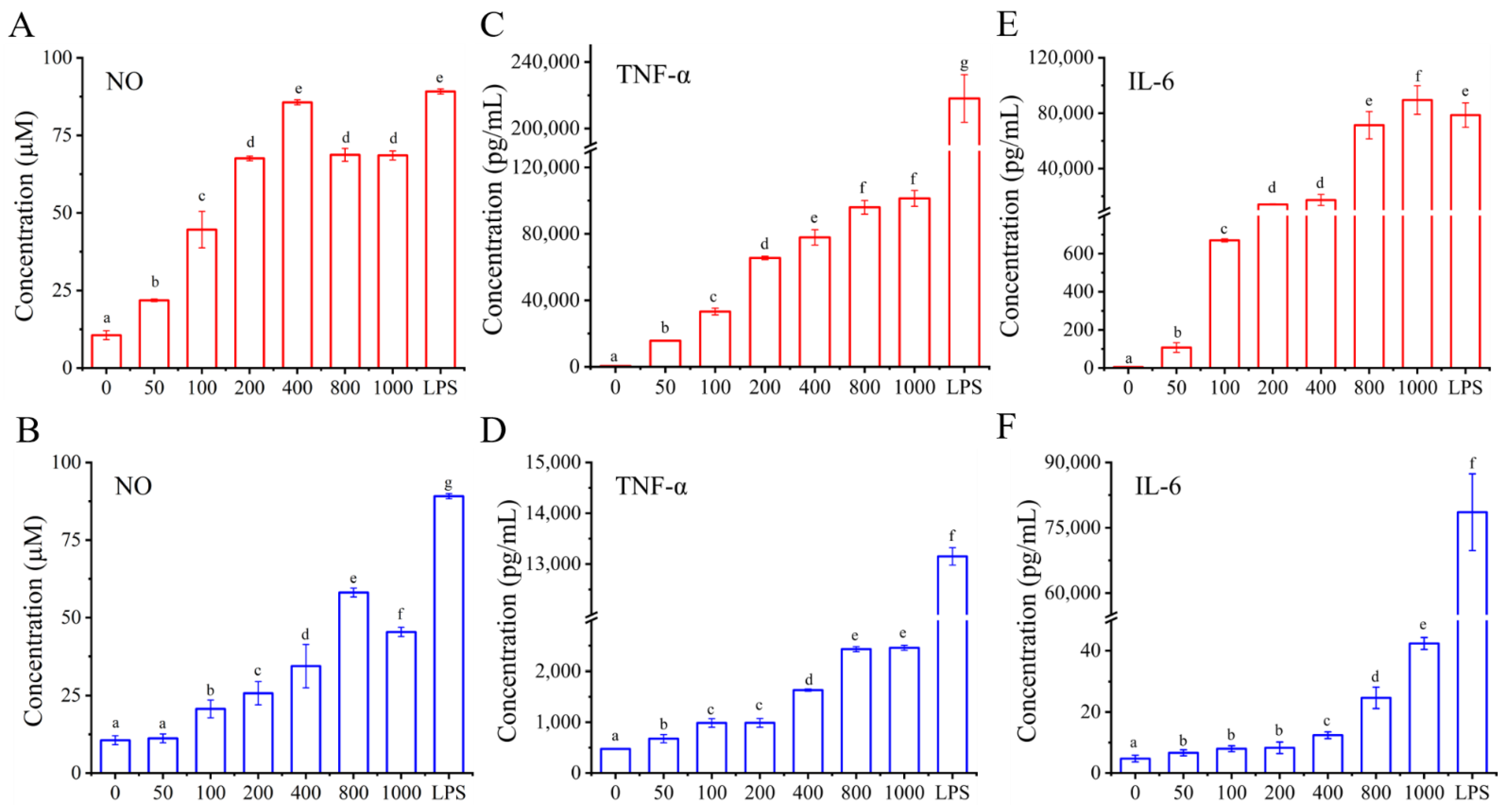
3.4.4. Effects of I01-2 and I01-4, Respectively, on the Generation of NO and the Secretion of IL-6 and TNF-α
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Freitas, F.; Torres, C.A.; Reis, M.A. Engineering aspects of microbial exopolysaccharide production. Bioresour. Technol. 2017, 245, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.M.; Coffey, A.; Arendt, E.K. Exopolysaccharide producing lactic acid bacteria: Their techno-functional role and potential application in gluten-free bread products. Food Res. Int. 2018, 110, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Buksa, K.; Kowalczyk, M.; Boreczek, J. Extraction, purification and characterisation of exopolysaccharides produced by newly isolated lactic acid bacteria strains and the examination of their influence on resistant starch formation. Food Chem. 2021, 362, 130221. [Google Scholar] [CrossRef]
- Lynch, K.M.; McSweeney, P.L.; Arendt, E.K.; Uniacke-Lowe, T.; Galle, S.; Coffey, A. Isolation and characterisation of exopolysaccharide-producing Weissella and Lactobacillus and their application as adjunct cultures in Cheddar cheese. Int. Dairy J. 2014, 34, 125–134. [Google Scholar] [CrossRef]
- Tiwari, S.; Kavitake, D.; Devi, P.B.; Shetty, P.H. Bacterial exopolysaccharides for improvement of technological, functional and rheological properties of yoghurt. Int. J. Biol. Macromol. 2021, 183, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Bachtarzi, N.; Kharroub, K.; Ruas-Madiedo, P. Exopolysaccharide-producing lactic acid bacteria isolated from traditional Algerian dairy products and their application for skim-milk fermentations. LWT 2019, 107, 117–124. [Google Scholar] [CrossRef]
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Z.; Tao, X.; Wei, H. Characterization and sulfated modification of an exopolysaccharide from Lactobacillus plantarum ZDY2013 and its biological activities. Carbohydr. Polym. 2016, 153, 25–33. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Galiwango, E.; Tamiello-Rosa, C.; Abdullah, H.; Esposito, G.; Hunashal, Y.; Obaid, R.S.; Hamed, F. Characterization, bioactivities, and rheological properties of exopolysaccharide produced by novel probiotic Lactobacillus plantarum C70 isolated from camel milk. Int. J. Biol. Macromol. 2019, 144, 938–946. [Google Scholar] [CrossRef]
- Jiang, B.; Tian, L.; Huang, X.; Liu, Z.; Jia, K.; Wei, H.; Tao, X. Characterization and antitumor activity of novel exopolysaccharide APS of Lactobacillus plantarum WLPL09 from human breast milk. Int. J. Biol. Macromol. 2020, 163, 985–995. [Google Scholar] [CrossRef]
- You, X.; Li, Z.; Ma, K.; Zhang, C.; Chen, X.; Wang, G.; Yang, L.; Dong, M.; Rui, X.; Zhang, Q.; et al. Structural characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus helveticus LZ-R-5. Carbohydr. Polym. 2020, 235, 115977. [Google Scholar] [CrossRef]
- Sarikaya, H.; Aslim, B.; Yuksekdag, Z. Assessment of anti-biofilm activity and bifidogenic growth stimulator (BGS) effect of lyophilized exopolysaccharides (l-EPSs) from Lactobacilli strains. Int. J. Food Prop. 2016, 20, 362–371. [Google Scholar] [CrossRef] [Green Version]
- Biliavska, L.; Pankivska, Y.; Povnitsa, O.; Zagorodnya, S. Antiviral Activity of Exopolysaccharides Produced by Lactic Acid Bacteria of the Genera Pediococcus, Leuconostoc and Lactobacillus against Human Adenovirus Type 5. Medicina 2019, 55, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yılmaz, T.; Şimşek, Ö. Potential Health Benefits of Ropy Exopolysaccharides Produced by Lactobacillus plantarum. Molecules 2020, 25, 3293. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.; López, P.; Strahinic, I.; Suárez, A.; Kojic, M.; Fernández-García, M.; Topisirovic, L.; Golic, N.; Ruas-Madiedo, P. Characterisation of the exopolysaccharide (EPS)-producing Lactobacillus paraplantarum BGCG11 and its non-EPS producing derivative strains as potential probiotics. Int. J. Food Microbiol. 2012, 158, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, T.; Fang, X.; Min, W.; Yang, Z. Characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus plantarum JLK0142 isolated from fermented dairy tofu. Int. J. Biol. Macromol. 2018, 115, 985–993. [Google Scholar] [CrossRef]
- Khalil, M.A.; Sonbol, F.I.; Al-Madboly, L.A.; Aboshady, T.A.; Alqurashi, A.S.; Ali, S.S. Exploring the therapeutic potentials of exopolysaccharides derived from lactic acid bacteria and bifidobacteria: Antioxidant, antitumor, and periodontal regeneration. Front. Microbiol. 2022, 13, 803688. [Google Scholar] [CrossRef]
- Park, H.-R.; Hwang, D.; Suh, H.-J.; Yu, K.-W.; Kim, T.Y.; Shin, K.-S. Antitumor and antimetastatic activities of rhamnogalacturonan-II-type polysaccharide isolated from mature leaves of green tea via activation of macrophages and natural killer cells. Int. J. Biol. Macromol. 2017, 99, 179–186. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Nikolic, M.; López, P.; Suárez, A.; Miljkovic, M.; Kojic, M.; Margolles, A.; Golic, N.; Ruas-Madiedo, P. Exopolysaccharide-producing Bifidobacterium animalis subsp. lactis strains and their polymers elicit different responses on immune cells from blood and gut associated lymphoid tissue. Anaerobe 2014, 26, 24–30. [Google Scholar] [CrossRef]
- Lodemann, U.; Hübener, K.; Jansen, N.; Martens, H. Effects of Enterococcus faecium NCIMB 10415 as probiotic supplement on intestinal transport and barrier function of piglets. Arch. Anim. Nutr. 2006, 60, 35–48. [Google Scholar] [CrossRef]
- Awad, W.; Ghareeb, K.; Böhm, J. Intestinal Structure and Function of Broiler Chickens on Diets Supplemented with a Synbiotic Containing Enterococcus faecium and Oligosaccharides. Int. J. Mol. Sci. 2008, 9, 2205–2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamid, N.H.; Daud, H.M.; Kayansamruaj, P.; Abu Hassim, H.; Yusoff, S.M.; Abu Bakar, S.N.; Srisapoome, P. Short- and long-term probiotic effects of Enterococcus hirae isolated from fermented vegetable wastes on the growth, immune responses, and disease resistance of hybrid catfish (Clarias gariepinus × Clarias macrocephalus). Fish Shellfish Immunol. 2021, 114, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jayamanohar, J.; Devi, P.B.; Kavitake, D.; Rajendran, S.; Priyadarisini, V.B.; Shetty, P.H. Characterization of α-D-glucan produced by a probiont Enterococcus hirae KX577639 from feces of south Indian Irula tribals. Int. J. Biol. Macromol. 2018, 118, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Bhat, B.; Bajaj, B.K. Hypocholesterolemic and bioactive potential of exopolysaccharide from a probiotic Enterococcus faecium K1 isolated from kalarei. Bioresour. Technol. 2018, 254, 264–267. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Jia, K.; Tao, X.; Liu, Z.; Zhan, H.; He, W.; Zhang, Z.; Zeng, Z.; Wei, H. Characterization of novel exopolysaccharide of Enterococcus faecium WEFA23 from infant and demonstration of its in vitro biological properties. Int. J. Biol. Macromol. 2018, 128, 710–717. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, M.; Wan, C.; Chen, X.; Chen, X.; Tao, X.; Shah, N.P.; Wei, H. Screening probiotic strains for safety: Evaluation of virulence and antimicrobial susceptibility of enterococci from healthy Chinese infants. J. Dairy Sci. 2016, 99, 4282–4290. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.; Gu, E.; Luo, J.; Zhang, Z.; Xu, D.; Tao, X.; Shah, N.P.; Wei, H. Enterococcus hirae WEHI01 isolated from a healthy Chinese infant ameliorates the symptoms of type 2 diabetes by elevating the abundance of Lactobacillales in rats. J. Dairy Sci. 2020, 103, 2969–2981. [Google Scholar] [CrossRef]
- Zhang, F.; Qiu, L.; Xu, X.; Liu, Z.; Zhan, H.; Tao, X.; Shah, N.P.; Wei, H. Beneficial effects of probiotic cholesterol-lowering strain of Enterococcus faecium WEFA23 from infants on diet-induced metabolic syndrome in rats. J. Dairy Sci. 2017, 100, 1618–1628. [Google Scholar] [CrossRef]
- Panda, S.; Ding, J.L. Natural Antibodies Bridge Innate and Adaptive Immunity. J. Immunol. 2014, 194, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Yan, M.; Yang, J.; Li, F.; Wang, Y.; Feng, K.; Wang, S.; Lin, N.; Wang, Y.; Yang, B. Structural characterization of a polysaccharide from Trametes sanguinea Lloyd with immune-enhancing activity via activation of TLR4. Int. J. Biol. Macromol. 2022, 206, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-D.; Wu, Q.-X.; Zhao, J.; Su, T.; Lu, Y.-M.; Zhang, W.-N.; Wang, Y.; Chen, Y. Immunomodulatory effect of a polysaccharide fraction on RAW 264.7 macrophages extracted from the wild Lactarius deliciosus. Int. J. Biol. Macromol. 2019, 128, 732–739. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Cui, S.-H.; Zha, X.-Q.; Bansal, V.; Xue, L.; Li, X.-L.; Hao, R.; Pan, L.-H.; Luo, J.-P. Jellyfish skin polysaccharides: Extraction and inhibitory activity on macrophage-derived foam cell formation. Carbohydr. Polym. 2014, 106, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhou, R.-R.; Xie, H.-L.; Yu, Y.; Zhang, S.-H.; Zhao, C.-X.; Huang, J.-H.; Huang, L.-Q. Application of near infrared spectroscopy for rapid determination the geographical regions and polysaccharides contents of Lentinula edodes. Int. J. Biol. Macromol. 2018, 122, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Fu, X.; Luo, F.; Huang, Q. Effects of maltose on stability and rheological properties of orange oil-in-water emulsion formed by OSA modified starch. Food Hydrocoll. 2012, 32, 79–86. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, D.; Shen, Y.; Tao, X.; Liu, L.; Zhong, Y.; Fang, S. DPF2 regulates OCT4 protein level and nuclear distribution. Biochim. Biophys. Acta 2015, 1853, 3279–3293. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, C.; Wang, Y.; Yu, H.; Liu, H.; Wang, L.; Yang, X.; Liu, Z.; Wen, X.; Sun, Y.; et al. Structural elucidation of a heteroglycan from the fruiting bodies of Agaricus blazei Murill. Int. J. Biol. Macromol. 2011, 49, 716–720. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Wang, Z.; Wang, Y.; Xie, M.; Xie, J. Sulfated polysaccharide from Cyclocarya paliurus enhances the immunomodulatory activity of macrophages. Carbohydr. Polym. 2017, 174, 669–676. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, G.; Lai, F.; Wu, H. Structural Characterization and Immunomodulatory Activity of a Novel Polysaccharide from Lepidium meyenii. J. Agric. Food Chem. 2016, 64, 1921–1931. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Liu, P.; Ahmed, Z.; Xiao, P.; Bai, X. Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet Kefir. Carbohydr. Polym. 2010, 82, 895–903. [Google Scholar] [CrossRef]
- Wang, X.; Shao, C.; Liu, L.; Guo, X.; Xu, Y.; Lü, X. Optimization, partial characterization and antioxidant activity of an exopolysaccharide from Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2017, 103, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Isfahani, F.M.; Tahmourespour, A.; Hoodaji, M.; Ataabadi, M.; Mohammadi, A. Characterizing the new bacterial isolates of high yielding exopolysaccharides under hypersaline conditions. J. Clean. Prod. 2018, 185, 922–928. [Google Scholar] [CrossRef]
- Yang, X.H.; Yang, J.T.; Liu, H.H.; Ma, Z.R.; Guo, P.H.; Chen, H.; Gao, D.D. Extraction, structure analysis and antioxidant activity of Sibiraea laevigata (L.) Maxim polysaccharide. Int. J. Food Prop. 2022, 25, 2267–2285. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, F.; Wei, X. Characterization and antioxidant activities of polysaccharides from leaves, flowers and seeds of green tea. Carbohydr. Polym. 2012, 88, 146–153. [Google Scholar] [CrossRef]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. Production, characterization and antioxidant activities in vitro of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 2009, 78, 275–281. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.; Van Griensven, L.J. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011, 129, 1667–1675. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, L.; Yu, B.; Chen, K.; Liu, B.; Liu, J.; Qin, G.; Liu, C.; Liu, H.; Chen, K. Exopolysaccharide from Trichoderma pseudokoningii induces macrophage activation. Carbohydr. Polym. 2016, 149, 112–120. [Google Scholar] [CrossRef]
- Wang, S.; Liu, R.; Yu, Q.; Dong, L.; Bi, Y.; Liu, G. Metabolic reprogramming of macrophages during infections and cancer. Cancer Lett. 2019, 452, 14–22. [Google Scholar] [CrossRef]
- Liu, C.-F.; Tseng, K.-C.; Chiang, S.-S.; Lee, B.-H.; Hsu, W.-H.; Pan, T.-M. Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides. J. Sci. Food Agric. 2011, 91, 2284–2291. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; You, L.; Fu, X.; Liu, R.H. Fractionation, preliminary structural characterization and bioactivities of polysaccharides from Sargassum pallidum. Carbohydr. Polym. 2017, 155, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yin, J.; Nie, S.; Wan, Y.; Xie, M. Fractionation, physicochemical property and immunological activity of polysaccharides from Cassia obtusifolia. Int. J. Biol. Macromol. 2016, 91, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cui, Y.; Wang, X.; Yue, F.; Shan, Y.; Liu, B.; Zhou, Y.; Yi, Y.; Lü, X. Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2019, 128, 480–492. [Google Scholar] [CrossRef]
- Wen, Z.-S.; Xiang, X.-W.; Jin, H.-X.; Guo, X.-Y.; Liu, L.-J.; Huang, Y.-N.; OuYang, X.-K.; Qu, Y.-L. Composition and anti-inflammatory effect of polysaccharides from Sargassum horneri in RAW264.7 macrophages. Int. J. Biol. Macromol. 2016, 88, 403–413. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Wang, W.; Zou, Y.; Li, Q.; Mao, R.; Shao, X.; Jin, D.; Zheng, D.; Zhao, T.; Zhu, H.; Zhang, L.; et al. Immunomodulatory effects of a polysaccharide purified from Lepidium meyenii Walp. on macrophages. Process Biochem. 2016, 51, 542–553. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, P.; Zhao, S.; Nie, C.; Wang, N.; Du, X.; Zhou, Y. Characterization, antioxidant activity and immunomodulatory activity of polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2017, 95, 809–817. [Google Scholar] [CrossRef]
- Nie, C.; Zhu, P.; Ma, S.; Wang, M.; Hu, Y. Purification, characterization and immunomodulatory activity of polysaccharides from stem lettuce. Carbohydr. Polym. 2018, 188, 236–242. [Google Scholar] [CrossRef]
- Zheng, D.; Zou, Y.; Cobbina, S.J.; Wang, W.; Li, Q.; Chen, Y.; Feng, W.; Zou, Y.; Zhao, T.; Zhang, M.; et al. Purification, characterization and immunoregulatory activity of a polysaccharide isolated from Hibiscus sabdariffa L. J. Sci. Food Agric. 2016, 97, 1599–1606. [Google Scholar] [CrossRef]
- Habijanic, J.; Berovic, M.; Boh, B.; Plankl, M.; Wraber, B. Submerged cultivation of Ganoderma lucidum and the effects of its polysaccharides on the production of human cytokines TNF-α, IL-12, IFN-γ, IL-2, IL-4, IL-10 and IL-17. New Biotechnol. 2015, 32, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Hashizume, M.; Yoshida, H.; Suzuki, M.; Shiina, M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. 2011, 122, 143–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciszek-Lenda, M.; Nowak, B.; Śróttek, M.; Gamian, A.; Marcinkiewicz, J. Immunoregulatory potential of exopolysaccharide from Lactobacillus rhamnosus KL37. Effects on the production of inflammatory mediators by mouse macrophages. Int. J. Exp. Pathol. 2011, 92, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nie, Z.-K.; Zhou, Q.; Zhang, J.-L.; Yin, J.-J.; Xu, W.; Qiu, Y.; Ming, Y.-L.; Liang, S. Antitumor efficacy in H22 tumor bearing mice and immunoregulatory activity on RAW 264.7 macrophages of polysaccharides from Talinum triangulare. Food Funct. 2014, 5, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, X.; Pan, W.; Shen, X.; He, Y.; Yin, H.; Zhou, K.; Zou, L.; Chen, S.; Liu, S. Exopolysaccharides produced by yogurt-texture improving Lactobacillus plantarum RS20D and the immunoregulatory activity. Int. J. Biol. Macromol. 2018, 121, 342–349. [Google Scholar] [CrossRef]
- Georgiev, Y.N.; Ognyanov, M.H.; Kiyohara, H.; Batsalova, T.G.; Dzhambazov, B.M.; Ciz, M.; Denev, P.N.; Yamada, H.; Paulsen, B.S.; Vasicek, O.; et al. Acidic polysaccharide complexes from purslane, silver linden and lavender stimulate Peyer’s patch immune cells through innate and adaptive mechanisms. Int. J. Biol. Macromol. 2017, 105, 730–740. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, S.; Zhu, P.; Nie, C.; Ma, S.; Wang, N.; Du, X.; Zhou, Y. Purification, characterization and immunomodulatory activity of water extractable polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2018, 107, 882–890. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Lopez-Suarez, P.; Gueimonde, M.; Reyes-Gavilan, C.D.L.; Suarez-Diaz, A.M.; Margolles, A.; Ruas-Madiedo, P. Immune Modulation Capability of Exopolysaccharides Synthesised by Lactic Acid Bacteria and Bifidobacteria. Probiotics Antimicrob. Proteins 2012, 4, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-C.; Wu, Y.-J.; Hu, C.-Y. Monosaccharide composition influence and immunomodulatory effects of probiotic exopolysaccharides. Int. J. Biol. Macromol. 2019, 133, 575–582. [Google Scholar] [CrossRef]

| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| iNOS | GCGAAAGGTCATGGCTTCAC | TCTTCCAAGGTGCTTGCCTT |
| TNF-α | CGAGTGACAAGCCTGTAGCC | ACAAGGTACAACCCATCGGC |
| IL-6 | GTCCTTCCTACCCCAATTTCCA | CGCACTAGGTTTGCCGAGTA |
| β-actin | GCTCCTCCTGAGCGCAAGTA | CAGCTCAGTAACAGTCCGCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, K.; Wei, M.; He, Y.; Wang, Y.; Wei, H.; Tao, X. Characterization of Novel Exopolysaccharides from Enterococcus hirae WEHI01 and Its Immunomodulatory Activity. Foods 2022, 11, 3538. https://doi.org/10.3390/foods11213538
Jia K, Wei M, He Y, Wang Y, Wei H, Tao X. Characterization of Novel Exopolysaccharides from Enterococcus hirae WEHI01 and Its Immunomodulatory Activity. Foods. 2022; 11(21):3538. https://doi.org/10.3390/foods11213538
Chicago/Turabian StyleJia, Kaiying, Min Wei, Yao He, Yujie Wang, Hua Wei, and Xueying Tao. 2022. "Characterization of Novel Exopolysaccharides from Enterococcus hirae WEHI01 and Its Immunomodulatory Activity" Foods 11, no. 21: 3538. https://doi.org/10.3390/foods11213538





