Changes in the Physical–Chemical Properties and Volatile Flavor Components of Dry-Cured Donkey Leg during Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Manufacturing Process of Dry-Cured Donkey Leg
2.2. Sampling of Dry-Cured Donkey Leg
2.3. Physicochemical Analysis
2.4. Free Amino Acid Analysis
2.4.1. Sample Pretreatment
2.4.2. Determination of Free Amino Acids
2.5. Free Fatty Acid Analysis
2.5.1. Fat Extraction
2.5.2. Elution of Free Fatty Acids
2.5.3. Methyl Esterification of Free Fatty Acid
2.5.4. Determination of Free Fatty Acids
2.6. Volatile Flavor Compounds Analysis
2.6.1. Extraction of Flavor Compounds
2.6.2. Chromatographic Conditions
2.7. Statistical Analysis
3. Results and Discussion
3.1. Changes in Physicochemical Parameters
3.2. Changes in Free Amino Acids
3.3. Changes in Free Fatty Acids
3.4. Volatile Flavor Compounds Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, X.; Wang, G.; Zou, Y.; Zhao, Y.; Ge, C.; Liao, G. Evaluation of small molecular metabolites and sensory properties of Xuanwei ham salted with partial replacement of NaCl by KCl. Meat Sci. 2021, 175, 108465. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Zhou, X.; Li, C.; Liu, Y. Changes in the extent and products of In vitro protein digestion during the ripening periods of Chinese dry-cured hams. Meat Sci. 2021, 171, 108290. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-Y.; Pan, D.-D.; Bai, Y.; Li, C.-B.; Xu, X.-L.; Zhou, G.-H.; Cao, J.-X. Evaluating endogenous protease of salting exudates during the salting process of Jinhua ham. LWT 2019, 101, 76–82. [Google Scholar] [CrossRef]
- Martínez-Onandi, N.; Rivas-Cañedo, A.; Ávila, M.; Garde, S.; Nuñez, M.; Picon, A. Influence of physicochemical characteristics and high pressure processing on the volatile fraction of Iberian dry-cured ham. Meat Sci. 2017, 131, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Arellano, I.; Flores, M.; Toldrá, F. The ability of peptide extracts obtained at different dry cured ham ripening stages to bind aroma compounds. Food Chem. 2016, 196, 9–16. [Google Scholar] [CrossRef]
- Harkouss, R.; Astruc, T.; Lebert, A.; Gatellier, P.; Loison, O.; Safa, H.; Portanguen, S.; Parafita, E.; Mirade, P.-S. Quantitative study of the relationships among proteolysis, lipid oxidation, structure and texture throughout the dry-cured ham process. Food Chem. 2015, 166, 522–530. [Google Scholar] [CrossRef]
- Toldra, F. Proteolysis and lipolysis in flavour development of dry-cured meat products. Meat Sci. 1998, 49, S101–S110. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Lu, S.; Wang, J.; Fu, H.; Gu, B.; Lyu, B.; Wang, Q. Changes in proteolysis, protein oxidation, flavor, color and texture of dry-cured mutton ham during storage. LWT 2021, 149, 111860. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sarriés, M.V.; Tateo, A.; Polidori, P.; Franco, D.; Lanza, M. Carcass characteristics, meat quality and nutritional value of horsemeat: A review. Meat Sci. 2014, 96, 1478–1488. [Google Scholar] [CrossRef]
- Li, X.; Amadou, I.; Zhou, G.Y.; Qian, L.Y.; Zhang, J.L.; Wang, D.L.; Cheng, X.R. Flavor Components Comparison between the Neck Meat of Donkey, Swine, Bovine, and Sheep. Food Sci. Anim. Resour. 2020, 40, 527–540. [Google Scholar] [CrossRef]
- Polidori, P.; Di Girolami, P.; Vincenzetti, S. Vitamins and Minerals in Raw and Cooked Donkey Meat. In Meat and Nutrition; IntechOpen: London, UK, 2021. [Google Scholar]
- Polidori, P.; Pucciarelli, S.; Ariani, A.; Polzonetti, V.; Vincenzetti, S. A comparison of the carcass and meat quality of Martina Franca donkey foals aged 8 or 12 months. Meat Sci. 2015, 106, 6–10. [Google Scholar] [CrossRef]
- Guo, X.; Lu, S.; Wang, Y.; Dong, J.; Ji, H.; Wang, Q. Correlations among flavor compounds, lipid oxidation indices, and endogenous enzyme activity during the processing of Xinjiang dry-cured mutton ham. J. Food Process. Pres. 2019, 43, e14199. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, Y.; Wang, G.; Zhu, R.; Ge, C.; Liao, G. Changes in the physicochemical properties and volatile flavor compounds of dry-cured Chinese Laowo ham during processing. J. Food Process. Pres. 2020, 44, e14593. [Google Scholar] [CrossRef]
- Sugimoto, M.; Sugawara, T.; Obiya, S.; Enomoto, A.; Kaneko, M.; Ota, S.; Soga, T.; Tomita, M. Sensory properties and metabolomic profiles of dry-cured ham during the ripening process. Food Res. Int. 2020, 129, 108850. [Google Scholar] [CrossRef]
- Huan, Y.; Zhou, G.; Zhao, G.; Xu, X.; Peng, Z. Changes in flavor compounds of dry-cured Chinese Jinhua ham during processing. Meat Sci. 2005, 71, 291–299. [Google Scholar] [CrossRef]
- Zhou, C.-Y.; Wang, Y.; Pan, D.-D.; Cao, J.-X.; Chen, Y.-J.; Liu, Y.; Sun, Y.-Y.; Ou, C.-R. The changes in the proteolysis activity and the accumulation of free amino acids during chinese traditional dry-cured loins processing. Food Sci. Biotechnol. 2017, 26, 679–687. [Google Scholar] [CrossRef]
- Jin, G.; He, L.; Zhang, J.; Yu, X.; Wang, J.; Huang, F. Effects of temperature and NaCl percentage on lipid oxidation in pork muscle and exploration of the controlling method using response surface methodology (RSM). Food Chem. 2012, 131, 817–825. [Google Scholar] [CrossRef]
- Luccia, A.D.; Picariello, G.; Cacace, G.; Scaloni, A.; Faccia, M.; Liuzzi, V.; Alviti, G.; Musso, S.S. Proteomic analysis of water soluble and myofibrillar protein changes occurring in dry-cured hams. Meat Sci. 2005, 69, 479–491. [Google Scholar] [CrossRef]
- del Olmo, A.; Calzada, J.; Gaya, P.; Nuñez, M. Proteolysis, Texture, and Sensory Characteristics of Serrano Hams from Duroc and Large White Pigs during Dry-Curing. J. Food Sci. 2013, 78, C416–C424. [Google Scholar] [CrossRef]
- Marušić, N.; Vidaček, S.; Janči, T.; Petrak, T.; Medić, H. Determination of volatile compounds and quality parameters of traditional Istrian dry-cured ham. Meat Sci. 2014, 96, 1409–1416. [Google Scholar] [CrossRef]
- Shi, Y.; Li, X.; Huang, A. A metabolomics-based approach investigates volatile flavor formation and characteristic compounds of the Dahe black pig dry-cured ham. Meat Sci. 2019, 158, 107904. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, R.; Franco, D.; Carballo, J.; Sentandreu, M.Á.; Lorenzo, J.M. Influence of muscle type on the evolution of free amino acids and sarcoplasmic and myofibrillar proteins through the manufacturing process of Celta dry-cured ham. Food Res. Int. 2014, 56, 226–235. [Google Scholar] [CrossRef]
- Frank, D.; Ball, A.; Hughes, J.; Krishnamurthy, R.; Piyasiri, U.; Stark, J.; Watkins, P.; Warner, R. Sensory and Flavor Chemistry Characteristics of Australian Beef: Influence of Intramuscular Fat, Feed, and Breed. J. Agric. Food Chem. 2016, 64, 4299–4311. [Google Scholar] [CrossRef]
- Ardö, Y. Flavour formation by amino acid catabolism. Biotechnol. Adv. 2006, 24, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Iida, F.; Miyazaki, Y.; Tsuyuki, R.; Kato, K.; Egusa, A.; Ogoshi, H.; Nishimura, T. Changes in taste compounds, breaking properties, and sensory attributes during dry aging of beef from Japanese black cattle. Meat Sci. 2016, 112, 46–51. [Google Scholar] [CrossRef]
- Flores, M.; Toldrá, F. Microbial enzymatic activities for improved fermented meats. Trends Food Sci. Technol. 2011, 22, 81–90. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, G.; Wang, J.; Zhang, W. Effect of intensifying high-temperature ripening on lipolysis and lipid oxidation of Jinhua ham. LWT Food Sci. Technol. 2011, 44, 473–479. [Google Scholar] [CrossRef]
- Lorenzo, J.M. Changes on physico-chemical, textural, lipolysis and volatile compounds during the manufacture of dry-cured foal “cecina”. Meat Sci. 2014, 96, 256–263. [Google Scholar] [CrossRef]
- Purriños, L.; Bermúdez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Development of Volatile Compounds during the Manufacture of Dry-Cured “Lacón,” a Spanish Traditional Meat Product. J. Food Sci. 2011, 76, C89–C97. [Google Scholar] [CrossRef]
- Toldrá, F.; Flores, M. The Role of Muscle Proteases and Lipases in Flavor Development During the Processing of Dry-Cured Ham. Crit. Rev. Food Sci. 1998, 38, 331–352. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Pan, D.; Wang, Y.; Cao, J. Effects of high pressure treatment on lipolysis-oxidation and volatiles of marinated pork meat in soy sauce. Meat Sci. 2018, 145, 186–194. [Google Scholar] [CrossRef]
- Gandemer, G. Lipids in muscles and adipose tissues, changes during processing and sensory properties of meat products. Meat Sci. 2002, 62, 309–321. [Google Scholar] [CrossRef]
- Pugliese, C.; Sirtori, F.; Škrlep, M.; Piasentier, E.; Calamai, L.; Franci, O.; Čandek-Potokar, M. The effect of ripening time on the chemical, textural, volatile and sensorial traits of Bicep femoris and Semimembranosus muscles of the Slovenian dry-cured ham Kraški pršut. Meat Sci. 2015, 100, 58–68. [Google Scholar] [CrossRef]
- Antequera, T.; López-Bote, C.J.; Córdoba, J.J.; García, C.; Asensio, M.A.; Ventanas, J.; García-Regueiro, J.A.; Díaz, I. Lipid oxidative changes in the processing of Iberian pig hams. Food Chem. 1992, 45, 105–110. [Google Scholar] [CrossRef]
- Wang, J.; Jin, G.; Zhang, W.; Ahn, D.U.; Zhang, J. Effect of curing salt content on lipid oxidation and volatile flavour compounds of dry-cured turkey ham. LWT Food Sci. Technol. 2012, 48, 102–106. [Google Scholar] [CrossRef]
- Aliño, M.; Grau, R.; Toldrá, F.; Barat, J.M. Physicochemical changes in dry-cured hams salted with potassium, calcium and magnesium chloride as a partial replacement for sodium chloride. Meat Sci. 2010, 86, 331–336. [Google Scholar] [CrossRef]
- Sørensen, G.; Jørgensen, S.S. A critical examination of some experimental variables in the 2-thiobarbituric acid (TBA) test for lipid oxidation in meat products. Z. Lebensm. -Unters. Forsch. 1996, 202, 205–210. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Carballo, J. Changes in physico-chemical properties and volatile compounds throughout the manufacturing process of dry-cured foal loin. Meat Sci. 2015, 99, 44–51. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Regueiro, J.A.G.; Gibert, J.; Díaz, I. Determination of neutral lipids from subcutaneous fat of cured ham by capillary gas chromatography and liquid chromatography. J. Chromatogr. A. 1994, 667, 225–233. [Google Scholar] [CrossRef]
- Giovanelli, G.; Buratti, S.; Laureati, M.; Pagliarini, E. Evolution of physicochemical, morphological and aromatic characteristics of Italian PDO dry-cured hams during processing. Eur. Food Res. Technol. 2016, 242, 1117–1127. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.M.; Zhou, G.H.; Xu, X.L.; Peng, Z.Q.; Huan, Y.J.; Jing, Z.M.; Chen, M.W. Studies on time-related changes of dipeptidyl peptidase during processing of Jinhua ham using response surface methodology. Meat Sci. 2005, 69, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, G.; Zou, Y.; Zhao, Y.; Ge, C.; Liao, G. Changes in physicochemical properties and water-soluble small molecular compounds of dry-cured Xuanwei ham during processing. J. Food Process. 2021, 45, e15711. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, G.; He, C.; Zhang, L. Studies on the changes of physical and chemical index during the processing of Xuanwei ham. J. Henan Inst. Sci. Technol. 2009, 37, 39–41. [Google Scholar]
- Ventanas, J.; CÓRdoba, J.J.; Antequera, T.; Garcia, C.; LÓPez-Bote, C.; Asensio, M.A. Hydrolysis and Maillard Reactions During Ripening of Iberian Ham. J. Food Sci. 1992, 57, 813–815. [Google Scholar] [CrossRef]
- Martín, L.; Córdoba, J.J.; Antequera, T.; Timón, M.L.; Ventanas, J. Effects of salt and temperature on proteolysis during ripening of Iberian ham. Meat Sci. 1998, 49, 145–153. [Google Scholar] [CrossRef]
- Gou, P.; Guerrero, L.; Arnau, J. Sex and crossbreed effects on the characteristics of dry-cured ham. Meat Sci. 1995, 40, 21–31. [Google Scholar] [CrossRef]
- Cilla, I.; Martínez, L.; Beltrán, J.A.; Roncalés, P. Factors affecting acceptability of dry-cured ham throughout extended maturation under “bodega” conditions. Meat Sci. 2005, 69, 789–795. [Google Scholar] [CrossRef]
- Mariscal, C.; Garcia Ruiz, A.; Soriano, A.; Cabezudo, M. Study of the proteolysis and cathepsin D activity of commercial dry-cured Iberian and Serrano hams. Sci. Aliment. 2004, 24, 221–232. [Google Scholar] [CrossRef]
- Karolyi, D. Chemical properties and quality of Istrian dry-cured ham. MESO: Prvi Hrvat. Časopis Mesu 2006, 8, 224–228. [Google Scholar]
- Cava, R.; Ruiz, J.; Ventanas, J.; Antequera, T. Oxidative and lipolytic changes during ripening of Iberian hams as affected by feeding regime: Extensive feeding and alpha-tocopheryl acetate supplementation. Meat Sci. 1999, 52, 165–172. [Google Scholar] [CrossRef]
- Marcuse, R. Antioxidative effect of amino-acids. Nature 1960, 186, 886–887. [Google Scholar] [CrossRef]
- Peña-Ramos, E.A.; Xiong, Y.L.; Arteaga, G.E. Fractionation and characterisation for antioxidant activity of hydrolysed whey protein. J. Sci. Food Agric. 2004, 84, 1908–1918. [Google Scholar] [CrossRef]
- Cilla, I.; Altarriba, J.; Guerrero, L.; Gispert, M.; Martínez, L.; Moreno, C.; Beltrán, J.A.; Guàrdia, M.D.; Diestre, A.; Arnau, J.; et al. Effect of different Duroc line sires on carcass composition, meat quality and dry-cured ham acceptability. Meat Sci. 2006, 72, 252–260. [Google Scholar] [CrossRef]
- Aksu, M.İ.; Kaya, M. Effect of storage temperatures and time on shelf-life of sliced and modified atmosphere packaged Pastırma, a dried meat product, produced from beef. J. Sci. Food Agric. 2005, 85, 1305–1312. [Google Scholar] [CrossRef]
- Sforza, S.; Pigazzani, A.; Motti, M.; Porta, C.; Virgili, R.; Galaverna, G.; Dossena, A.; Marchelli, R. Oligopeptides and free amino acids in Parma hams of known cathepsin B activity. Food Chem. 2001, 75, 267–273. [Google Scholar] [CrossRef]
- Zhao, G.M.; Zhou, G.H.; Tian, W.; Xu, X.L.; Wang, Y.L.; Luo, X. Changes of alanyl aminopeptidase activity and free amino acid contents in biceps femoris during processing of Jinhua ham. Meat Sci. 2005, 71, 612–619. [Google Scholar] [CrossRef]
- Virgili, R.; Saccani, G.; Gabba, L.; Tanzi, E.; Soresi Bordini, C. Changes of free amino acids and biogenic amines during extended ageing of Italian dry-cured ham. LWT Food Sci. Technol. 2007, 40, 871–878. [Google Scholar] [CrossRef]
- Salazar, E.; Cayuela, J.M.; Abellán, A.; Bueno-Gavilá, E.; Tejada, L. Fatty Acids and Free Amino Acids Changes during Processing of a Mediterranean Native Pig Breed Dry-Cured Ham. Foods 2020, 9, 1170. [Google Scholar] [CrossRef]
- Cordoba, J.J.; Antequera, T.; García, C.; Ventanas, J.; Lopez Bote, C.; Asensio, M.A. Evolution of free amino acids and amines during ripening of Iberian cured ham. J. Sci. Food Agric. 1994, 42, 2296–2301. [Google Scholar] [CrossRef]
- Zhu, S.; Hu, J. Studies on Jinhua ham tastes and taste substances. J. Food Sci. 1993, 159, 8–11. [Google Scholar]
- Yu, Y.; Wang, G.; Sun, Y.; Ge, C.; Liao, G. Changes in physicochemical parameters, free fatty acid profile and water-soluble compounds of Yunnan dry-cured beef during processing. J. Food Process Preserv. 2020, 44, e14380. [Google Scholar] [CrossRef]
- Corino, C.; Magni, S.; Pastorelli, G.; Rossi, R.; Mourot, J. Effect of conjugated linoleic acid on meat quality, lipid metabolism, and sensory characteristics of dry-cured hams from heavy pigs1. J. Anim. Sci. 2003, 81, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Martín, L.; Córdoba, J.J.; Ventanas, J.; Antequera, T. Changes in intramuscular lipids during ripening of Iberian dry-cured ham. Meat Sci. 1999, 51, 129–134. [Google Scholar] [CrossRef]
- Zhu, C.-Z.; Zhao, J.-L.; Tian, W.; Liu, Y.-X.; Li, M.-Y.; Zhao, G.-M. Contribution of Histidine and Lysine to the Generation of Volatile Compounds in Jinhua Ham Exposed to Ripening Conditions Via Maillard Reaction. J. Food Sci. 2018, 83, 46–52. [Google Scholar] [CrossRef]
- Martínez-Onandi, N.; Rivas-Cañedo, A.; Nuñez, M.; Picon, A. Effect of chemical composition and high pressure processing on the volatile fraction of Serrano dry-cured ham. Meat Sci. 2016, 111, 130–138. [Google Scholar] [CrossRef]
- Li, F.; Feng, X.; Zhang, D.; Li, C.; Xu, X.; Zhou, G.; Liu, Y. Physical properties, compositions and volatile profiles of Chinese dry-cured hams from different regions. J. Food Meas. Charact. 2020, 14, 492–504. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Franco, D.; Carballo, J. Effect of the inclusion of chestnut in the finishing diet on volatile compounds during the manufacture of dry-cured “Lacón” from Celta pig breed. Meat Sci. 2014, 96, 211–223. [Google Scholar] [CrossRef]
- Wang, W.; Feng, X.; Zhang, D.; Li, B.; Sun, B.; Tian, H.; Liu, Y. Analysis of volatile compounds in Chinese dry-cured hams by comprehensive two-dimensional gas chromatography with high-resolution time-of-flight mass spectrometry. Meat Sci. 2018, 140, 14–25. [Google Scholar] [CrossRef]
- Carrapiso, A.I.; Noseda, B.; García, C.; Reina, R.; Sánchez del Pulgar, J.; Devlieghere, F. SIFT-MS analysis of Iberian hams from pigs reared under different conditions. Meat Sci. 2015, 104, 8–13. [Google Scholar] [CrossRef]
- Muriel, E.; Antequera, T.; Petrón, M.J.; Andrés, A.I.; Ruiz, J. Volatile compounds in Iberian dry-cured loin. Meat Sci. 2004, 68, 391–400. [Google Scholar] [CrossRef]
- Zhang, J.; Zhen, Z.; Zhang, W.; Zeng, T.; Zhou, G. Effect of intensifying high-temperature ripening on proteolysis, lipolysis and flavor of Jinhua ham. J. Sci. Food Agric. 2009, 89, 834–842. [Google Scholar] [CrossRef]
- Lecanu, L.; Ducruet, V.; Jouquand, C.; Gratadoux, J.J.; Feigenbaum, A. Optimization of Headspace Solid-Phase Microextraction (SPME) for the Odor Analysis of Surface-Ripened Cheese. J. Agric. Food Chem. 2002, 50, 3810–3817. [Google Scholar] [CrossRef]
- Flores, M.; Aristoy, M.; Spanier, A.; Toldrá, F. Correlations of non-volatile components and sensory properties of Spanish Serrano dry-cured ham, Effect of processing time. J. Food Sci. 1997, 62, 1235–1242. [Google Scholar] [CrossRef]
| Physicochemical Parameters | Green Leg (0 d) | Start of Salting (10 d) | Middle of Salting (20 d) | End of Salting (35 d) | Start of Fermentation (65 d) | End of Fermentation (105 d) | Aging (165 d) |
|---|---|---|---|---|---|---|---|
| Moisture (%) | 72 ± 0.03 a | 66 ± 0.02 b | 51 ± 0.02 c | 47 ± 0.01 d | 44 ± 0.02 d | 37 ± 0.01 e | 35 ± 0.01 e |
| Water activity | 0.90 ± 0.00 a | 0.90 ± 0.00 a | 0.89 ± 0.01 a | 0.87 ± 0.01 b | 0.84 ± 0.00 c | 0.80 ± 0.00 d | 0.78 ± 0.01 e |
| pH | 6.5 ± 0.03 a | 6.32 ± 0.05 bc | 6.28 ± 0.03 bc | 6.24 ± 0.05 c | 6.38 ± 0.04 b | 6.56 ± 0.11 a | 6.6 ± 0.07 a |
| Chloride (% of DM) | 0.45 ± 0.01 g | 1.36 ± 0.01 f | 3.16 ± 0.02 e | 6.46 ± 0.04 d | 9.87 ± 0.10 c | 10.89 ± 0.04 b | 12.39 ± 0.03 a |
| TBARS (mg MDA/kg meat) | 0.03 ± 0.00 f | 0.12 ± 0.01 e | 0.22 ± 0.01 d | 0.39 ± 0.01 a | 0.36 ± 0.02 ab | 0.33 ± 0.02 c | 0.34 ± 0.01 bc |
| POV (g/100 g) | 1.39 ± 0.01 e | 1.71 ± 0.03 d | 2.30 ± 0.09 c | 4.78 ± 0.03 b | 5.12 ± 0.21 a | 5.23 ± 0.01 a | 5.26 ± 0.01 a |
| TVB-N (mg/kg) | 1.43 ± 0.08 g | 2.00 ± 0.57 f | 3.34 ± 0.02 e | 3.72 ± 0.10 d | 5.59 ± 0.16 c | 6.74 ± 0.02 b | 8.58 ± 0.02 a |
| Ash (%) | 3 ± 0.00 e | 9 ± 0.00 d | 11 ± 0.01 c | 13 ± 0.00 b | 16 ± 0.01 a | 16 ± 0.00 a | 17 ± 0.00 a |
| Green Leg (0 d) | Start of Salting (10 d) | Middle of Salting (20 d) | End of Salting (35 d) | Start of Fermentation (65 d) | End of Fermentation (105 d) | Aging (165 d) | |
|---|---|---|---|---|---|---|---|
| Glutamate (Glu) | 0.85 ± 0.04 d | 0.92 ± 0.05 d | 0.99 ± 0.01 d | 1.11 ± 0.10 d | 2.61 ± 0.10 c | 6.51 ± 0.29 b | 8.64 ± 0.15 a |
| Aspartate (Asp) | 0.20 ± 0.02 c | 0.22 ± 0.02 c | 0.25 ± 0.02 c | 0.27 ± 0.02 c | 0.60 ± 0.15 c | 1.03 ± 0.17 b | 1.68 ± 0.51 a |
| Umami AAS | 1.05 ± 0.06 d | 1.15 ± 0.04 d | 1.24 ± 0.01 d | 1.38 ± 0.12 d | 3.21 ± 0.15 c | 7.54 ± 0.40 b | 10.32 ± 0.36 a |
| Alanine (Ala) | 0.37 ± 0.03 d | 0.40 ± 0.02 d | 0.47 ± 0.02 d | 0.61 ± 0.03 d | 1.75 ± 0.43 c | 4.50 ± 0.39 b | 6.57 ± 0.44 a |
| Glycine (Gly) | 0.19 ± 0.03 d | 0.23 ± 0.01 d | 0.25 ± 0.04 d | 0.26 ± 0.02 d | 0.60 ± 0.07 c | 1.74 ± 0.05 b | 2.16 ± 0.05 a |
| Serine (Ser) | 0.20 ± 0.02 d | 0.24 ± 0.01 d | 0.24 ± 0.02 d | 0.27 ± 0.02 d | 0.66 ± 0.07 c | 1.80 ± 0.05 b | 2.26 ± 0.13 a |
| Threonine (Thr) | 0.14 ± 0.01 d | 0.15 ± 0.02 d | 0.16 ± 0.02 d | 0.18 ± 0.03 d | 0.55 ± 0.13 c | 0.74 ± 0.14 b | 1.16 ± 0.11 a |
| Sweet AAs | 0.90 ± 0.05 d | 1.03 ± 0.05 d | 1.13 ± 0.07 d | 1.32 ± 0.06 d | 3.55 ± 0.60 c | 8.78 ± 0.47 b | 12.15 ± 0.45 a |
| Arginine (Arg) | 0.11 ± 0.02 d | 0.14 ± 0.03 d | 0.18 ± 0.02 d | 0.22 ± 0.01 d | 0.39 ± 0.13 cd | 0.62 ± 0.07 b | 1.02 ± 0.19 a |
| Isoleucine (Ile) | 0.12 ± 0.02 d | 0.14 ± 0.03 d | 0.16 ± 0.02 d | 0.19 ± 0.02 d | 0.36 ± 0.07 c | 0.66 ± 0.11 b | 1.12 ± 0.11 a |
| Leucine (Leu) | 0.36 ± 0.03 d | 0.40 ± 0.02 d | 0.45 ± 0.06 d | 0.46 ± 0.04 d | 1.63 ± 0.11 c | 3.00 ± 0.30 b | 5.09 ± 0.19 a |
| Methionine (Met) | 0.06 ± 0.03 d | 0.06 ± 0.03 d | 0.07 ± 0.01 d | 0.12 ± 0.06 d | 0.22 ± 0.05 c | 0.55 ± 0.06 b | 1.02 ± 0.06 a |
| Phenylalanine (Phe) | 0.22 ± 0.03 d | 0.25 ± 0.02 d | 0.28 ± 0.02 d | 0.29 ± 0.02 d | 0.53 ± 0.09 c | 0.68 ± 0.11 b | 1.25 ± 0.09 a |
| Tyrosine (Tyr) | 0.14 ± 0.02 c | 0.17 ± 0.01 c | 0.22 ± 0.03 c | 0.24 ± 0.03 c | 0.44 ± 0.15 b | 0.56 ± 0.13 b | 1.18 ± 0.15 a |
| Valine (Val) | 0.12 ± 0.02 c | 0.16 ± 0.01 c | 0.24 ± 0.05 c | 0.25 ± 0.05 c | 0.52 ± 0.08 b | 0.67 ± 0.21 b | 1.25 ± 0.22 a |
| Histidine (His) | 0.55 ± 0.02 d | 0.60 ± 0.02 d | 0.66 ± 0.02 d | 0.75 ± 0.05 d | 2.03 ± 0.40 c | 5.66 ± 0.22 b | 7.85 ± 0.05 a |
| Tryptophan (Try) | 0.05 ± 0.01 d | 0.05 ± 0.02 d | 0.04 ± 0.01 d | 0.06 ± 0.02 d | 0.09 ± 0.02 cd | 0.16 ± 0.03 b | 0.22 ± 0.03 a |
| Bitter AAs | 1.73 ± 0.13 e | 1.98 ± 0.09 de | 2.30 ± 0.05 de | 2.59 ± 0.24 d | 6.21 ± 0.82 c | 12.57 ± 0.44 b | 19.99 ± 0.38 a |
| Lysine (Lys) | 0.29 ± 0.01 d | 0.33 ± 0.03 d | 0.37 ± 0.06 d | 0.40 ± 0.08 d | 1.60 ± 0.07 c | 2.47 ± 0.10 b | 4.69 ± 0.25 a |
| Proline (Pro) | 0.11 ± 0.02 c | 0.14 ± 0.02 c | 0.14 ± 0.05 c | 0.16 ± 0.02 c | 0.63 ± 0.10 b | 0.70 ± 0.04 b | 0.94 ± 0.08 a |
| Tasteless AAs | 0.40 ± 0.03 d | 0.46 ± 0.05 d | 0.51 ± 0.03 d | 0.56 ± 0.09 d | 2.23 ± 0.07 c | 3.17 ± 0.13 b | 5.63 ± 0.31 a |
| Other Cysteine (Cys) | 0.02 ± 0.01 c | 0.04 ± 0.01 c | 0.02 ± 0.02 c | 0.04 ± 0.01 c | 0.05 ± 0.03 c | 0.11 ± 0.02 b | 0.29 ± 0.02 a |
| Total | 4.10 ± 0.27 e | 4.66 ± 0.23 de | 5.20 ± 0.10 de | 5.89 ± 0.44 d | 15.26 ± 1.40 c | 32.18 ± 0.92 b | 48.37 ± 0.89 a |
| Free Fatty Acids | Green Leg (0 d) | Start of Salting (10 d) | Middle of Salting (20 d) | End of Salting (35 d) | Start of Fermentation (65 d) | End of Fermentation (105 d) | Aging (165 d) |
|---|---|---|---|---|---|---|---|
| C4:0 | 0.37 ± 0.07 ab | 0.27 ± 0.04 bcde | 0.26 ± 0.05 ae | 0.31 ± 0.09 ac | 0.41 ± 0.13 a | 0.25 ± 0.03 bcde | 0.29 ± 0.03 ad |
| C14:0 | 0.00 ± 0.00 d | 0.07 ± 0.02 d | 0.36 ± 0.12 bc | 0.49 ± 0.27 ab | 0.20 ± 0.01 cd | 0.50 ± 0.01 b | 0.78 ± 0.20 a |
| C16:0 | 3.43 ± 0.94 d | 3.83 ± 0.55 d | 5.76 ± 1.26 c | 6.24 ± 0.36 c | 8.49 ± 0.92 b | 8.40 ± 0.81 b | 10.51 ± 0.91 a |
| C18:0 | 2.11 ± 0.92 e | 2.50 ± 0.12 e | 4.43 ± 0.28 bc | 3.00 ± 0.73 de | 5.07 ± 0.36 b | 3.92 ± 0. 79 cd | 6.91 ± 0.50 a |
| C20:0 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.69 ± 0.58 a | 0.00 ± 0.00 b | 0.14 ± 0.03 b | 0.00 ± 0.00 b | 0.38 ± 0.14 ab |
| SFAs | 6.12 ± 1.58 e | 6.67 ± 0.81 e | 10.81 ± 1.2 cd | 10.04 ± 0.96 d | 14.32 ± 1.28 b | 13.08 ± 0.75 bc | 18.86 ± 1.23 a |
| C16:1 | 0.00 ± 0.00 b | 0.32 ± 0.02 ab | 0.42 ± 0.06 a | 0.49 ± 0.12 a | 0.41 ± 0.14 a | 5.25 ± 0.34 a | 1.31 ± 0.21 a |
| C18:1n 9c | 0.21 ± 0.02 c | 0.00 ± 0.00 c | 1.51 ± 0.11 b | 0.27 ± 0.11 c | 1.19 ± 0.09 b | 0.63 ± 0.30 a | 5.25 ± 0.24 a |
| C18:1n 9t | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.82 ± 0.39 b | 0.00 ± 0.00 c | 1.58 ± 0.23 a |
| C20:1 | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.70 ± 0.27 b | 0.00 ± 0.00 c | 1.75 ± 0.21 a |
| MUFAs | 0.21 ± 0.02 e | 0.32 ± 0.02 e | 1.93 ± 0.13 d | 0.76 ± 0.19 e | 3.12 ± 0.80 c | 5.88 ± 0.32 b | 9.89 ± 0.58 a |
| C18:2n 6c | 0.40 ± 0.28 d | 0.60 ± 0.05 d | 1.35 ± 028 c | 3.16 ± 0.20 b | 2.80 ± 0.48 b | 3.39 ± 0.06 b | 10.43 ± 0.60 a |
| C18:2n6t | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.22 ± 0.10 b | 0.28 ± 0.25 b | 0.62 ± 0.02 a |
| C18:3n3 | 0.19 ± 0.12 bcde | 0.29 ± 0.11 ac | 0.51 ± 0.07 a | 0.27 ± 0.02 bcd | 0.28 ± 0.13 ad | 0.21 ± 0.02 e | 0.41 ± 0.08 ab |
| C18:3n6 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.64 ± 0.15 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.32 ± 0.08 ab |
| C20:2 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.34 ± 0.05 a |
| C20:3 | 0.00 ± 0.00 c | 0.17 ± 0.03 c | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.88 ± 0.16 b | 0.42 ± 0.26 b | 3.10 ± 0.48 a |
| PUFAs | 0.59 ± 0.40 d | 1.07 ± 0.08 cd | 1.86 ± 0.45 c | 4.06 ± 0.33 b | 4.17 ± 0.69 b | 4.30 ± 0.33 b | 15.52 ± 1.12 a |
| Total | 6.71 ± 1.25 d | 8.05 ± 0.61 d | 15.29 ± 0.8 c | 14.86 ± 1.19 c | 21.61 ± 2.70 b | 23.25 ± 1.42 b | 44.27 ± 2.70 a |
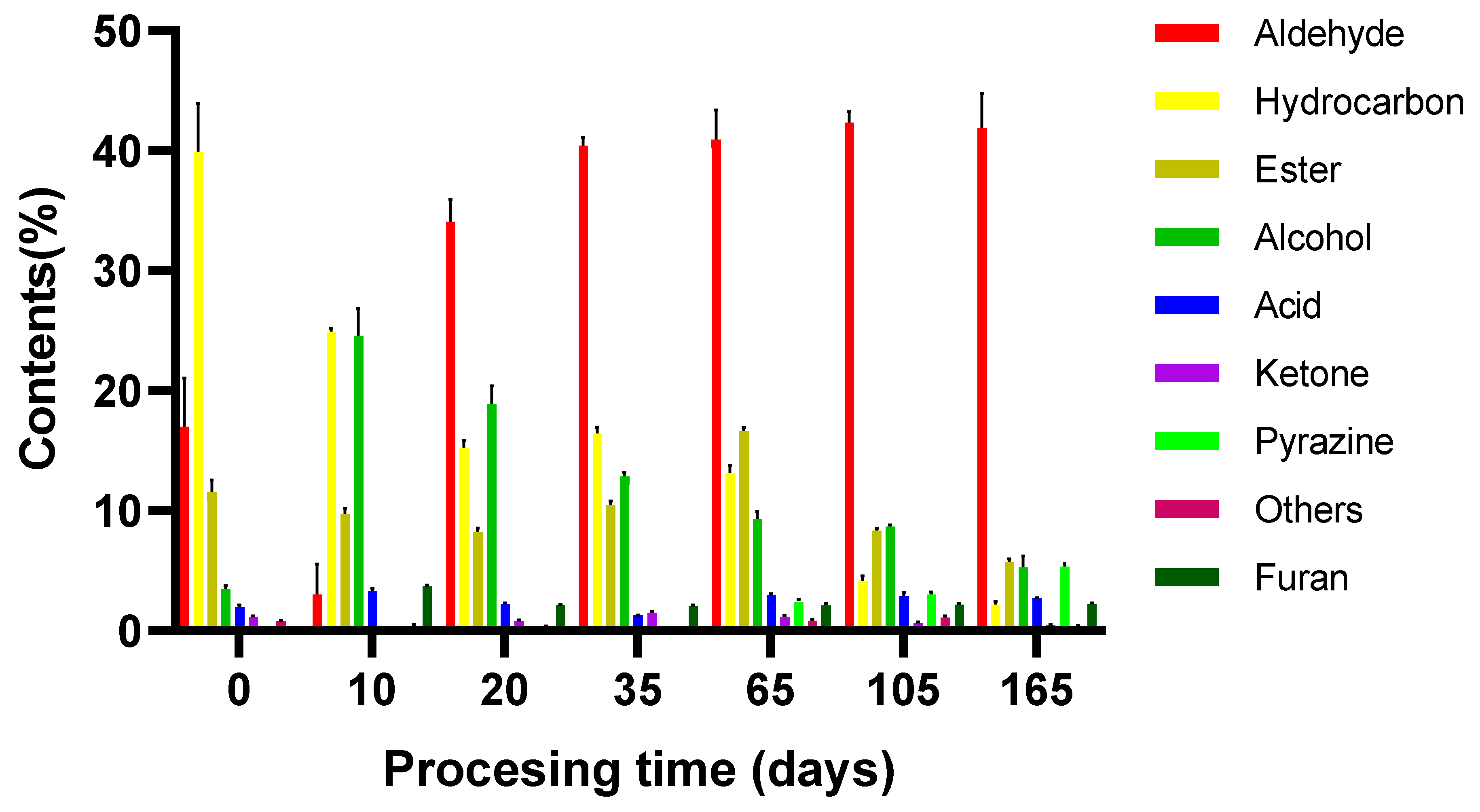
| Volatile Flavor Compounds | Green Leg (0 d) | Start of Salting (10 d) | Middle of Salting (20 d) | End of Salting (35 d) | Start of Fermentation (65 d) | End of Fermentation (105 d) | Aging (165 d) |
|---|---|---|---|---|---|---|---|
| Aldehydes | 17.00 ± 4.07 c | 30.53 ± 2.56 b | 34.12 ± 1.82 b | 40.42 ± 0.7 a | 40.95 ± 2.43 a | 42.36 ± 0.89 a | 41.88 ± 2.89 a |
| Hexanal | 12.55 ± 3.15 d | 22.0 ± 2.14 bc | 22.6 ± 1.47 b | 18.44 ± 2.06 c | 27.18 ± 1.94 a | 28.38 ± 1.52 a | 27.27 ± 1.07 a |
| N-octanal | ND | 1.79 ± 0.52 c | ND | 4.36 ± 0.44 a | ND | 3.09 ± 0.37 b | 3.45 ± 0.45 b |
| N-nonanal | 3.48 ± 0.41 cde | 2.68 ± 1.02 de | 5.07 ± 1.32 b | 8.45 ± 1.09 a | 2.00 ± 0.30 e | 4.98 ± 0.21 bc | 3.97 ± 0.12 bcd |
| 2-phenyl-2-butenal | ND | ND | 0.74 ± 0.25 a | ND | ND | ND | 0.03 ± 0.04 b |
| Trans-2-Octenal | ND | 1.55 ± 0.48 c | 2.19 ± 0.15 b | 1.67 ± 0.22 c | 2.91 ± 0.18 a | ND | ND |
| Benzaldehyde | 0.77 ± 0.43 c | 0.49 ± 0.14 c | 1.56 ± 0.17 b | 1.52 ± 0.11 b | 1.54 ± 0.05 b | 1.4 ± 0.16 b | 2.52 ± 0.19 a |
| 3-Ethylbenzaldehyde | ND | 0.13 ± 0.03 bc | 0.29 ± 0.05 a | 0.29 ± 0.08 a | ND | 0.20 ± 0.02 b | 0.11 ± 0.02 c |
| Trans-2-Nonenal | ND | ND | ND | 1.33 ± 0.31 a | 1.44 ± 0.05 a | 0.7 ± 0.03 b | 0.92 ± 0.05 b |
| 2,4-Nonadienal | 0.09 ± 0.11 c | 0.18 ± 0.03 c | 0.24 ± 0.08 bc | 0.42 ± 0.1 b | 1.02 ± 0.24 a | 0.18 ± 0.02 c | 0.2 ± 0.06 bc |
| 2,4-Decadienal | ND | 0.25 ± 0.04 b | 0.07 ± 0.07 cd | 0.28 ± 0.02 b | 1.11 ± 0.14 a | ND | 0.16 ± 0.03 bc |
| (E)-Tetradec-2-enal | ND | ND | ND | ND | ND | 0.28 ± 0.01 b | 0.34 ± 0.04 a |
| Trans,trans-2,4-Heptadienal | ND | ND | ND | 0.13 ± 0.05 b | 0.13 ± 0.03 b | 0.15 ± 0.05 ab | 0.2 ± 0.02 a |
| (E)-2-Decenal | ND | ND | ND | 1.6 ± 0.14 a | 1.37 ± 0.1 b | 0.8 ± 0.02 c | 0.63 ± 0.02 d |
| 2-Dodecenal | ND | 0 b | ND | ND | ND | ND | 0.24 ± 0.07 a |
| Vanillin | 0.11 ± 0.04 a | ND | ND | 0.05 ± 0.05 a | 0.05 ± 0.07 a | ND | ND |
| Trans-2-Heptenal | ND | 0.76 ± 0.04 cde | 0.85 ± 0.06 de | 0.97 ± 0.08 bcd | 1.7 ± 0.17 abc | 1.88 ± 0.13 a | 1.79 ± 1.13 ab |
| Tetradecanal | ND | ND | ND | ND | ND | ND | 0.05 ± 0.02 a |
| Bourgeonal | ND | ND | 0.49 ± 0.06 a | ND | ND | ND | ND |
| 2-Undecenal | ND | 0.16 ± 0.02 cd | ND | 0.91 ± 0.17 a | 0.5 ± 0.13 b | 0.32 ± 0.1 bc | ND |
| 4-(1,1-dimethyl)Benzenepropanal | ND | 0.48 ± 0.70 a | ND | ND | ND | ND | ND |
| Hydrocarbons | 39.93 ± 4.01 a | 24.94 ± 0.23 b | 15.29 ± 0.56 cd | 16.42 ± 0.51 c | 13.13 ± 0.62 d | 4.18 ± 0.37 e | 2.19 ± 0.26 e |
| Octamethylcyclotetrasiloxane | 17.53 ± 0.98 a | 6.63 ± 0.85 b | 5.35 ± 0.56 c | 4.4 ± 0.36 c | 4.53 ± 0.13 c | ND | ND |
| Decamethylcyclopentasiloxane | 17.65 ± 1.14 a | 13.13 ± 1.41 b | 5.16 ± 0.15 c | 6.47 ± 0.19 c | ND | ND | ND |
| Dodecamethylcyclohexasiloxane | 1.97 ± 0.79 b | 3.37 ± 0.51 a | 0.79 ± 0.32 c | 1.08 ± 0.1 c | 2.69 ± 0.16 ab | 0.58 ± 0.12 c | 0.42 ± 0.05 c |
| Tetradecane | ND | 0.72 ± 0.05 b | 1.5 ± 0.22 a | 1.61 ± 0.07 a | 0.62 ± 0.06 b | 0.59 ± 0.05 b | 0.53 ± 0.04 b |
| Pentadecane | ND | 0.9 ± 0.2 a | 0.57 ± 0.13 b | 0.44 ± 0.03 bc | 0.27 ± 0.06 c | 0.51 ± 0.04 b | 0.55 ± 0.12 b |
| N-Hexadecane | ND | ND | ND | 0.11 ± 0.03 a | ND | ND | ND |
| Octadecane | ND | ND | ND | 0.52 ± 0.07 a | ND | ND | ND |
| 1,4-di-tert-butylbenzene | 0.46 ± 0.15 a | ND | ND | ND | ND | ND | ND |
| Tetradecamethylcycloheptasiloxane | 0.19 ± 0.1 bc | 0.18 ± 0.1 c | 0.23 ± 0.06 bc | 0.38 ± 0.15 ab | 0.53 ± 0.13 a | 0.15 ± 0.04 c | 0.12 ± 0.03 c |
| Naphthalene | ND | ND | 0.04 ± 0.04 ab | 0.03 ± 0.04 ab | ND | ND | 0.05 ± 0.03 a |
| 2-Methylnaphthalene | 0.13 ± 0.05 a | 0.01 ± 0.01 c | 0.04 ± 0.04 bc | 0.07 ± 0.02 b | 0.13 ± 0.03 a | ND | 0.08 ± 0.01 ab |
| 1-Methylnaphthalene | ND | ND | 0.08 ± 0.05 a | ND | 0.07 ± 0.05 a | 0.04 ± 0.03 ab | 0.03 ± 0.03 ab |
| N-Tridecane | ND | ND | 0.95 ± 0.14 a | ND | 0.59 ± 0.13 b | ND | ND |
| 2-Methyltridecane | ND | ND | ND | ND | 0.24 ± 0.05 a | ND | 0.23 ± 0.04 a |
| Dichloromethane | ND | ND | ND | ND | 1.04 ± 0.06 a | 1.04 ± 0.06 a | ND |
| 1,3-Hexadiene | ND | ND | ND | ND | ND | 0.3 ± 0.15 a | ND |
| Styrene | ND | ND | ND | ND | 2.42 ± 0.07 a | ND | ND |
| Propyl cyclopropane | ND | ND | ND | ND | ND | 0.64 ± 0.03 a | ND |
| Benzocycloheptatriene | ND | ND | ND | 0.05 ± 0.02 b | ND | ND | 0.18 ± 0.03 a |
| Esters | 11.58 ± 1 b | 9.72 ± 0.47 c | 8.21 ± 0.37 d | 10.51 ± 0.29 c | 16.64 ± 0.3 a | 8.37 ± 0.16 d | 5.72 ± 0.25 e |
| Methyl octanoate | 1.44 ± 0.23 b | 1.46 ± 0.19 b | 1.2 ± 0.04 bc | 0.98 ± 0.12 c | 1.82 ± 0.06 a | 1.43 ± 0.09 b | 1.37 ± 0.11 b |
| Methyl pelargonate | 0.5 ± 0.11 c | 0.26 ± 0.05 d | 0.78 ± 0.09 b | 0.39 ± 0.12 cd | 1.35 ± 0.05 a | 0.85 ± 0.08 b | 0.55 ± 0.09 c |
| Methyl decanoate | 2.77 ± 0.53 a | 0.12 ± 0.04 b | 0.26 ± 0.08 b | 0.3 ± 0.05 b | 0.31 ± 0.11 b | 0.41 ± 0.06 b | 0.4 ± 0.11 b |
| Ethyl decanoate | ND | ND | ND | ND | ND | ND | 0.05 ± 0.02 a |
| Hexyl hexoate | ND | ND | ND | ND | 0.18 ± 0.04 a | 0.18 ± 0.02 a | ND |
| Ethyl caproate | ND | ND | ND | ND | 0.2 ± 0.08 a | 0.22 ± 0.04 a | ND |
| Amyl hexoate | ND | ND | ND | ND | ND | 0.2 ± 0.05 a | ND |
| Gamma-Butyrolactone | ND | ND | ND | ND | 0.05 ± 0.03 a | 0.05 ± 0.04 a | 0.05 ± 0.01 a |
| Gamma-Octanoiclacton | ND | ND | ND | ND | 0.3 ± 0.09 a | 0.13 ± 0.04 b | 0.11 ± 0.04 b |
| Methyl dodecanoate | 0.16 ± 0.06 a | ND | ND | ND | ND | ND | ND |
| Delta-Octalactone | 6.15 ± 0.29 d | 7.88 ± 0.4 b | 5.97 ± 0.22 d | 7.17 ± 0.62 c | 9.75 ± 0.42 a | 2.33 ± 0.1 e | 2.09 ± 0.09 e |
| Gamma-Nonalactone | 0.15 ± 0.04 c | ND | ND | ND | 0.68 ± 0.04 a | 0.24 ± 0.06 b | 0.14 ± 0.04 c |
| Methyl myristate | 0.14 ± 0.02 a | ND | ND | ND | ND | ND | ND |
| Methyl palmitate | 0.17 ± 0.03 a | ND | ND | ND | ND | ND | ND |
| Methyl palmitoleate | 0.1 ± 0.03 a | ND | ND | ND | ND | ND | ND |
| Delta-Dodecalactone | ND | ND | ND | ND | ND | ND | 0.03 ± 0.03 a |
| Methyl benzoate | ND | ND | ND | ND | 0.07 ± 0.05 a | 0.07 ± 0.03 a | ND |
| Methyl phenylacetate | ND | ND | ND | 0.27 ± 0.07 b | 0.24 ± 0.04 b | 0.76 ± 0.11 a | 0.84 ± 0.14 a |
| Ethyl phenylacetate | ND | ND | ND | ND | ND | 0.06 ± 0.03 b | 0.09 ± 0.01 a |
| Methyl 2-octenate | ND | ND | ND | ND | 0.75 ± 0.1 a | 0.75 ± 0.01 a | ND |
| Undecanolactone | ND | ND | ND | ND | 0.29 ± 0.03 a | 0.04 ± 0.01 b | ND |
| Butyl decyl ester | ND | ND | ND | 0.15 ± 0.04 a | ND | ND | ND |
| Alcohols | 3.48 ± 0.3 e | 24.59 ± 2.26 a | 18.88 ± 1.5 b | 12.86 ± 0.31 c | 9.3 ± 0.62 d | 8.68 ± 0.12 d | 5.25 ± 0.96 e |
| 1-Octen-3-Ol | 2.00 ± 0.08 e | 9.10 ± 0.32 a | 7.17 ± 0.22 b | 5.31 ± 0.09 c | 3.53 ± 0.37 d | 3.55 ± 0.08 d | ND |
| Heptanol | 0.80 ± 0.14 d | 0.85 ± 0.08 d | 1.39 ± 0.16 b | 1.66 ± 0.08 a | 0.97 ± 0.10 cd | 1.4 ± 0.07 b | 1.17 ± 0.03 c |
| N-Octanol | 0.68 ± 0.13 d | 0.7 ± 0.06 d | 1.73 ± 0.11 a | 1.85 ± 0.07 a | 1.35 ± 0.06 b | 1.3 ± 0.05 b | 1.02 ± 0.06 c |
| 1-Pentanol | ND | 2.05 ± 0.17 a | 0 ± 0 d | 1.53 ± 0.1 c | 1.61 ± 0.12 c | 1.84 ± 0.05 b | 0 ± 0 d |
| N-Hexanol | ND | 11.89 ± 1.89 a | 6.83 ± 0.09 b | 2.43 ± 0.29 c | 1.49 ± 0.05 cd | 0 d | 2.31 ± 1.02 c |
| Benzyl alcohol | ND | ND | 0.09 ± 0.03 a | 0.02 ± 0.03 b | 0.03 ± 0.03 b | 0.03 ± 0.03 b | 0.02 ± 0.03 b |
| Phenethyl alcohol | ND | ND | 0.25 ± 0.07 b | 0.06 ± 0.02 c | 0.32 ± 0.09 b | 0.52 ± 0.06 a | 0.32 ± 0.07 b |
| trans-2-Octen-1-ol | ND | ND | ND | ND | ND | ND | 0.41 ± 0.12 a |
| Hexaethylene glycol | ND | ND | ND | ND | ND | 0.04 ± 0.02 a | ND |
| Epoxydihydrolinalool | ND | ND | 0.36 ± 0.06 a | ND | ND | ND | ND |
| Acids | 1.99 ± 0.17 c | 3.32 ± 0.21 a | 2.21 ± 0.1 c | 1.3 ± 0.02 d | 2.98 ± 0.12 b | 2.9 ± 0.29 b | 2.74 ± 0.04 b |
| Hexanoic acid | 0.46 ± 0.08 b | 1.98 ± 0.13 a | 1.96 ± 0.08 a | 0.66 ± 0.03 b | 2.11 ± 0.15 a | 0.67 ± 0.14 b | 0.65 ± 0.08 b |
| Acetic acid | ND | ND | ND | ND | ND | 0.58 ± 0.05 a | 0.41 ± 0.06 b |
| Dimethyldithiocarbamate | 0.08 ± 0.03 a | ND | ND | ND | ND | ND | ND |
| N-Hexadecanoic acid | 1.2 ± 0.14 b | 1.34 ± 0.08 a | ND | ND | ND | ND | ND |
| Butanoic acid | ND | ND | ND | 0.28 ± 0.03 c | 0.17 ± 0.02 d | 0.74 ± 0.08 a | 0.5 ± 0.02 b |
| Isobutyric acid | ND | ND | ND | ND | ND | 0.24 ± 0.03 a | 0.25 ± 0.01 a |
| Butanoic acid, 2-methyl-, 3-methy | ND | ND | ND | ND | ND | ND | 0.34 ± 0.06 a |
| Butanoic acid, 2-methyl-, hexyl | ND | ND | ND | ND | ND | ND | 0.43 ± 0.04 a |
| Octanoic acid | ND | ND | 0.13 ± 0.02 bc | 0.08 ± 0.04 c | 0.23 ± 0.07 a | 0.14 ± 0.01 b | ND |
| Nonoic acid | ND | ND | 0.06 ± 0.04 b | ND | 0.13 ± 0.02 a | 0.05 ± 0.04 b | ND |
| Heptanoic acid | ND | ND | 0.06 ± 0.02 a | ND | 0.06 ± 0.03 a | 0.05 ± 0.02 a | 0.04 ± 0.02 a |
| Decoic acid | 0.25 ± 0.04 a | ND | ND | ND | ND | ND | ND |
| Valeric acid | ND | ND | ND | ND | ND | 0.28 ± 0.04 a | 0.12 ± 0.03 b |
| Dodecanoic acid | ND | ND | ND | ND | ND | 0.02 ± 0.03 a | ND |
| 2-methyl-propionic acid | ND | ND | ND | 0.28 ± 0.06 a | 0.28 ± 0.04 a | 0.13 ± 0.02 b | ND |
| Ketones | 1.2 ± 0.02 b | 0.13 ± 0.05 f | 0.81 ± 0.1 c | 1.52 ± 0.07 a | 1.18 ± 0.11 b | 0.66 ± 0.08 d | 0.48 ± 0.04 e |
| 2(3H)-Furanone, 5-butyldihydro- | ND | ND | ND | 0.02 ± 0.01 a | ND | ND | ND |
| Methyl heptenone | ND | ND | 0.37 ± 0.04 a | ND | ND | ND | ND |
| 6-Methyl-3,5-heptadiene-2-one | ND | ND | 0.26 ± 0.03 a | ND | ND | 0.09 ± 0.03 b | 0.11 ± 0.04 b |
| 6-Methyl-5-heptadiene-2-one | ND | ND | ND | ND | 0.16 ± 0.01 a | ND | ND |
| Nerylacetone | ND | ND | 0.18 ± 0.03 a | 0.14 ± 0.02 b | ND | ND | ND |
| Geranylaceto | ND | 0.13 ± 0.05 a | ND | ND | 0.1 ± 0.06 a | 0.1 ± 0.04 a | ND |
| Acetophenone | ND | ND | ND | ND | 0.09 ± 0.04 a | 0.09 ± 0.03 a | ND |
| 2-Piperidinone | ND | ND | ND | 0.07 ± 0.01 a | ND | ND | ND |
| 2,3-Octanedione | ND | ND | ND | 1.29 ± 0.07 a | ND | ND | ND |
| 2,5-Octanedione | 1.2 ± 0.02 a | ND | ND | ND | ND | ND | ND |
| 3,5-Octadien-2-one | ND | ND | ND | ND | 0.83 ± 0.04 a | 0.38 ± 0.03 b | 0.37 ± 0.05 b |
| 2,5-dimethylpyrazine | ND | ND | ND | ND | 1.18 ± 0.04 b | 1.35 ± 0.07 a | 1.33 ± 0.09 a |
| Pyrazines | ND | ND | ND | ND | 2.44 ± 0.17 c | 3.03 ± 0.18 b | 5.35 ± 0.24 a |
| 2,6-dimethylpyrazine | ND | ND | ND | ND | ND | ND | 0.77 ± 0.08 a |
| Trimethyl-pyrazine | ND | ND | ND | ND | ND | ND | 2.38 ± 0.24 a |
| 2,3,5-Trimethyl-6-ethylpyrazine | ND | ND | ND | ND | 0.11 ± 0.01 b | 0.13 ± 0.05 b | 0.27 ± 0.05 a |
| Trimethyl-pyrazine | ND | ND | ND | ND | 0.88 ± 0.13 b | 1.3 ± 0.04 a | ND |
| Tetramethylpyrazine | ND | ND | ND | ND | 0.27 ± 0.06 b | 0.25 ± 0.07 b | 0.6 ± 0.06 a |
| Furans | 0 c | 3.68 ± 0.12 a | 2.13 ± 0.04 b | 2.06 ± 0.08 b | 2.1 ± 0.16 b | 2.18 ± 0.11 b | 2.23 ± 0.08 b |
| 2-Pentylfuran | 0 c | 3.68 ± 0.12 a | 2.13 ± 0.04 b | 2.06 ± 0.08 b | 2.1 ± 0.16 b | 2.18 ± 0.11 b | 2.23 ± 0.08 b |
| Others | 0.8 ± 0.07 b | 0.4 ± 0.1 c | 0.36 ± 0.04 cd | 0.24 ± 0.03 d | 0.85 ± 0.08 b | 1.12 ± 0.12 a | 0.36 ± 0.08 cd |
| Methoxy phenoxime | 0.64 ± 0.06 a | ND | ND | ND | 0.53 ± 0.09 b | 0.68 ± 0.04 a | ND |
| 1H-Indene,1-methilene- | ND | ND | ND | ND | 0.1 ± 0.03 a | 0.09 ± 0.04 a | ND |
| 1,3-dimethoxy-Benzene | ND | ND | ND | ND | 0.22 ± 0.02 b | 0.35 ± 0.07 a | ND |
| Sec-Butylamine | ND | 0.4 ± 0.1 a | ND | ND | ND | ND | ND |
| N,N-dibutyl-Formamide | ND | ND | 0.36 ± 0.04 a | 0.24 ± 0.03 b | ND | ND | ND |
| N-Hydroxymethylacetamide | ND | ND | ND | ND | ND | ND | 0.36 ± 0.08 a |
| Azulene | 0.16 ± 0.03 a | ND | ND | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wei, Z.; Zhang, H.; Xie, L.; Vincenzetti, S.; Polidori, P.; Li, L.; Liu, G. Changes in the Physical–Chemical Properties and Volatile Flavor Components of Dry-Cured Donkey Leg during Processing. Foods 2022, 11, 3542. https://doi.org/10.3390/foods11213542
Zhang J, Wei Z, Zhang H, Xie L, Vincenzetti S, Polidori P, Li L, Liu G. Changes in the Physical–Chemical Properties and Volatile Flavor Components of Dry-Cured Donkey Leg during Processing. Foods. 2022; 11(21):3542. https://doi.org/10.3390/foods11213542
Chicago/Turabian StyleZhang, Jingjing, Zixiang Wei, Huachen Zhang, Lan Xie, Silvia Vincenzetti, Paolo Polidori, Lanjie Li, and Guiqin Liu. 2022. "Changes in the Physical–Chemical Properties and Volatile Flavor Components of Dry-Cured Donkey Leg during Processing" Foods 11, no. 21: 3542. https://doi.org/10.3390/foods11213542
APA StyleZhang, J., Wei, Z., Zhang, H., Xie, L., Vincenzetti, S., Polidori, P., Li, L., & Liu, G. (2022). Changes in the Physical–Chemical Properties and Volatile Flavor Components of Dry-Cured Donkey Leg during Processing. Foods, 11(21), 3542. https://doi.org/10.3390/foods11213542







