A Comprehensive Review of the Cardioprotective Effect of Marine Algae Polysaccharide on the Gut Microbiota
Abstract
:1. Introduction
2. Structure–Function Relationship of MAPs to Cardioprotective Activity
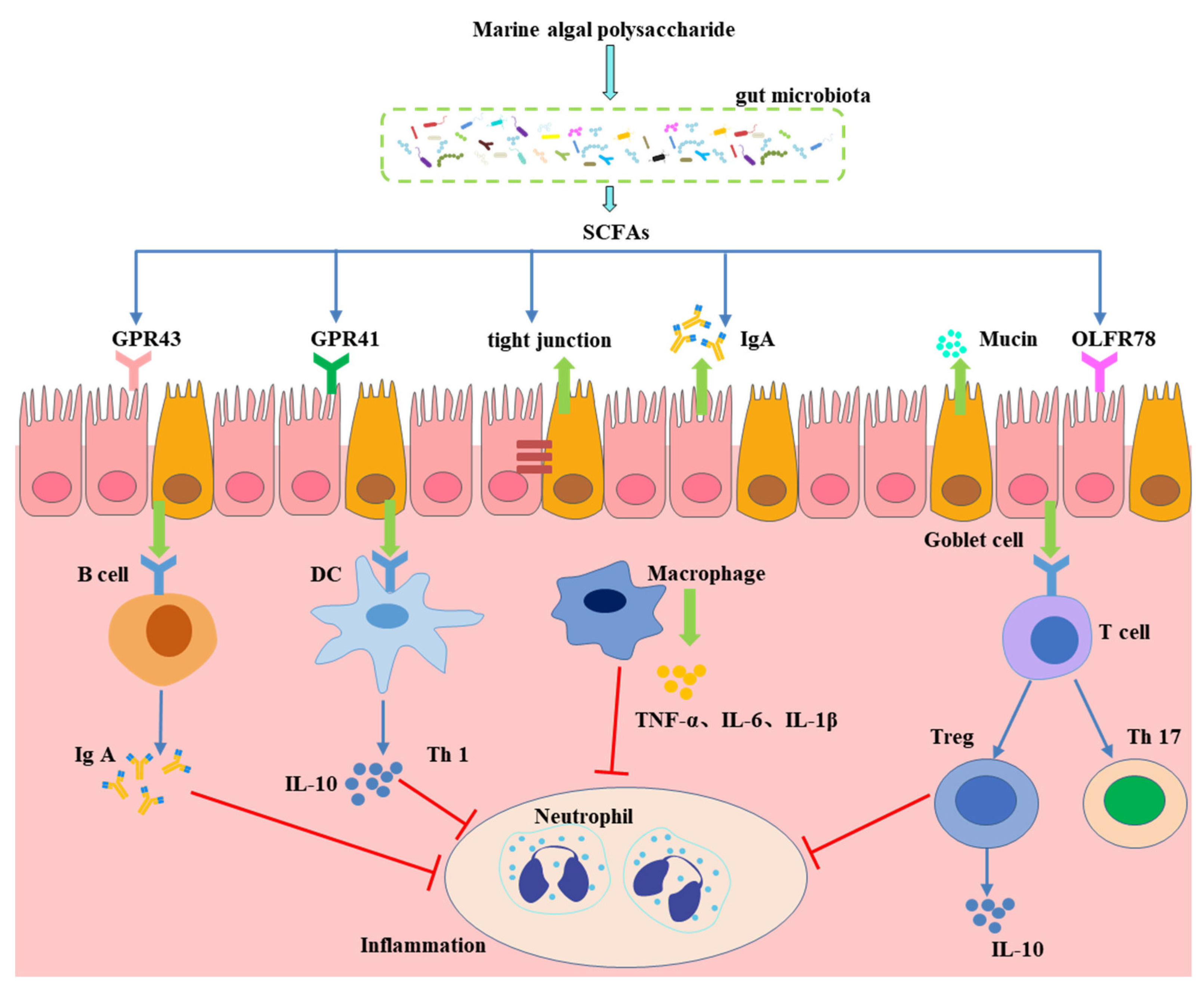
3. Cardioprotective Effect of MAPs Associated with Gut Microbiota Modulation
| Type of Polysaccharides | Marine Algae Sources | Influence on Intestinal Microbiota | Treatment and Prevention of CVD | Ref. |
|---|---|---|---|---|
| alginate | Sargassum fusiforme | Lactobacillus, Bacteroides, Akkermansia Alloprevotella, Weissella, and Enterorhabdus ↑ Turicibacter and Helicobacter ↓ | attenuated pathological changes in adipose, hepatic, and heart tissues; diminished oxidative stress | [48] |
| carrageenan | Kappaphycus Alvarezii | Parasutterella, Alloprevotella, Oscillibacter, Melainabacteria, and Butyricimonas ↑ Clostridia, Erysipelotrichaceae, Blautia, and Lachnospiraceae ↓ | decreased total cholesterol and high-density level cholesterol; reduced adipocyte size and levels of adiponectin and leptin | [49] |
| fucan | Saccharina japonica | Bacteroides sartorii, Bacteroides acidifaciens, Akkermansia, and Lachnospiraceae NK4A136 ↑ | prevented high-fat diet-induced obesity; regulated blood glucose/lipid metabolism | [50] |
| fucoidan | Laminaria japonica | phylum Bacteroidetes and families Muribaculaceae and Bacteroidaceae ↑ | ameliorated high-fat diet-induced body weight gain, fat accumulation, serum lipid profiles, insulin resistance, hepatic steatosis, and adipocyte hypertrophy | [51] |
| fucoidan | Sargassum fusiforme | Bacteroides, Faecalibacterium, and Blautia ↑ | reduced epididymal fat deposition, decreased oxidative stress, and attenuated the pathological changes in heart tissues | [52] |
| fucoidan | Sargassum fusiforme | Bacteroides, Ruminococcaceae, and Butyricoccus↑ Helicobacter↓ | reduced fat accumulation; enhanced the energy expenditure through increasing the expression of uncoupling protein 1 in adipose tissues | [53] |
| porphyran | Porphyra haitanensis | Roseburia and Eubacterium ↑, Helicobacter ↓ | ameliorated body fat accumulation in liver, serum, and adipose tissues; increased the pathway of PGC 1α-UCP 1-mitochondrial to produce more energy | [54] |
| porphyran | Neoporphyra haitanensis | Parabacteroides and Coriobacteriaceae UCG-002 ↑ | inhibited G6Pase and PEPCK enzymes related to hepatic gluconeogenesis; enhanced the expression of the GLUT4 enzyme involved in peripheral glucose uptake | [55] |
| ulvan | Enteromorpha prolifera | Desulfovibrio ↑, modulated Verrucomicrobiaceae, Odoribacteraceae, Mogibacteriaceae, Planococcaceae, and Coriobacteriaceae | decreased levels of inflammatory factors, including IFN-γ, TNF-α, and IL-6; increased total antioxidant capacity and superoxide dismutase, glutathione, catalase, and telomerase levels | [56] |
| ulvan | Ulva lactuca | Dubosiella, Lactobacillus, and Parasutterella ↑ Staphylococcus, Escherichia−Shigella, and Ruminococcus ↓ | reduced the amount of blood urea nitrogen, serum uric acid, and creatinine; suppressed the activities of serum and hepatic xanthine oxidase | [57] |
4. Effect of Gut Microbiota-Generated Short-Chain Fatty Acids in CVD
5. Bile Acids as a Link between the Gut Microbiota and CVD
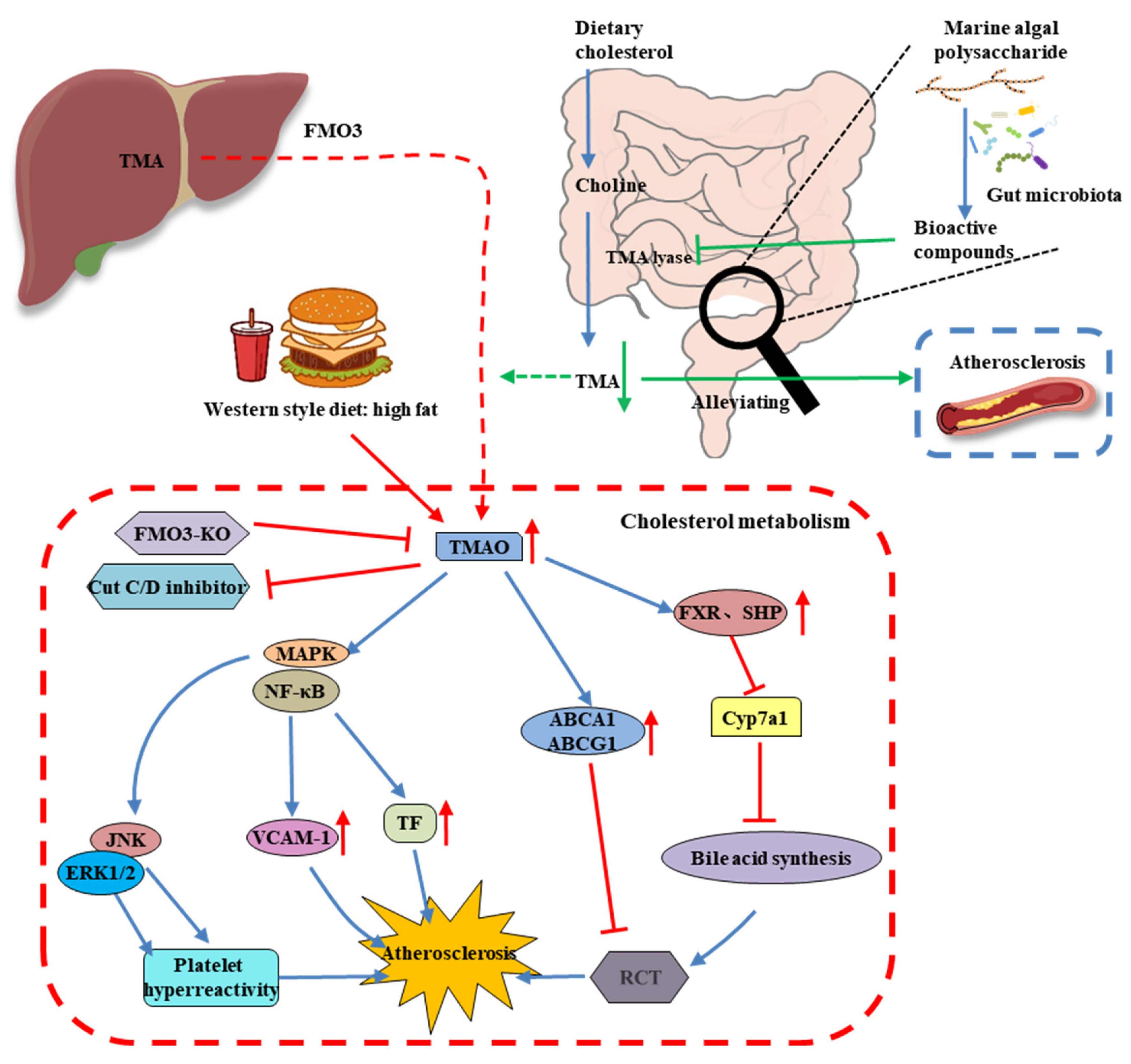
6. MAP Modulates the Gut Microbiota-Derived Metabolite TMAO
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, L.Y.; Chen, W.W.; Gao, R.L.; Liu, L.S.; Zhu, M.L.; Wang, Y.J.; Wu, Z.S.; Li, H.J.; Gu, D.F.; Yang, Y.J.; et al. China cardiovascular diseases report 2018: An updated summary. J. Geriatr. Cardiol. 2020, 17, 1–8. [Google Scholar] [PubMed]
- Celermajer, D.S.; Chow, C.K.; Marijon, E.; Anstey, N.M.; Woo, K.S. Cardiovascular Disease in the Developing World. J. Am. Coll. Cardiol. 2012, 60, 1207–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mainous, A.G., III; Tanner, R.J.; Jo, A.; Park, K.; De Rochars, V.M.B. Trends in Cardiovascular Disease Risk in the US, 1999–2014. Am. J. Prev. Med. 2018, 55, 384–388. [Google Scholar] [CrossRef]
- Moonesinghe, R.; Yang, Q.; Zhang, Z.; Khoury, M.J. Prevalence and Cardiovascular Health Impact of Family History of Premature Heart Disease in the United States: Analysis of the National Health and Nutrition Examination Survey, 2007–2201. J. Am. Heart Assoc. 2019, 8, e012364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olinic, D.-M.; Spinu, M.; Olinic, M.; Homorodean, C.; Tataru, D.-A.; Liew, A.; Schernthaner, G.-H.; Stanek, A.; Fowkes, G.; Catalano, M. Epidemiology of peripheral artery disease in Europe: VAS Educational Paper. Int. Angiol. 2018, 37, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Freedman, N.D.; Albert, P.S.; Huxley, R.R.; Shiels, M.S.; Withrow, D.R.; Spillane, S.; Powell-Wiley, T.M.; Berrington de González, A. Association of cardiovascular disease with premature mortality in the United States. JAMA Cardiol. 2019, 4, 1230–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Costello, E.K.; Gordon, J.I.; Secor, S.M.; Knight, R. Postprandial remodeling of the gut microbiota in Burmese pythons. ISME J. 2010, 4, 1375–1385. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Huang, X.; Cheong, K.-L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef]
- Xie, X.-T.; Cheong, K.-L. Recent advances in marine algae oligosaccharides: Structure, analysis, and potential prebiotic activities. Crit. Rev. Food Sci. Nutr. 2021, 62, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Manlusoc, J.K.; Hsieh, C.-L.; Hsieh, C.-Y.; Salac, E.S.; Lee, Y.-T.; Tsai, P.-W. Pharmacologic application potentials of sulfated polysaccharide from marine algae. Polymers 2019, 11, 1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Arokiarajan, M.S.; Thirunavukkarasu, R.; Joseph, J.; Ekaterina, O.; Aruni, W. Advance research in biomedical applications on marine sulfated polysaccharide. Int. J. Biol. Macromol. 2022, 194, 870–881. [Google Scholar] [CrossRef]
- Yao, W.; Chen, X.; Li, X.; Chang, S.; Zhao, M.; You, L. Current trends in the anti-photoaging activities and mechanisms of dietary non-starch polysaccharides from natural resources. Crit. Rev. Food Sci. Nutr. 2021, 1–15. [Google Scholar] [CrossRef]
- Zheng, L.-X.; Chen, X.-Q.; Cheong, K.-L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol. 2020, 151, 344–354. [Google Scholar] [CrossRef]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic effects of fucoidan: A review on recent studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Lan, Y.; Liu, J.; Zhang, F.; Zhang, L.; Li, B.; Zhao, X. The structure property and endothelial protective activity of fucoidan from Laminaria japonica. Int. J. Biol. Macromol. 2017, 105, 1421–1429. [Google Scholar] [CrossRef]
- Sanchez-Ballester, N.M.; Bataille, B.; Soulairol, I. Sodium alginate and alginic acid as pharmaceutical excipients for tablet formulation: Structure-function relationship. Carbohydr. Polym. 2021, 270, 118399. [Google Scholar] [CrossRef]
- Qiang, T.; Wang, J.; Jiang, L.; Xiong, K. Modulation of hyperglycemia by sodium alginate is associated with changes of serum metabolite and gut microbiota in mice. Carbohydr. Polym. 2022, 291, 119359. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Qiu, H.-M.; Cheong, K.-L.; Zhong, S. Advances in anti-cancer effects and underlying mechanisms of marine algae polysaccharides. Int. J. Biol. Macromol. 2022, 221, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, H.; Zhang, N.; Cai, C.; Li, G.; Hao, J.; Yu, G. Anti-diabetic activities of agaropectin-derived oligosaccharides from Gloiopeltis furcata via regulation of mitochondrial function. Carbohydr. Polym. 2020, 229, 115482. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, W.; Xiao, L.; Liu, C.; Qi, H.; Zhang, Z. In vivo antihyperlipidemic and antioxidant activity of porphyran in hyperlipidemic mice. Carbohydr. Polym. 2017, 174, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Campo, V.L.; Kawano, D.F.; Silva, D.B.d.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Valado, A.; Pereira, M.; Caseiro, A.; Figueiredo, J.P.; Loureiro, H.; Almeida, C.; Cotas, J.; Pereira, L. Effect of carrageenans on vegetable jelly in humans with hypercholesterolemia. Mar. Drugs 2020, 18, 19. [Google Scholar] [CrossRef] [Green Version]
- Tziveleka, L.-A.; Ioannou, E.; Roussis, V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wu, H.; Liu, R. Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar. Drugs 2014, 12, 4984–5020. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Sheng, J. The antihyperlipidemic mechanism of high sulfate content ulvan in rats. Mar. Drugs 2015, 13, 3407–3421. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.-F.; Ye, H.; Zhu, Y.-J.; Li, Y.-P.; Wang, J.-F.; Wang, P. Characterization and hypoglycemic activity of a rhamnan-type sulfated polysaccharide derivative. Mar. Drugs 2019, 17, 21. [Google Scholar] [CrossRef]
- Cao, S.; He, X.; Qin, L.; He, M.; Yang, Y.; Liu, Z.; Mao, W. Anticoagulant and antithrombotic properties in vitro and in vivo of a novel sulfated polysaccharide from marine green alga Monostroma nitidum. Mar. Drugs 2019, 17, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Li, H. The role of gut microbiota in atherosclerosis and hypertension. Front. Pharmacol. 2018, 9, 1082. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Zhang, X.; Aweya, J.J.; Huang, Z.-X.; Kang, Z.-Y.; Bai, Z.-H.; Li, K.-H.; He, X.-T.; Liu, Y.; Chen, X.-Q.; Cheong, K.-L. In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohydr. Polym. 2020, 234, 115894. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-T.; An, L.-Y.; Liu, W.; Hu, Y.-C.; Wang, S.-P.; Zou, L. In vitro fecal fermentation properties of polysaccharides from Tremella fuciformis and related modulation effects on gut microbiota. Food Res. Int. 2022, 156, 111185. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-T.; He, Y.; Yuan, Q.; Wang, S.; Gan, R.-Y.; Hu, Y.-C.; Zou, L. Effects of molecular weight and degree of branching on microbial fermentation characteristics of okra pectic-polysaccharide and its selective impact on gut microbial composition. Food Hydrocolloid. 2022, 132, 107897. [Google Scholar] [CrossRef]
- Nguyen, S.G.; Kim, J.; Guevarra, R.B.; Lee, J.-H.; Kim, E.; Kim, S.-i.; Unno, T. Laminarin favorably modulates gut microbiota in mice fed a high-fat diet. Food Funct. 2016, 7, 4193–4201. [Google Scholar] [CrossRef]
- Grigor’eva, I.N. Gallstone disease, obesity and the Firmicutes/Bacteroidetes ratio as a possible biomarker of gut dysbiosis. J. Pers. Med. 2021, 11, 13. [Google Scholar] [CrossRef]
- Yuan, D.; Li, C.; You, L.; Dong, H.; Fu, X. Changes of digestive and fermentation properties of Sargassum pallidum polysaccharide after ultrasonic degradation and its impacts on gut microbiota. Int. J. Biol. Macromol. 2020, 164, 1443–1450. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Zhang, H.; Liu, Y.; Sarker, M.M.R.; Wu, Y.; Chen, X.; Zhao, C. The anti-hyperuricemic effects of green alga Enteromorpha prolifera polysaccharide via regulation of the uric acid transporters in vivo. Food Chem. Toxicol. 2021, 158, 112630. [Google Scholar] [CrossRef]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Q.; Wang, Y.; Pan, L.; Niu, Q.; Li, C.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary polysaccharide from Enteromorpha clathrata modulates gut microbiota and promotes the growth of Akkermansia muciniphila, Bifidobacterium spp. and Lactobacillus spp. Mar. Drugs 2018, 16, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Q.; Song, G.; Zhang, M.; Shi, J.; Xu, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice. J. Funct. Food. 2017, 28, 138–146. [Google Scholar] [CrossRef]
- Nowak, A.; Paliwoda, A.; Błasiak, J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: A review of mechanisms and therapeutic perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 3456–3467. [Google Scholar] [CrossRef] [PubMed]
- Miremadi, F.; Ayyash, M.; Sherkat, F.; Stojanovska, L. Cholesterol reduction mechanisms and fatty acid composition of cellular membranes of probiotic Lactobacilli and Bifidobacteria. J. Funct. Food. 2014, 9, 295–305. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Ye, C.; Yuan, J.; Qin, S. Alginate oligosaccharide improves lipid metabolism and inflammation by modulating gut microbiota in high-fat diet fed mice. Appl. Microbiol. Biotechnol. 2020, 104, 3541–3554. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-R.; Jia, R.-B.; Luo, D.; Lin, L.; Zheng, Q.; Zhao, M. The positive effects and underlying mechanisms of Undaria pinnatifida polysaccharides on type 2 diabetes mellitus in rats. Food Funct. 2021, 12, 11898–11912. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.; Cheng, Y.; Liu, Q.; Su, L.; Yang, Y.; Zhang, X.; Wu, M.; Choi, J.-I.; Tong, H. Sargassum fusiforme alginate relieves hyperglycemia and modulates intestinal microbiota and metabolites in type 2 diabetic mice. Nutrients 2021, 13, 2887. [Google Scholar] [CrossRef]
- Chin, Y.X.; Mi, Y.; Cao, W.X.; Lim, P.E.; Xue, C.H.; Tang, Q.J. A pilot study on anti-obesity mechanisms of Kappaphycus alvarezii: The role of native κ-carrageenan and the leftover sans-carrageenan fraction. Nutrients 2019, 11, 1133. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.; Zhang, B.; Du, A.-Q.; Zhou, Z.-Y.; Lu, D.-Z.; Zhu, Z.-H.; Ke, S.-Z.; Wang, S.-J.; Yu, Y.-L.; Chen, J.-W.; et al. Saccharina japonica fucan suppresses high fat diet-induced obesity and enriches fucoidan-degrading gut bacteria. Carbohydr. Polym. 2022, 290, 119411. [Google Scholar] [CrossRef]
- Zhang, X.; You, Y.; Wang, L.; Ai, C.; Huang, L.; Wang, S.; Wang, Z.; Song, S.; Zhu, B. Anti-obesity effects of Laminaria japonica fucoidan in high-fat diet-fed mice vary with the gut microbiota structure. Food Funct. 2022, 13, 6259–6270. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wu, S.; Cheng, Y.; Zhang, Z.; Mao, G.; Li, S.; Yang, Y.; Zhang, X.; Wu, M.; Tong, H. Sargassum fusiforme fucoidan modifies gut microbiota and intestinal metabolites during alleviation of hyperglycemia in type 2 diabetic mice. Food Funct. 2021, 12, 3572–3585. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Zhang, Y.; Wu, Y.; Liu, J.; Wu, Q.; Shen, Y.; Jin, L.; Wu, M.; Ma, Z.; Tong, H. Sargassum fusiforme fucoidan ameliorates diet-induced obesity through enhancing thermogenesis of adipose tissues and modulating gut microbiota. Int. J. Biol. Macromol. 2022, 216, 728–740. [Google Scholar] [CrossRef]
- Wang, X.; Dong, J.; Liang, W.; Fang, Y.; Liang, M.; Xu, L.; Sun, W.; Li, X. Porphyran from Porphyra haitanensis alleviate obesity by reducing lipid accumulation and modulating gut microbiota homeostasis. Front. Pharmacol. 2022, 2600. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Jiang, J.; Li, C.; Xue, C.; Kong, B.; Chang, Y.; Tang, Q. The compound enzymatic hydrolysate of Neoporphyra haitanensis improved hyperglycemia and regulated the gut microbiome in high-fat diet-fed mice. Food Funct. 2022, 13, 6777–6791. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Liu, D.; Lin, G.-P.; Wu, Y.-J.; Gao, L.-Y.; Ai, C.; Huang, Y.-F.; Wang, M.-F.; El-Seedi, H.R.; Chen, X.-H.; et al. Anti-ageing and antioxidant effects of sulfate oligosaccharides from green algae Ulva lactuca and Enteromorpha prolifera in SAMP8 mice. Int. J. Biol. Macromol. 2019, 139, 342–351. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Gao, X.; Wu, Y.; El-Seedi, H.R.; Cao, Y.; Zhao, C. Antihyperuricemic effect of green alga Ulva lactuca ulvan through regulating urate transporters. J. Agric. Food. Chem. 2021, 69, 11225–11235. [Google Scholar] [CrossRef]
- Scheppach, W. Effects of short chain fatty acids on gut morphology and function. Gut 1994, 35 (Suppl. S1), S35. [Google Scholar] [CrossRef] [Green Version]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2020, 80, 37–49. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396.e10–406.e10. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Duan, M.; Liu, Y.; Luo, T.; Ma, N.; Song, S.; Ai, C. The beneficial effects of Gracilaria lemaneiformis polysaccharides on obesity and the gut microbiota in high fat diet-fed mice. J. Funct. Food. 2018, 46, 48–56. [Google Scholar] [CrossRef]
- Olaniyi, K.S.; Areloegbe, S.E. Suppression of PCSK9/NF-kB-dependent pathways by acetate ameliorates cardiac inflammation in a rat model of polycystic ovarian syndrome. Life Sci. 2022, 300, 120560. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Lv, Y.-W.; Long, J.; Chen, J.-M.; He, J.-M.; Ruan, X.-Z.; Zhu, H.-b. Butyrate improves the metabolic disorder and gut microbiome dysbiosis in mice induced by a high-fat diet. Front. Pharmacol. 2019, 10, 1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, E.; Grootaert, C.; Verstraete, W.; Van de Wiele, T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 2011, 69, 245–258. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Zeybek, N.; Rastall, R.A.; Buyukkileci, A.O. Utilization of xylan-type polysaccharides in co-culture fermentations of Bifidobacterium and Bacteroides species. Carbohydr. Polym. 2020, 236, 116076. [Google Scholar] [CrossRef]
- Amiri, P.; Hosseini, S.A.; Ghaffari, S.; Tutunchi, H.; Ghaffari, S.; Mosharkesh, E.; Asghari, S.; Roshanravan, N. Role of butyrate, a gut microbiota derived metabolite, in cardiovascular diseases: A comprehensive narrative review. Front. Pharmacol. 2022, 4178. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, Y.; Zhou, C.; Zhao, Q.; Zhong, H.; Zhu, X.; Fu, T.; Pan, L.; Shang, Q.; Yu, G. Dietary polysaccharide from Enteromorpha clathrata attenuates obesity and increases the intestinal abundance of butyrate-producing bacterium, Eubacterium xylanophilum, in mice fed a high-fat diet. Polymers 2021, 13, 3286. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Aweya, J.J.; Li, N.; Deng, R.-Y.; Chen, W.-Y.; Tang, J.; Cheong, K.-L. Microbial catabolism of Porphyra haitanensis polysaccharides by human gut microbiota. Food Chem. 2019, 289, 177–186. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Chen, X.-Q.; Aweya, J.J.; Cheong, K.-L. Catabolism of Saccharina japonica polysaccharides and oligosaccharides by human fecal microbiota. LWT 2020, 130, 109635. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Liu, Y.; Tang, S.; Zhang, W.; Yu, Q.; Shi, C.; Cheong, K.-L. Gracilaria lemaneiformis polysaccharides alleviate colitis by modulating the gut microbiota and intestinal barrier in mice. Food Chem. X 2022, 13, 100197. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-T.; Zheng, L.-X.; Duan, H.-M.; Liu, Y.; Chen, X.-Q.; Cheong, K.-L. Structural characteristics of Gracilaria lemaneiformis oligosaccharides and their alleviation of dextran sulphate sodium-induced colitis by modulating the gut microbiota and intestinal metabolites in mice. Food Funct. 2021, 12, 8635–8646. [Google Scholar] [CrossRef] [PubMed]
- Copple, B.L.; Li, T. Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol. Res. 2016, 104, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelakos, I.; Heeren, J.; Verkade, E.; Kuipers, F. Role of bile acids in inflammatory liver diseases. Seminars in Immunopathology 2021, 43, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, Y.; Cai, W.; Wang, Q.; Chang, Y.; Sun, Y.; Dong, P.; Wang, J. Novel ι-carrageenan tetrasaccharide alleviates liver lipid accumulation via the bile acid–FXR–SHP/PXR pathway to regulate cholesterol conversion and fatty acid metabolism in insulin-resistant mice. J. Agric. Food. Chem. 2021, 69, 9813–9821. [Google Scholar] [CrossRef] [PubMed]
- Busnelli, M.; Manzini, S.; Chiesa, G. The gut microbiota affects host pathophysiology as an endocrine organ: A focus on cardiovascular disease. Nutrients 2020, 12, 79. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Liu, M.; Zhang, P.; Fan, S.; Huang, J.; Yu, S.; Zhang, C.; Li, H. Fucoidan and galactooligosaccharides ameliorate high-fat diet–induced dyslipidemia in rats by modulating the gut microbiota and bile acid metabolism. Nutrition 2019, 65, 50–59. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, G.; Wang, Y.; Yin, J.; Wang, J.; Xia, B.; Li, T.; Yang, X.; Hou, P.; Hu, S.; et al. Fucoidan A2 from the brown seaweed Ascophyllum nodosum lowers lipid by improving reverse cholesterol transport in C57BL/6J mice fed a high-fat diet. J. Agric. Food. Chem. 2019, 67, 5782–5791. [Google Scholar] [CrossRef]
- Hoyles, L.; Jiménez-Pranteda, M.L.; Chilloux, J.; Brial, F.; Myridakis, A.; Aranias, T.; Magnan, C.; Gibson, G.R.; Sanderson, J.D.; Nicholson, J.K.; et al. Metabolic retroconversion of trimethylamine N-oxide and the gut microbiota. Microbiome 2018, 6, 73. [Google Scholar] [CrossRef]
- Nam, H.S. Gut microbiota and ischemic stroke: The role of trimethylamine N-oxide. J. Stroke 2019, 21, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Praveenraj, S.S.; Sonali, S.; Anand, N.; Tousif, H.A.; Vichitra, C.; Kalyan, M.; Kanna, P.V.; Chandana, K.A.; Shasthara, P.; Mahalakshmi, A.M.; et al. The role of a gut microbial-derived metabolite, trimethylamine N-oxide (TMAO), in neurological disorders. Mol. Neurobiol. 2022, 59, 6684–6700. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhu, S.; Li, S.; Feng, Y.; Wu, H.; Zeng, M. Microalgae polysaccharides ameliorates obesity in association with modulation of lipid metabolism and gut microbiota in high-fat-diet fed C57BL/6 mice. Int. J. Biol. Macromol. 2021, 182, 1371–1383. [Google Scholar] [CrossRef]
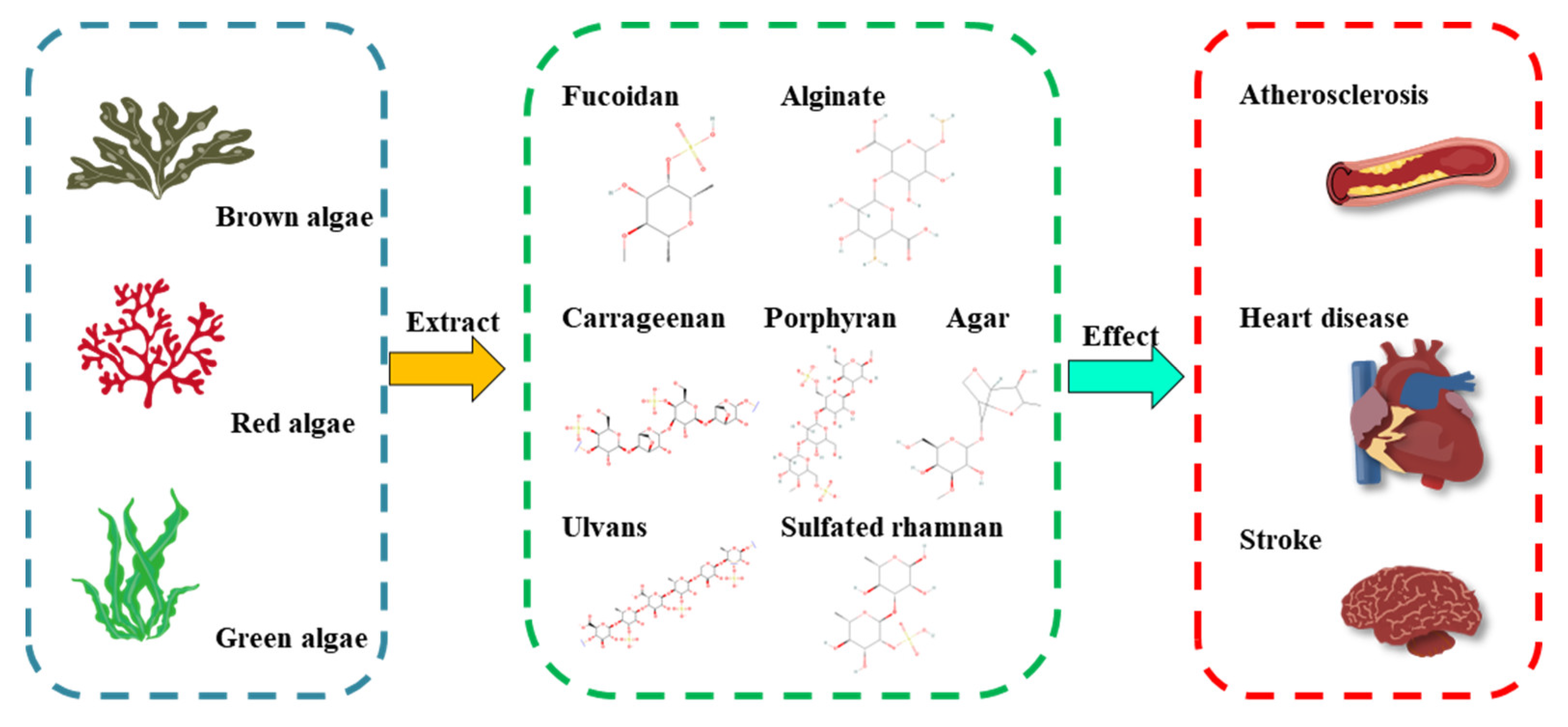


| Source | Polysaccharide Type | Main Composition | Linkage Units | Bioactive Activities | Refs |
|---|---|---|---|---|---|
| Laminaria japonica | Fucoidan | L-fucose | α-(1→2)-linked fucose or α-(1→3)-linked fucose | endothelial protective activity ↑ | [18,19] |
| Laminaria, Saccharina, Ascophyllum, Durvillaea, Macrocystis, Ecklonia, and Lessonia spp. | Alginate | mannuronic acid, guluronic acid | (1,4)-linked β-D-mannuronic acid (ManA) and α-L-guluronic acid (GluA) | fasting blood glucose ↓, total cholesterol ↓, total-body fat ↓ | [20,21] |
| Gelidium and Gracilaria spp. | Agar | agarose and agaropectin | 3,6-anhydro-L-galactopyranose and D-galactose/L-galactose and D-galactose linked sulfate groups | antidiabetic effects ↑ | [22,23] |
| Porphyra spp. | Porphyran | galactose, galactose 6-sulfate | (1→4)-linked α-L-galactose 6-sulfate units and (1→3)-linked β-D-galactose units | antihyperlipidemic activity ↑, antioxidant capacities ↑ | [24] |
| Eucheuma cottonii, Chondrus crispus | Carrageenan | D-galactose, 3,6-anhydro-D-galactose | α-1,3-glucosidic and β-1,4-glycosidic linkages | total cholesterol ↓, low-density lipoprotein cholesterol ↓ | [25,26] |
| Ulva, Enteromorpha spp. | Ulvan | rhamnose, L-rhamnose 3-sulfate | O-3-sulfate rhamnose and β-D-glucuronic acid(1→4)-L-rhamnose 3-sulfate, O-3-sulfate rhamnose and α-L-iduronic acid(1→4)-L-rhamnose 3-sulfate | antihyperlipidemic activity ↑ | [27,28] |
| Monostroma nitidum | Sulfated rhamnan | rhamnose | →3)-α-L-Rhap-(1→ and →2)-α-L-Rhap-(1→ residues | thrombolytic activity ↑, antithrombotic activity ↑ | [29,30,31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheong, K.-L.; Yu, B.; Chen, J.; Zhong, S. A Comprehensive Review of the Cardioprotective Effect of Marine Algae Polysaccharide on the Gut Microbiota. Foods 2022, 11, 3550. https://doi.org/10.3390/foods11223550
Cheong K-L, Yu B, Chen J, Zhong S. A Comprehensive Review of the Cardioprotective Effect of Marine Algae Polysaccharide on the Gut Microbiota. Foods. 2022; 11(22):3550. https://doi.org/10.3390/foods11223550
Chicago/Turabian StyleCheong, Kit-Leong, Biao Yu, Jing Chen, and Saiyi Zhong. 2022. "A Comprehensive Review of the Cardioprotective Effect of Marine Algae Polysaccharide on the Gut Microbiota" Foods 11, no. 22: 3550. https://doi.org/10.3390/foods11223550
APA StyleCheong, K.-L., Yu, B., Chen, J., & Zhong, S. (2022). A Comprehensive Review of the Cardioprotective Effect of Marine Algae Polysaccharide on the Gut Microbiota. Foods, 11(22), 3550. https://doi.org/10.3390/foods11223550










