Characterization of Traditional Chinese Sesame Oil by Using Headspace Solid-Phase Microextraction/Gas Chromatography–Mass Spectrometry, Electronic Nose, Sensory Evaluation, and RapidOxy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. HS-SMPE/GC-MS Analysis of Sesame Oil Samples
2.3. Identification, Quantification, and Odor Activity Value (OAV) Calculation of the Volatile Components
2.4. Electronic Nose Analysis
2.5. Sensory Analysis
2.6. Oxidation Stability Analysis
2.7. Statistical Analysis
3. Results and Discussion
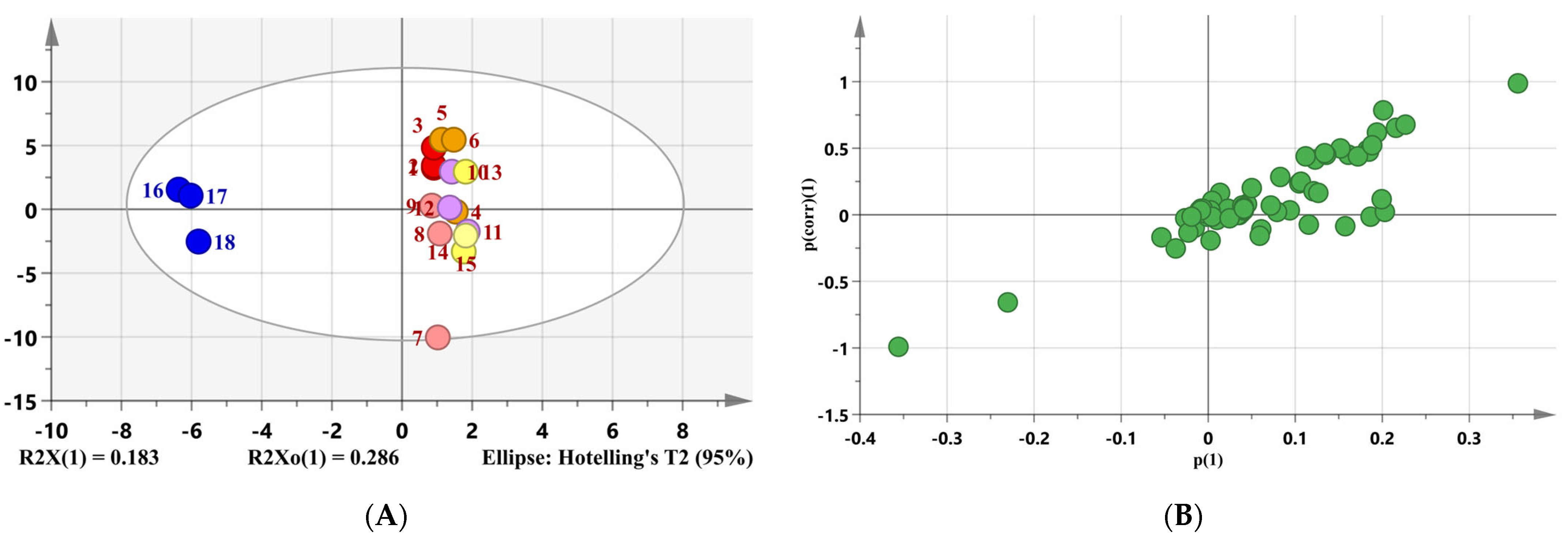
3.1. HS-SPME/GC-MS Analysis of Sesame Oil Samples
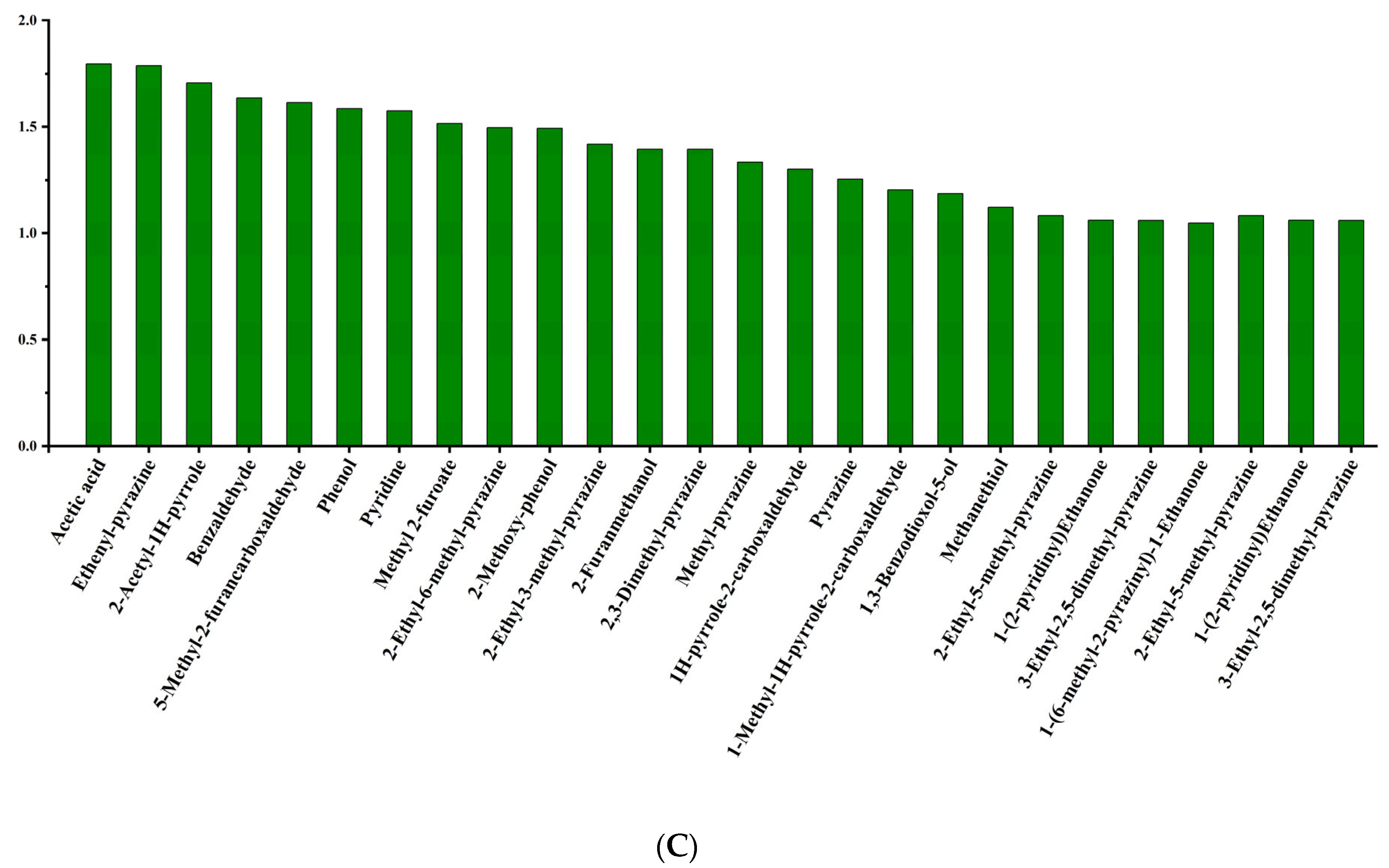
3.2. Identification of Aroma Active Compounds by OAVs
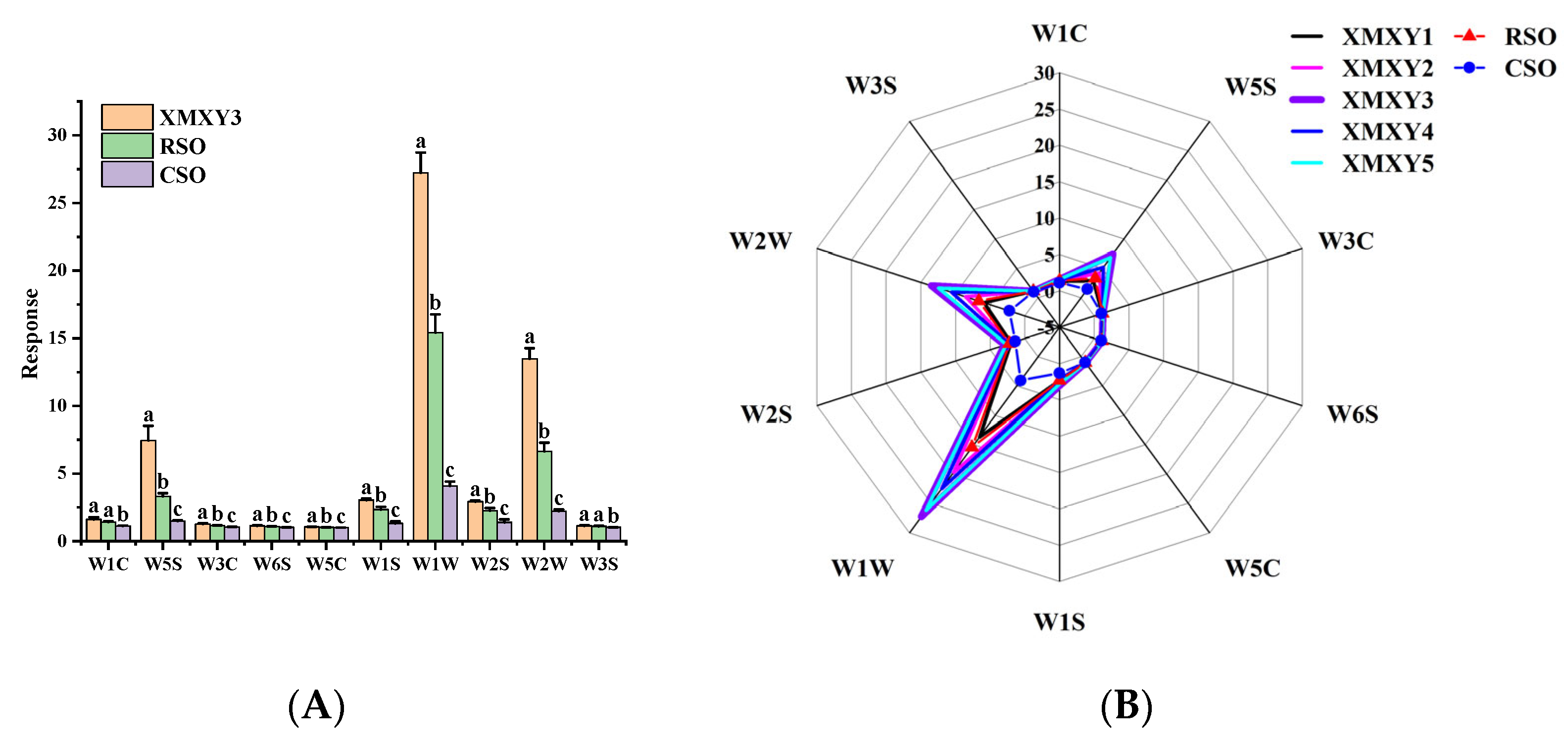
3.3. Electronic Nose Analysis of Sesame oil Samples
3.4. Sensory Analysis of Sesame Oil Samples
3.5. Oxidation Stability Analysis of Sesame Oil Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wan, Y.; Li, H.; Fu, G.; Chen, X.; Chen, F.; Xie, M. The relationship of antioxidant components and antioxidant activity of sesame seed oil. J. Sci. Food Agric. 2015, 95, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.S.; Aquino, L.B.B.; Barbaza, M.Y.U.; Hsieh, C.L.; Castro-Cruz, K.A.; Yang, L.L.; Tsai, P.W. Anti-Inflammatory and Anticancer Properties of Bioactive Compounds from Sesamum indicum L.—A Review. Molecules 2019, 24, 4426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, J.B.; Wang, M.L.; Tonnis, B.D. Variability for oil, protein, lignan, tocopherol, and fatty acid concentrations in eight sesame (Sesamum indicum L.) genotypes. Ind. Crops Prod. 2021, 164, 113355. [Google Scholar] [CrossRef]
- Jayaraj, P.; Narasimhulu, C.A.; Rajagopalan, S.; Parthasarathy, S.; Desikan, R. Sesamol: A powerful functional food ingredient from sesame oil for cardioprotection. Food Funct. 2020, 11, 1198–1210. [Google Scholar] [CrossRef]
- Dalibalta, S.; Majdalawieh, A.F.; Manjikian, H. Health benefits of sesamin on cardiovascular disease and its associated risk factors. Saudi Pharm. J. 2020, 28, 1276–1289. [Google Scholar] [CrossRef]
- Shi, L.; Zheng, L.; Zhang, Y.; Liu, R.; Chang, M.; Huang, J.; Jin, Q.; Zhang, H.; Wang, X. Evaluation and Comparison of Lipid Composition, Oxidation Stability, and Antioxidant Capacity of Sesame Oil: An Industrial-Scale Study Based on Oil Extraction Method. Eur. J. Lipid Sci. Technol. 2018, 120, 158. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, X.; Liu, S.Q. Aroma modulation of vegetable oils—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1538–1551. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Lu, X.; Sun, H.; Wang, F. Effect of oilseed roasting on the quality, flavor and safety of oil: A comprehensive review. Food Res. Int. 2021, 150, 110791. [Google Scholar] [CrossRef]
- Park, M.H.; Jeong, M.K.; Yeo, J.; Son, H.J.; Lim, C.L.; Hong, E.J.; Noh, B.S.; Lee, J. Application of solid phase-microextraction (SPME) and electronic nose techniques to differentiate volatiles of sesame oils prepared with diverse roasting conditions. J. Food Sci. 2011, 76, C80–C88. [Google Scholar] [CrossRef]
- Xu-Yan, D.; Ping-Ping, L.; Fang, W.; Mu-lan, J.; Ying-Zhong, Z.; Guang-Ming, L.; Hong, C.; Yuan-Di, Z. The impact of processing on the profile of volatile compounds in sesame oil. Eur. J. Lipid Sci. Technol. 2012, 114, 277–286. [Google Scholar] [CrossRef]
- Zhou, Q.; Geng, F.; Deng, Q.; Huang, F.; Wang, J. Dynamic analysis of polar metabolites and volatile compounds in sesame seeds during roasting. Cereal Chem. 2019, 96, 358–369. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Zhang, X.; Qu, Z.; Gao, Y.; Li, Q.; Yu, X. Mechanism, indexes, methods, challenges, and perspectives of edible oil oxidation analysis. Crit. Rev. Food Sci. Nutr. 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tenyang, N.; Ponka, R.; Tiencheu, B.; Djikeng, F.T.; Azmeera, T.; Karuna, M.S.L.; Prasad, R.B.N.; Womeni, H.M. Effects of boiling and roasting on proximate composition, lipid oxidation, fatty acid profile and mineral content of two sesame varieties commercialized and consumed in Far-North Region of Cameroon. Food Chem. 2017, 221, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Liu, Y.; Shi, L.; Wang, N.; Wang, X. Effect of roasting treatment on the chemical composition of sesame oil. LWT 2019, 101, 191–200. [Google Scholar] [CrossRef]
- Liu, R.; Chen, H.; Wang, S.; Wei, L.; Yu, Y.; Lan, W.; Yang, J.; Guo, L.; Fu, H. Maillard reaction products and guaiacol as production process and raw material markers for the authentication of sesame oil. J. Sci. Food Agric. 2022, 102, 250–258. [Google Scholar] [CrossRef]
- Bordón, M.G.; Bodoira, R.M.; Cittadini, M.C.; Marin, M.A.; Ribotta, P.D.; Martínez, M.L. Influence of fluidized-bed roasting conditions of white sesame seeds on the physico-chemical properties and sensory acceptability of the cold-pressed oils. J. Food Process. Preserv. 2020, 45, e15079. [Google Scholar] [CrossRef]
- Mohamed Ahmed, I.A.; Uslu, N.; Musa Ozcan, M.; Al Juhaimi, F.; Ghafoor, K.; Babiker, E.E.; Osman, M.A.; Alqah, H.A.S. Effect of conventional oven roasting treatment on the physicochemical quality attributes of sesame seeds obtained from different locations. Food Chem. 2021, 338, 128109. [Google Scholar] [CrossRef]
- Jia, X.; Zhou, Q.; Wang, J.; Liu, C.; Huang, F.; Huang, Y. Identification of key aroma-active compounds in sesame oil from microwaved seeds using E-nose and HS-SPME-GCxGC-TOF/MS. J. Food Biochem. 2019, 43, e12786. [Google Scholar] [CrossRef]
- Mansur, A.R.; Jeong, H.-R.; Lee, B.H.; Koo, M.; Seo, D.-H.; Hwang, S.H.; Park, J.S.; Kim, D.-O.; Nam, T.G. Comparative evaluation of triacylglycerols, fatty acids, and volatile organic compounds as markers for authenticating sesame oil. Int. J. Food Prop. 2018, 21, 2509–2516. [Google Scholar] [CrossRef]
- Nakamura, S.; Nishimura, O.; Masuda, H.; Mihara, S. Identification of Volatile Flavor Components of the Oil from Roasted Sesame Seeds. Agric. Biol. Chem. 2014, 53, 1891–1899. [Google Scholar] [CrossRef]
- Ivanova-Petropulos, V.; Mitrev, S.; Stafilov, T.; Markova, N.; Leitner, E.; Lankmayr, E.; Siegmund, B. Characterisation of traditional Macedonian edible oils by their fatty acid composition and their volatile compounds. Food Res. Int. 2015, 77, 506–514. [Google Scholar] [CrossRef]
- Dou, X.; Zhang, L.; Yang, R.; Wang, X.; Yu, L.; Yue, X.; Ma, F.; Mao, J.; Wang, X.; Li, P. Adulteration detection of essence in sesame oil based on headspace gas chromatography-ion mobility spectrometry. Food Chem. 2022, 370, 131373. [Google Scholar] [CrossRef] [PubMed]
- Symoniuk, E.; Wroniak, M.; Napiorkowska, K.; Brzezinska, R.; Ratusz, K. Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils and Oils Mixtures. Foods 2022, 11, 111597. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.R.; Song, H.-W.; Lee, J.-G.; Yoon, H.-J.; Chung, M.-S.; Kim, Y.-S. Comparison of the contents of benzo(a)pyrene, sesamol and sesamolin, and volatiles in sesame oils according to origins of sesame seeds. Appl. Biol. Chem. 2016, 59, 129–141. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.; Wen, S.; Sun, Y.; Chen, J.; Gao, Y.; Sagymbek, A.; Yu, X. Analytical methods for determining the peroxide value of edible oils: A mini-review. Food Chem. 2021, 358, 129834. [Google Scholar] [CrossRef]
- Gebremeskel, A.F.; Ngoda, P.N.; Kamau-Mbuthia, E.W.; Mahungu, S.M. The effect of roasting, storage temperature, and ethanoic basil (Ocimum basilicum L.) extract on the oxidative stability of crude sesame (Sesamum indicum L.) oil. Food Sci. Nutr. 2022, 10, 2736–2748. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.-Y.; Li, M.-k.; Song, H.-L.; Zou, T.-t.; Zhang, L.; Xiong, J. Characterization of aroma in response surface optimized no-salt bovine bone protein extract by switchable GC/GC×GC-olfactometry-mass spectrometry, electronic nose, and sensory evaluation. LWT 2021, 147, 111559. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Manousi, N.; Rosenberg, E.; Zachariadis, G.A.; Paraskevopoulou, A.; Samanidou, V. Exploring the volatile metabolome of conventional and organic walnut oils by solid-phase microextraction and analysis by GC-MS combined with chemometrics. Food Chem. 2021, 363, 130331. [Google Scholar] [CrossRef]
- Ni, R.; Wang, P.; Zhan, P.; Tian, H.; Li, T. Effects of different frying temperatures on the aroma profiles of fried mountain pepper (Litsea cubeba (Lour.) Pers.) oils and characterization of their key odorants. Food Chem. 2021, 357, 129786. [Google Scholar] [CrossRef]
- Rodriguez, G.; Squeo, G.; Estivi, L.; Quezada Berru, S.; Buleje, D.; Caponio, F.; Brandolini, A.; Hidalgo, A. Changes in stability, tocopherols, fatty acids and antioxidant capacity of sacha inchi (Plukenetia volubilis) oil during French fries deep-frying. Food Chem. 2021, 340, 127942. [Google Scholar] [CrossRef]
- Baker, G.L.; Cornell, J.A.; Gorbet, D.W.; O’Keefe, S.F.; Sims, C.A.; Talcott, S.T. Determination of Pyrazine and Flavor Variations in Peanut Genotypes during Roasting. J. Food Sci. 2003, 68, 394–400. [Google Scholar] [CrossRef]
- Liu, X.; Jin, Q.; Liu, Y.; Huang, J.; Wang, X.; Mao, W.; Wang, S. Changes in volatile compounds of peanut oil during the roasting process for production of aromatic roasted peanut oil. J. Food Sci. 2011, 76, C404–C412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, R.; Yuan, Y.; Yang, T.; Liu, S. Changes in volatiles of palm kernel oil before and after kernel roasting. LWT 2016, 73, 432–441. [Google Scholar] [CrossRef]
- Gracka, A.; Jelen, H.H.; Majcher, M.; Siger, A.; Kaczmarek, A. Flavoromics approach in monitoring changes in volatile compounds of virgin rapeseed oil caused by seed roasting. J. Chromatogr. A 2016, 1428, 292–304. [Google Scholar] [CrossRef]
- Jia, X.; Wang, L.; Zheng, C.; Yang, Y.; Wang, X.; Hui, J.; Zhou, Q. Key Odorant Differences in Fragrant Brassica napus and Brassica juncea Oils Revealed by Gas Chromatography-Olfactometry, Odor Activity Values, and Aroma Recombination. J. Agric. Food Chem. 2020, 68, 14950–14960. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.P.; Blank, I.; Li, F.; Li, C.; Liu, Y. GC x GC-ToF-MS and GC-IMS based volatile profile characterization of the Chinese dry-cured hams from different regions. Food Res. Int. 2021, 142, 110222. [Google Scholar] [CrossRef]
- Pollner, G.; Schieberle, P. Characterization of the Key Odorants in Commercial Cold-Pressed Oils from Unpeeled and Peeled Rapeseeds by the Sensomics Approach. J. Agric. Food Chem. 2016, 64, 627–636. [Google Scholar] [CrossRef]
- Wang, Z.; Gan, S.; Sun, W.; Chen, Z. Widely Targeted Metabolomics Analysis Reveals the Differences of Nonvolatile Compounds in Oolong Tea in Different Production Areas. Foods 2022, 11, 1057. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, L.; Zhan, P.; Tian, H.; Liu, J. Characterization of the aroma compounds of Millet Huangjiu at different fermentation stages. Food Chem. 2022, 366, 130691. [Google Scholar] [CrossRef]
- Jia, X.; Deng, Q.; Yang, Y.; Xiang, X.; Zhou, X.; Tan, C.; Zhou, Q.; Huang, F. Unraveling of the Aroma-Active Compounds in Virgin Camellia Oil (Camellia oleifera Abel) Using Gas Chromatography-Mass Spectrometry-Olfactometry, Aroma Recombination, and Omission Studies. J. Agric. Food Chem. 2021, 69, 9043–9055. [Google Scholar] [CrossRef]
- Cao, W.; Shu, N.; Wen, J.; Yang, Y.; Jin, Y.; Lu, W. Characterization of the Key Aroma Volatile Compounds in Nine Different Grape Varieties Wine by Headspace Gas Chromatography–Ion Mobility Spectrometry (HS-GC-IMS), Odor Activity Values (OAV) and Sensory Analysis. Foods 2022, 11, 2767. [Google Scholar] [CrossRef] [PubMed]
- Karami, H.; Rasekh, M.; Mirzaee-Ghaleh, E. Qualitative analysis of edible oil oxidation using an olfactory machine. J. Food Meas. Charact. 2020, 14, 2600–2610. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, J.; Hong, Q.; Dong, W.; Chen, X.; Wu, G.; Zhang, Z. Identification of changes in the volatile compounds of robusta coffee beans during drying based on HS-SPME/GC-MS and E-nose analyses with the aid of chemometrics. LWT 2022, 161, 113317. [Google Scholar] [CrossRef]
- Hai, Z.; Wang, J. Detection of adulteration in camellia seed oil and sesame oil using an electronic nose. Eur. J. Lipid Sci. Technol. 2006, 108, 116–124. [Google Scholar] [CrossRef]
- Chau, C.-F.; Ciou, J.-Y.; Wu, C.-L. Commercialized Sesame Oil Analysis: Quality Characterization and Oxidative Stability of Blended Sesame Oil. ACS Food Sci. Technol. 2021, 1, 1222–1227. [Google Scholar] [CrossRef]
- Namiki, M.; Fukuda, Y.; Takei, Y.; Namiki, K.; Koizumi, Y. Changes in Functional Factors of Sesame Seed and Oil during Various Types of Processing. In Bioactive Compounds in Foods; ACS Publications: Washington, DC, USA, 2002; pp. 85–104. [Google Scholar] [CrossRef]
- Shi, L.K.; Zheng, L.; Liu, R.J.; Chang, M.; Jin, Q.Z.; Wang, X.G. Chemical Characterization, Oxidative Stability, and In Vitro Antioxidant Capacity of Sesame Oils Extracted by Supercritical and Subcritical Techniques and Conventional Methods: A Comparative Study Using Chemometrics. Eur. J. Lipid Sci. Technol. 2017, 120, 1700326. [Google Scholar] [CrossRef]
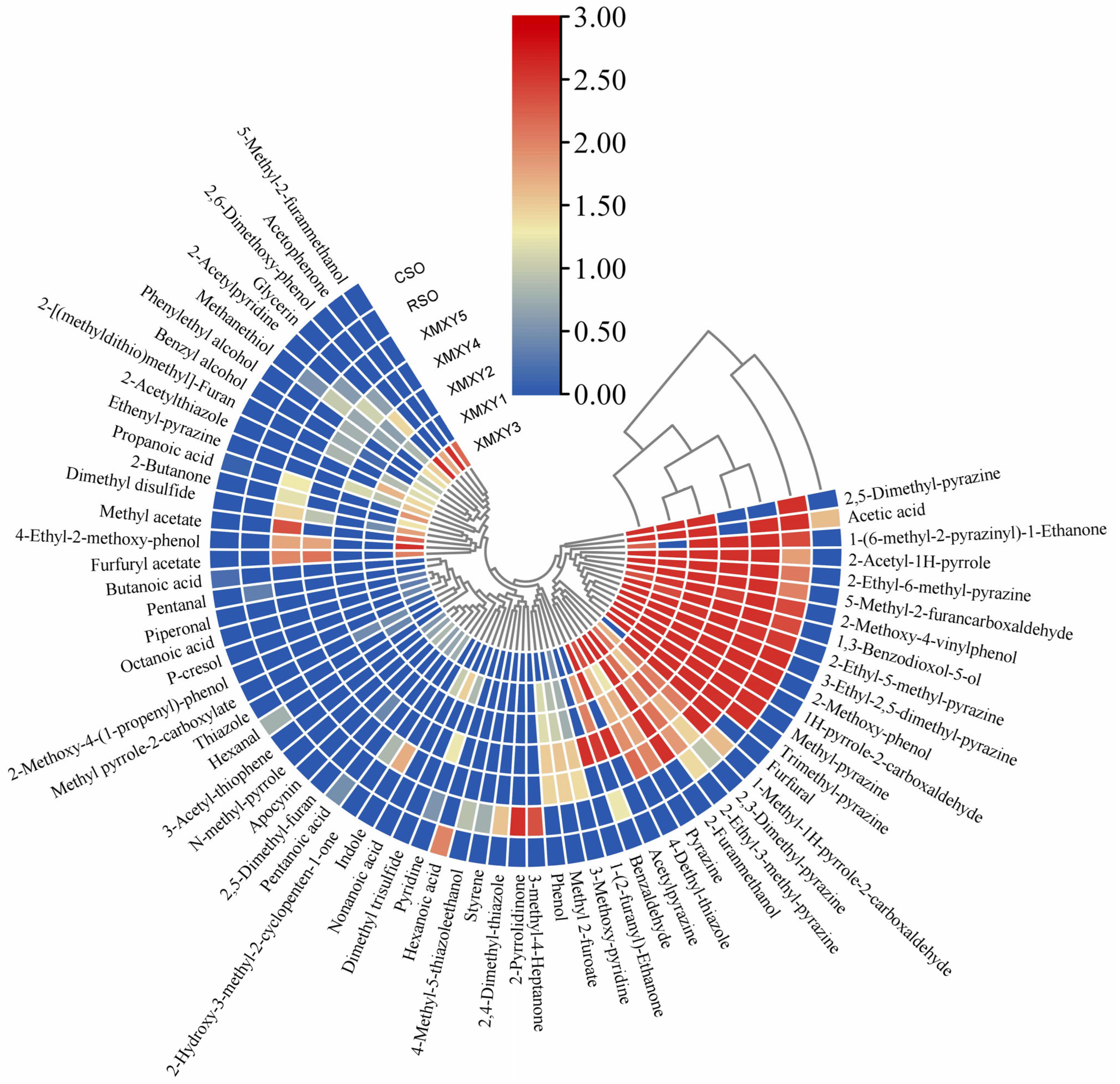

| Name | CAS ID | OTi a (mg/kg) | OAVs b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| XMXY1 | XMXY2 | XMXY3 | XMXY4 | XMXY5 | RSO | CSO | |||
| Methanethiol | 74-93-1 | 0.00006 | -- | 10,333 | 26,333 | 10,666 | 17,000 | 7166 | -- |
| Methyl acetate | 79-20-9 | 2 | -- | -- | 2 | -- | 2 | -- | -- |
| Pentanal | 110-62-3 | 0.24 | -- | -- | -- | -- | -- | 1 | -- |
| Dimethyl disulfide | 624-92-0 | 0.012 | 32 | -- | 208 | 77 | 145 | -- | -- |
| Hexanal | 66-25-1 | 0.12 | -- | -- | -- | -- | -- | -- | 16 |
| Thiazole | 288-47-1 | 0.038 | 8 | -- | -- | -- | -- | -- | -- |
| Methyl-pyrazine | 109-08-0 | 0.06 | 613 | 639 | 1031 | 688 | 717 | 475 | -- |
| 4-Dethyl-thiazole | 693-95-8 | 0.055 | 35 | 39 | 75 | 51 | 64 | -- | -- |
| 2,4-Dimethyl-thiazole | 541-58-2 | 0.018 | -- | -- | -- | -- | -- | 107 | -- |
| 2,5-Dimethyl-pyrazine | 123-32-0 | 2.6 | 6 | 5 | 10 | -- | -- | 7 | -- |
| 2,3-Dimethyl-pyrazine | 5910-89-4 | 0.4 | 4 | 9 | 8 | 5 | 4 | 2 | -- |
| Dimethyl trisulfide | 3658-80-8 | 0.0025 | 712 | -- | -- | -- | -- | -- | -- |
| 2-Ethyl-6-methyl-pyrazine | 13925-03-6 | 0.04 | 168 | 146 | 366 | 196 | 158 | 80 | -- |
| 2-Ethyl-5-methyl-pyrazine | 13360-64-0 | 0.32 | 22 | 15 | 29 | 17 | 14 | 14 | -- |
| 2-Ethyl-3-methyl-pyrazine | 15707-23-0 | 0.13 | 26 | 18 | 44 | 25 | 20 | 12 | -- |
| Trimethyl-pyrazine | 14667-55-1 | 0.29 | 23 | 22 | 44 | 23 | 19 | 23 | -- |
| Ethenyl-pyrazine | 4177-16-6 | 0.7 | 1 | 1 | 3 | -- | -- | -- | -- |
| Acetic acid | 64-19-7 | 0.124 | 57 | 60 | 49 | -- | 183 | 247 | 5 |
| 3-Ethyl-2,5-dimethyl-pyrazine | 13360-65-1 | 0.024 | 487 | 424 | 815 | 419 | 431 | 365 | -- |
| Furfural | 98-01-1 | 0.77 | 20 | 22 | -- | 15 | 25 | -- | -- |
| Benzaldehyde | 100-52-7 | 0.32 | 7 | 9 | -- | 14 | -- | 4 | -- |
| Propanoic acid | 79-09-4 | 0.72 | -- | -- | 4 | -- | 2 | -- | -- |
| 5-Methyl-2-furancarboxaldehyde | 620-02-0 | 0.5 | 33 | 36 | 10 | 28 | 49 | 6 | -- |
| 2-Acetylpyridine | 1122-62-9 | 0.019 | 41 | 29 | 97 | 58 | 27 | -- | -- |
| Acetylpyrazine | 22047-25-2 | 0.06 | 202 | -- | 109 | 150 | -- | -- | -- |
| 2-Acetylthiazole | 24295-03-2 | 0.01 | 216 | -- | 168 | -- | -- | -- | -- |
| Acetophenone | 98-86-2 | 5.629 | -- | -- | 1 | -- | -- | -- | -- |
| 2-Furanmethanol | 98-00-0 | 0.0123 | 1086 | 1302 | 1513 | 1315 | 1560 | -- | -- |
| 1-(6-methyl-2-pyrazinyl)-1-Ethanone | 22047-26-3 | 0.3 | 0.3 | -- | 19 | 12 | 23 | 33 | 14 |
| 2-[(methyldithio)methyl]-Furan | 57500-00-2 | 0.00004 | 21499 | -- | 31250 | 19500 | -- | -- | -- |
| Pentanoic acid | 109-52-4 | 0.061 | -- | -- | 10 | -- | -- | -- | 6 |
| Hexanoic acid | 142-62-1 | 0.7 | -- | -- | -- | -- | -- | -- | 4 |
| 2-Methoxy-phenol | 32994 | 0.016 | 927 | 1772 | 5343 | 3423 | 2826 | 347 | -- |
| Phenol | 108-95-2 | 0.1 | 12 | 12 | -- | 18 | 17 | -- | -- |
| 4-Ethyl-2-methoxy-phenol | 2785-89-9 | 0.05 | -- | -- | 261 | 48 | 47 | -- | -- |
| P-cresol | 106-44-5 | 0.025 | -- | -- | 14 | -- | -- | -- | -- |
| 1-Methyl-1H-pyrrole-2-carboxaldehyde | 1192-58-1 | 0.37 | 0.37 | 8 | 8 | 6 | 8 | 16 | 5 |
| 2-Methoxy-4-vinylphenol | 7786-61-0 | 0.2 | 95 | 104 | 82 | 90 | 475 | 85 | -- |
| 2,6-Dimethoxy-phenol | 91-10-1 | 0.05 | -- | -- | 9 | -- | -- | -- | -- |
| 2-Methoxy-4-(1-propenyl)-phenol | 97-54-1 | 0.263 | 0.1 | -- | -- | 1 | -- | -- | -- |
| Indole | 120-72-9 | 0.1 | -- | -- | -- | -- | 7 | -- | -- |
| Apocynin | 498-02-2 | 0.3 | -- | -- | 1 | -- | -- | -- | -- |
| Pressure Drop (%) | Induction Time | ||||||
|---|---|---|---|---|---|---|---|
| XMXY1 | XMXY2 | XMXY3 | XMXY4 | XMXY5 | RSO | CSO | |
| 2% | 42.17 ± 0.68 | 39.48 ± 1.22 | 53.11 ± 1.11 | 50.44 ± 1.54 | 48.83 ± 1.13 | 42.74 ± 1.08 | 28.8 ± 1.96 |
| 4% | 45.59 ± 0.93 | 41.74 ± 1.44 | 58.18 ± 0.85 | 55.77 ± 1.59 | 54.18 ± 1.4 | 46.78 ± 1.09 | 29.76 ± 2.01 |
| 6% | 46.73 ± 0.92 | 42.64 ± 1.36 | 59.64 ± 0.73 | 57.25 ± 1.53 | 55.63 ± 1.53 | 49.08 ± 1.19 | 30.58 ± 2.04 |
| 8% | 47.61 ± 0.92 | 43.44 ± 1.31 | 60.59 ± 0.8 | 58.21 ± 1.55 | 56.49 ± 1.49 | 50.85 ± 1.38 | 31.34 ± 2.06 |
| 10% | 48.43 ± 0.92 | 44.21 ± 1.27 | 61.52 ± 0.82 | 59.09 ± 1.55 | 57.34 ± 1.46 | 52.27 ± 1.47 | 32.11 ± 2.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Fu, Y.; Li, P.; Xi, H.; Zhao, W.; Wang, D.; Mao, J.; Zhang, S.; Sun, S.; Xie, J. Characterization of Traditional Chinese Sesame Oil by Using Headspace Solid-Phase Microextraction/Gas Chromatography–Mass Spectrometry, Electronic Nose, Sensory Evaluation, and RapidOxy. Foods 2022, 11, 3555. https://doi.org/10.3390/foods11223555
Chen Y, Fu Y, Li P, Xi H, Zhao W, Wang D, Mao J, Zhang S, Sun S, Xie J. Characterization of Traditional Chinese Sesame Oil by Using Headspace Solid-Phase Microextraction/Gas Chromatography–Mass Spectrometry, Electronic Nose, Sensory Evaluation, and RapidOxy. Foods. 2022; 11(22):3555. https://doi.org/10.3390/foods11223555
Chicago/Turabian StyleChen, Yan, Yingjie Fu, Peng Li, Hui Xi, Wuduo Zhao, Dingzhong Wang, Jian Mao, Shusheng Zhang, Shihao Sun, and Jianping Xie. 2022. "Characterization of Traditional Chinese Sesame Oil by Using Headspace Solid-Phase Microextraction/Gas Chromatography–Mass Spectrometry, Electronic Nose, Sensory Evaluation, and RapidOxy" Foods 11, no. 22: 3555. https://doi.org/10.3390/foods11223555






