Dietary Heme-Containing Proteins: Structures, Applications, and Challenges
Abstract
1. Introduction
2. Structure
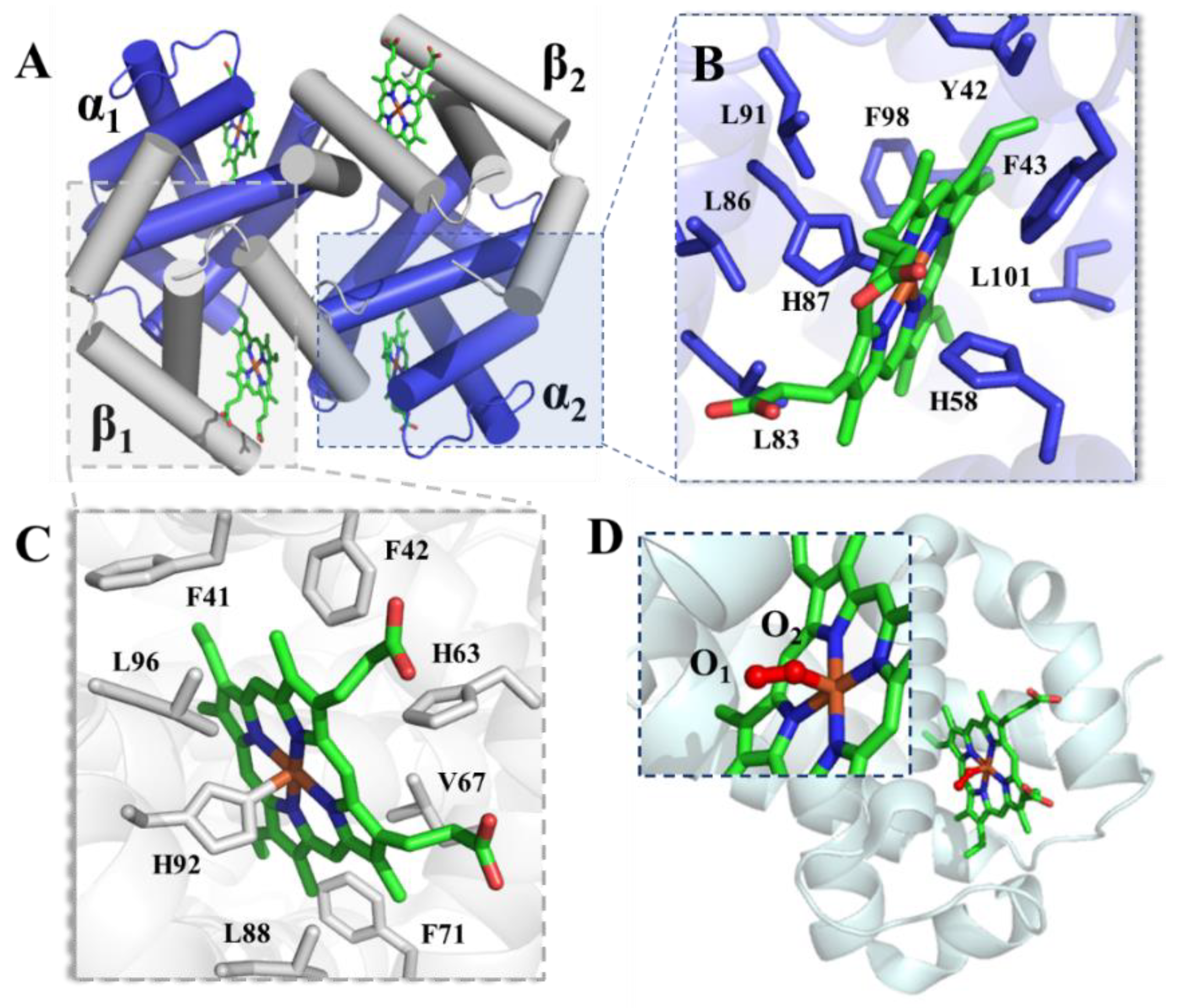
2.1. Hemoglobin
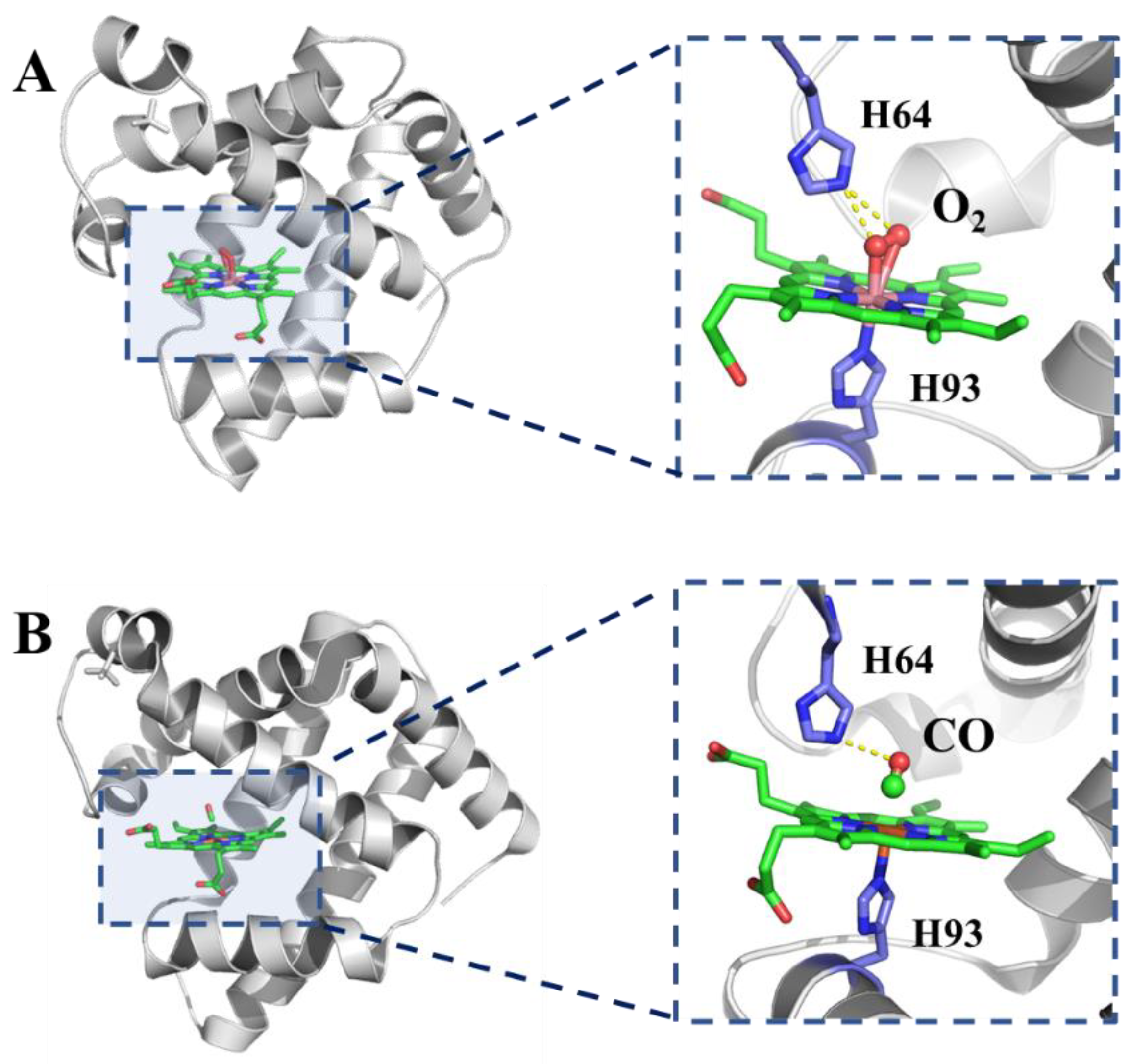
2.2. Myoglobin
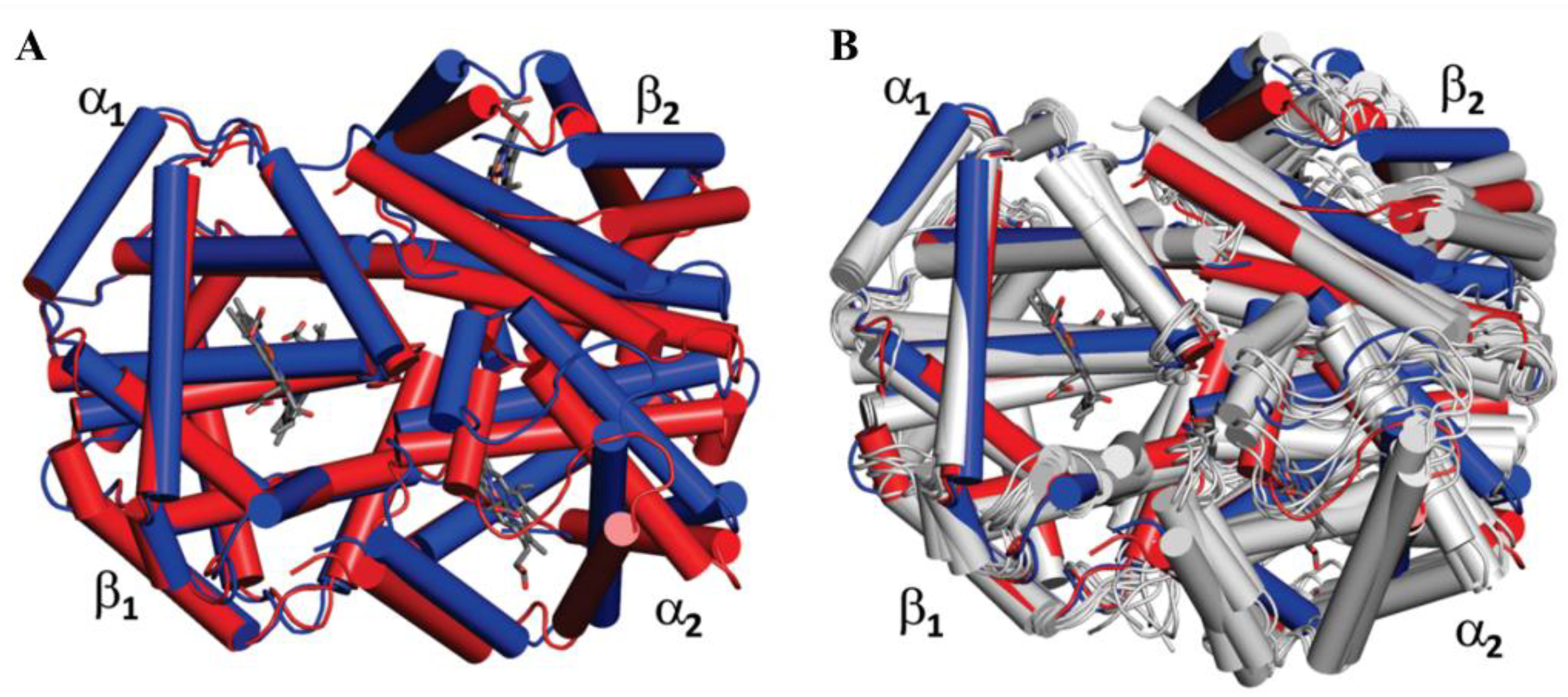
2.3. The Allosteric Effect
2.4. Other Heme Proteins
3. Applications in the Food Industry
3.1. Iron Supplements
3.2. Coloring & Pigments
3.3. Antibacterial Effects
3.4. Medical & Nutritional Applications
4. Discussion
4.1. Denaturation and Oxidation
4.2. Absorption Rate and Bioavailability
4.3. Nutritional Problems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Theuer, R.C. Iron-Fortified Infant Cereals. Food Rev. Int. 2008, 24, 277–310. [Google Scholar] [CrossRef]
- Lofti, M.; Venkatesh, M.; Merx, R.; Van den Heuvel, P. Micronutrient Fortification of Foods: Developing a Program. J. Food Technol. Afr. 2009, 4, 2–4. [Google Scholar] [CrossRef]
- Cook, J.D.; Flowers, C.H.; Skikne, B.S. The Quantitative Assessment of Body Iron. Blood 2003, 101, 3359–3363. [Google Scholar] [CrossRef] [PubMed]
- Springer, B.A.; Sligar, S.G.; Olson, J.S.; Phillips, G.N., Jr. Mechanisms of Ligand Recognition in Myoglobin. Chem. Rev. 1994, 94, 699–714. [Google Scholar] [CrossRef]
- Huang, X.; Groves, J.T. Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev. 2018, 118, 2491–2553. [Google Scholar] [CrossRef]
- Lynch, S.A.; Mullen, A.M.; O’Neill, E.E.; García, C.Á. Harnessing the Potential of Blood Proteins as Functional Ingredients: A Review of the State of the Art in Blood Processing: Blood Processing and Food Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Hou, C.; Gao, Y.; Zhu, J.; Zhang, D. Application of Hemoglobin and Its Derivatives in Food. J. Chin. Inst. Food Sci. Technol. 2018, 18, 314–322. [Google Scholar] [CrossRef]
- Morita, Y.; Yamada, T.; Kureishi, M.; Kihira, K.; Komatsu, T. Quaternary Structure Analysis of a Hemoglobin Core in Hemoglobin-Albumin Cluster. J. Phys. Chem. B 2018, 122, 12031–12039. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, X.; Liu, S.; Lu, G. The Structure of Greylag Goose Oxy Haemoglobin: The Roles of Four Mutations Compared with Bar-headed Goose Haemoglobin. Acta. Crystallogr. D Bio. Crystallogr. 2001, 57, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Balasco, N.; Vitagliano, L.; Merlino, A.; Verde, C.; Mazzarella, L.; Vergara, A. The Unique Structural Features of Carbonmonoxy Hemoglobin from the Sub-Antarctic Fish Eleginops Maclovinus. Sci. Rep. 2019, 9, 18987. [Google Scholar] [CrossRef] [PubMed]
- Khoshouei, M.; Radjainia, M.; Baumeister, W.; Danev, R. Cryo-EM Structure of Haemoglobin at 3.2 Å Determined with the Volta Phase Plate. Nat. Commun. 2017, 8, 16099. [Google Scholar] [CrossRef] [PubMed]
- Skikne, B.S. Current Concepts in Iron Deficiency Anemia. Food Rev. Int. 1988, 4, 137–173. [Google Scholar] [CrossRef]
- Narita, M.; Narita, M.; Itsuno, Y.; Itsuno, S. Autonomous Sequences in Myoglobin Emerging from X-Ray Structure of Holomyoglobin. ACS Omega 2019, 4, 992–999. [Google Scholar] [CrossRef]
- Brucker, E.; Olson, J.; Phillips, J.; Dou, Y.; Ikeda-Saito, M. High Resolution Crystal Structures of the Deoxy, Oxy, and Aquomet Forms of Cobalt Myoglobin. J. Biol. Chem. 1996, 271, 25419–25422. [Google Scholar] [CrossRef]
- Chu, K.; Vojtechovsky, J.; Mcmahon, B.; Sweet, R.; Berendzen, J.; Schlichting, I. Crystal Structure of a New Ligand Binding Intermediate in Wildtype Carbonmonoxy Myoglobin. Nature 2000, 403, 921. [Google Scholar] [CrossRef]
- Begun, A.; Molochkov, A.; Niemi, A.J. Protein Tertiary Structure and the Myoglobin Phase Diagram. Sci. Rep. 2019, 9, 10819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, J.; Wang, X.-J.; He, B.; Gao, S.-Q.; Lin, Y.-W. The Third Generation of Artificial Dye-Decolorizing Peroxidase Rationally Designed in Myoglobin. ACS Catal. 2019, 9, 7888–7893. [Google Scholar] [CrossRef]
- Liao, F.; Xu, J.-K.; Luo, J.; Gao, S.-Q.; Wang, X.-J.; Lin, Y.-W. Bioinspired Design of an Artificial Peroxidase: Introducing Key Residues of Native Peroxidases into F43Y Myoglobin with a Tyr-Heme Cross-Link. Dalton Trans. 2020, 49, 5029–5033. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Yuan, H.; Liu, C.; He, B.; Gao, S.-Q.; Wen, G.-B.; Tan, X.; Lin, Y.-W. A Rationally Designed Myoglobin Exhibits a Catalytic Dehalogenation Efficiency More than 1000-Fold That of a Native Dehaloperoxidase. ACS Catal. 2018, 8, 9619–9624. [Google Scholar] [CrossRef]
- Perutz, M.F. Regulation of Oxygen Affinity of Hemoglobin: Influence of Structure of the Globin on the Heme Iron. Annu. Rev. Biochem. 1979, 48, 327–386. [Google Scholar] [CrossRef]
- Yuan, Y.; Tam, M.F.; Simplaceanu, V.; Ho, C. New Look at Hemoglobin Allostery. Chem. Rev. 2015, 115, 1702–1724. [Google Scholar] [CrossRef]
- Fan, J.S.; Zheng, Y.; Choy, W.Y.; Simplaceanu, V.; Ho, N.T.; Ho, C.; Yang, D. Solution Structure and Dynamics of Human Hemoglobin in the Carbonmonoxy Form. Biochemistry 2013, 52, 5809–5820. [Google Scholar] [CrossRef]
- Antonini, E.; Brunori, M. Hemoglobin and Myoglobin in Their Reactions with Ligands. Front. Biol. 1971, 21, 27–31. [Google Scholar]
- Mathews, M.F. The Structure, Function and Evolution of Cytochromes. Prog. Biophys. Mol. Biol. 1985, 45, 1–56. [Google Scholar] [CrossRef]
- Samhan-Arias, A.K.; Fortalezas, S.; Cordas, C.M.; Moura, I.; Moura, J.J.G.; Gutierrez-Merino, C. Cytochrome B5 Reductase Is the Component from Neuronal Synaptic Plasma Membrane Vesicles That Generates Superoxide Anion upon Stimulation by Cytochrome c. Redox Biol. 2018, 15, 109–114. [Google Scholar] [CrossRef]
- Slater, B.; Kosmützky, D.; Nisbet, R.E.R.; Howe, C.J. The Evolution of the Cytochrome C6 Family of Photosynthetic Electron Transfer Proteins. Genome Biol. Evol. 2021, 13, evab146. [Google Scholar] [CrossRef] [PubMed]
- Im, S.-C.; Waskell, L. The Interaction of Microsomal Cytochrome P450 2B4 with Its Redox Partners, Cytochrome P450 Reductase and Cytochrome B5. Arch. Biochem. Biophys. 2011, 507, 144–153. [Google Scholar] [CrossRef]
- Churchfield, L.A.; Alberstein, R.G.; Williamson, L.M.; Tezca, F.A. Determining the Structural and Energetic Basis of Allostery in a De Novo Designed Metalloprotein Assembly. J. Am. Chem. Soc. 2018, 140, 31. [Google Scholar] [CrossRef]
- Song, W.J.; Yu, J.; Tezcan, F.A. Importance of Scaffold Flexibility/Rigidity in the Design and Directed Evolution of Artificial Metallo-β-Lactamases. J. Am. Chem. Soc. 2017, 139, 16772–16779. [Google Scholar] [CrossRef]
- Zang, J.; Chen, H.; Zhao, G.; Wang, F.; Ren, F. Ferritin Cage for Encapsulation and Delivery of Bioactive Nutrients: From Structure, Property to Applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 3673–3683. [Google Scholar] [CrossRef]
- Ma, Q.; Kim, E.Y.; Han, O. Heme Boosts Non-Heme Iron Absorption in Human Intestinal Caco-2 Cells. FASEB J. 2011, 25, 607–617. [Google Scholar]
- Tang, N.; Chen, L.; Zhuang, H. Effects of Heme Iron Enriched Peptide on Iron Deficiency Anemia in Rats. Food Funct 2014, 5, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zhu, Y.; Zhuang, H. Antioxidant and Anti-Anemia Activity of Heme Iron Obtained from Bovine Hemoglobin. Food Sci. Biotechnol. 2015, 24, 635–642. [Google Scholar] [CrossRef]
- Ksserumaga, K.J. Production and Use of a Shelf-Stable Bovine Blood Powder for Food Fortification as a Food-Based Strategy to Combat Iron Deficiency Anaemia in Sub-Saharan Africa. Afr. J. Food Agric. Nutr. Dev. 2005, 5, 1–17. [Google Scholar]
- Drvenica, I.; Stancic, A.; Kalusevic, A.; Markovic, S.; Ilic, V. Maltose-Mediated Long-Term Stabilization of Freeze- and Spray- Dried Forms of Bovine and Porcine Hemoglobin. J. Serbian Chem. Soc. 2019, 84, 67. [Google Scholar] [CrossRef]
- Jiang, X.; Fuller, D.; Hsieh, Y.-H.P.; Rao, Q. Monoclonal Antibody-Based ELISA for the Quantification of Porcine Hemoglobin in Meat Products. Food Chem. 2018, 250, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Song, X.; Li, Z.; Wang, W.; Shen, Q.; Zhang, D. Arginine Improves the Color Stability of Hemoglobin Powder during Freeze-drying and Storage. Food Sci. Nutr. 2019, 7, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhu, X.; Tan, S.; Qin, H.; Zhou, C. The Role of Monoxide Hemoglobin in Color Improvement of Chicken Sausage. Food Sci. Biotechnol. 2016, 25, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Chhem-Kieth, S.; Lametsch, R.; Hansen, E.T.; Ruiz-Carrascal, J. Storage and Thermal Stability of Novel Heme-based Pigments Prepared from Porcine Hemoglobin. J. Food Process Eng. 2019, 42, e12994. [Google Scholar] [CrossRef]
- Ofori, J.A.; Hsieh, Y.H.P. The Use of Blood and Derived Products as Food Additives; IntechOpen: Rijeka, Croatia, 2012; ISBN 953-51-0067-X. [Google Scholar]
- Sanchez-Reinoso, Z.; Cournoyer, A.; Thibodeau, J.; Said, L.B.; Fliss, I.; Bazinet, L.; Mikhaylin, S. Effect of PH on the Antimicrobial Activity and Peptide Population of Pepsin Hydrolysates Derived from Bovine and Porcine Hemoglobins. ACS Food Sci. Technol. 2021, 1, 1687–1701. [Google Scholar] [CrossRef]
- Álvarez, C.; Rendueles, M.; Díaz, M. Production of Porcine Hemoglobin Peptides at Moderate Temperature and Medium Pressure under a Nitrogen Stream. Functional and Antioxidant Properties. J. Agric. Food Chem. 2012, 60, 5636–5643. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, W.; Xin, X.; Chen, D.; Hu, H. Transferrin-Modified Nanoliposome Codelivery Strategies for Enhancing the Cancer Therapy. J. Pharm. Sci. 2020, 109, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Bak, K.; Petersen, M.; Lametsch, R.; Hansen, E.; Ruiz-Carrascal, J. Development of Volatile Compounds during Hydrolysis of Porcine Hemoglobin with Papain. Molecules 2018, 23, 357. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, J.; De Gobba, C.; Zhang, L.; Bredie, W.L.P.; Lametsch, R. Production of Taste Enhancers from Protein Hydrolysates of Porcine Hemoglobin and Meat Using Bacillus Amyloliquefaciens γ-Glutamyltranspeptidase. J. Agric. Food Chem. 2020, 68, 11782–11789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Luo, R.; Yalei, L.I.; Mengbin, M.A.; Zhou, Y.; Agriculture, S.O.; University, N. Structural Changes of Myoglobin during Beef Storage as Analyzed by Raman Spectroscopy. Food Sci. 2019, 40, 15–19. [Google Scholar]
- Dong, J.; Zhou, Y.; Lu, Y.; Lv, Y.; He, Q. Effect of Tea Polyphenols on the Oxidation and Color Stability of Porcine Hemoglobin. J. Food Sci. 2019, 84, 2086–2090. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Li, X.; Zhou, Y.; Lu, Y.; Lv, Y.; Chi, Y.; He, Q. Interactions of Gallic Acid with Porcine Hemoglobin: Effect on the Redox State and Structure of Hemoglobin. J. Agric. Food Chem. 2021, 69, 397–403. [Google Scholar] [CrossRef]
- Chhem-Kieth, S.; Skou, P.B.; Lametsch, R.; Hansen, E.T.; Ruiz-Carrascal, J. Investigation of Nitrite Alternatives for the Color Stabilization of Heme–Iron Hydrolysates. J. Food Sci. Technol. 2018, 55, 4287–4296. [Google Scholar] [CrossRef] [PubMed]
- Gianquinto, E.; Moscetti, I.; Bei, O.D.; Campanini, B.; Marchetti, M.; Luque, F.J.; Cannistraro, S.; Ronda, L.; Bizzarri, A.R.; Spyrakis, F. and Interaction of Human Hemoglobin and Semi-Hemoglobins with the Staphylococcus Aureus Hemophore IsdB: A Kinetic and Mechanistic Insight. Sci. Rep. 2019, 9, 18629. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and Polymersomes: A Comparative Review towards Cell Mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, J.; Pang, Q.; Ma, L.; Tong, W.; Gao, C. Construction of Microreactors for Cascade Reaction and Their Potential Application as Antibacterial Agents. Acs Appl. Mater. Interfaces 2019, 11, 6789–6795. [Google Scholar] [CrossRef] [PubMed]
- Gro, R.; Bauer, R.; Krüger, F.; Rücker-Braun, E.; Münch, J. A Placenta Derived C-Terminal Fragment of β-Hemoglobin with Combined Antibacterial and Antiviral Activity. Front. Microbiol. 2020, 11, 508. [Google Scholar]
- Carlsson, M.L.R.; Kanagarajan, S.; Bülow, L.; Zhu, L.H. Plant Based Production of Myoglobin—A Novel Source of the Muscle Heme-Protein. Sci. Rep. 2020, 10, 920. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.I.; Han, M.; Kim, H.J.; Suh, J.Y.; Jo, C. Mechanism Underlying Green Discolouration of Myoglobin Induced by Atmospheric Pressure Plasma. Sci. Rep. 2018, 8, 9790. [Google Scholar] [CrossRef] [PubMed]
- Hosta-Rigau, L. Optimization of Hemoglobin Encapsulation within PLGA Nanoparticles and Their Investigation as Potential Oxygen Carriers. Pharmaceutics 2021, 13, 1958. [Google Scholar]
- Silva, J.G.; Morais, H.A.; Oliveira, A.L.; Silvestre, M. Addition Effects of Bovine Blood Globin and Sodium Caseinate on the Quality Characteristics of Raw and Cooked Ham Paté. Meat Sci. 2003, 63, 177–184. [Google Scholar] [CrossRef]
- Brantley, R.E.; Smerdon, S.J.; Wilkinson, A.J.; Singleton, E.W.; Olson, J.S. The Mechanism of Autooxidation of Myoglobin. J. Biol. Chem. 1993, 268, 6995–7010. [Google Scholar] [CrossRef]
- Kohn, E.; Lee, J.; Calabro, A.; Vaden, T.; Caputo, G. Heme Dissociation from Myoglobin in the Presence of the Zwitterionic Detergent N,N-Dimethyl-N-Dodecylglycine Betaine: Effects of Ionic Liquids. Biomolecules 2018, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Weinborn, V.; Pizarro, F.; Olivares, M.; Brito, A.; Arredondo, M.; Flores, S.; Valenzuela, C. The Effect of Plant Proteins Derived from Cereals and Legumes on Heme Iron Absorption. Nutrients 2015, 7, 8977–8986. [Google Scholar] [CrossRef] [PubMed]
- Villarroel, P.; Flores, S.; Pizarro, F.; de Romaña, D.L.; Arredondo, M. Effect of Dietary Protein on Heme Iron Uptake by Caco-2 Cells. Eur. J. Nutr. 2011, 50, 637–643. [Google Scholar] [CrossRef]
- Lee, K.S.; Raymond, L.D.; Schoen, B.; Raymond, G.J.; Kett, L.; Moore, R.A.; Johnson, L.M.; Taubner, L.; Speare, J.O.; Onwubiko, H.A.; et al. Hemin Interactions and Alterations of the Subcellular Localization of Prion Protein *. J. Biol. Chem. 2007, 282, 36525–36533. [Google Scholar] [CrossRef] [PubMed]
- Group, I.N.A.C. Iron Absorption from Cereals and Legumes. In A Report of the Nutritional Anemia Consultative Group; The Nutrition Foundation: New York, NY, USA, 1982; pp. 1–44. [Google Scholar]
- Layrisse, M.; Martínez-Torres, C. Model for Measuring Dietary Absorption of Heme Iron: Test with a Complete Meal. Am. J. Clin. Nutr. 1972, 25, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Czerwonka, M.; Tokarz, A. Iron in Red Meat–Friend or Foe—ScienceDirect. Meat Sci. 2017, 123, 157–165. [Google Scholar] [CrossRef]
- Bastide, N.M.; Pierre, F.; Corpet, D.E. Heme Iron from Meat and Risk of Colorectal Cancer: A Meta-Analysis and a Review of the Mechanisms Involved. Cancer Prev. Res. 2011, 4, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, C.U.; Mller, J.K.S.; Skibsted, L.H. Heme-Iron in Lipid Oxidation. Coord. Chem. Rev. 2005, 249, 485–498. [Google Scholar] [CrossRef]
- Corpet, D.E.; Guéeraud, F.; O’brien, P.J. Iron from Meat Produces Endogenous Procarcinogenic Peroxides. In Endogenous Toxins; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 133–149. [Google Scholar]
- Tappel, A. Heme of Consumed Red Meat Can Act as a Catalyst of Oxidative Damage and Could Initiate Colon, Breast and Prostate Cancers, Heart Disease and Other Diseases. Med. Hypotheses 2007, 68, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Marmot, M.; Atinmo, T.; Byers, T.; Chen, J.; Hirohata, T.; Jackson, A.; James, W.; Kolonel, L.; Kumanyika, S.; Leitzmann, C.; et al. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; World Cancer Research Fund/American Institute for Cancer Research: Washington, DC, USA, 2007. [Google Scholar]
- Jiang, J.; Xiong, Y.L. Natural Antioxidants as Food and Feed Additives to Promote Health Benefits and Quality of Meat Products: A Review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef] [PubMed]

| Applications | Proteins | Advantages | References |
|---|---|---|---|
| Iron supplements | Hb-B | Readily available; less gastrointestinal discomfort | [34] |
| Hb-B & Hb-P | Good storage stability | [35] | |
| Hb-P | Higher content and good stability | [36] | |
| Heme-enriched peptide of Hb-B | Higher iron bioavailability and fewer side effects | [32] | |
| Coloring & pigments | Arg-Hb | Color stability during process | [37] |
| COHb-P | Significant increase in pigments | [38] | |
| Hb-P | Storage and thermal stability; reduction of nitrite addition | [39] | |
| Arg-Hb | Natural resource and color development | [40] | |
| Antibacterial & antioxidant | Peptides of Hb-B & Hb-P | Antibacterial, antifungal, and anti-yeast activities | [41] |
| Peptides of Hb-P | Better antioxidant properties at low concentrations; lasting | [42] | |
| Emulsifiers | Hb-P | Forming o/w emulsions | [38] |
| Hb-P & Peptides of Hb-P | Better emulsifiers to plant proteins; superior to casein | [40] | |
| Forming both o/w and w/o emulsions | [42] | ||
| Taste attributes | Peptides of Hb-P (exopeptidase hydrolysis) | Less bitter and higher umami taste | [43] |
| Peptides of Hb-P (papain hydrolysis) | Aroma development | [44] | |
| Both Hb-P and the peptides (γ-GT hydrolysis) | Overall sensory enhancement | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Gao, S.; Zhang, X.; Zang, J. Dietary Heme-Containing Proteins: Structures, Applications, and Challenges. Foods 2022, 11, 3594. https://doi.org/10.3390/foods11223594
Xing Y, Gao S, Zhang X, Zang J. Dietary Heme-Containing Proteins: Structures, Applications, and Challenges. Foods. 2022; 11(22):3594. https://doi.org/10.3390/foods11223594
Chicago/Turabian StyleXing, Yilin, Shanxing Gao, Xinyu Zhang, and Jiachen Zang. 2022. "Dietary Heme-Containing Proteins: Structures, Applications, and Challenges" Foods 11, no. 22: 3594. https://doi.org/10.3390/foods11223594
APA StyleXing, Y., Gao, S., Zhang, X., & Zang, J. (2022). Dietary Heme-Containing Proteins: Structures, Applications, and Challenges. Foods, 11(22), 3594. https://doi.org/10.3390/foods11223594





