Rapid Detection of Malathion, Phoxim and Thiram on Orange Surfaces Using Ag Nanoparticle Modified PDMS as Surface-Enhanced Raman Spectroscopy Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis and Characterization of AgNPs
2.3. Preparation of Amino-Functionalized PDMS Film
2.4. Preparation of AgNPs-PDMS SERS Substrate
2.5. Optimization and Evaluation of AgNPs-PDMS SERS Substrate
2.6. Semi-Quantitative Analysis of Three Pesticides and Determination of Intra-Batch and Inter-batch Precision
2.7. Detection of Three Pesticides on Orange Surfaces
2.8. Statistical Analysis
3. Results and Discussion
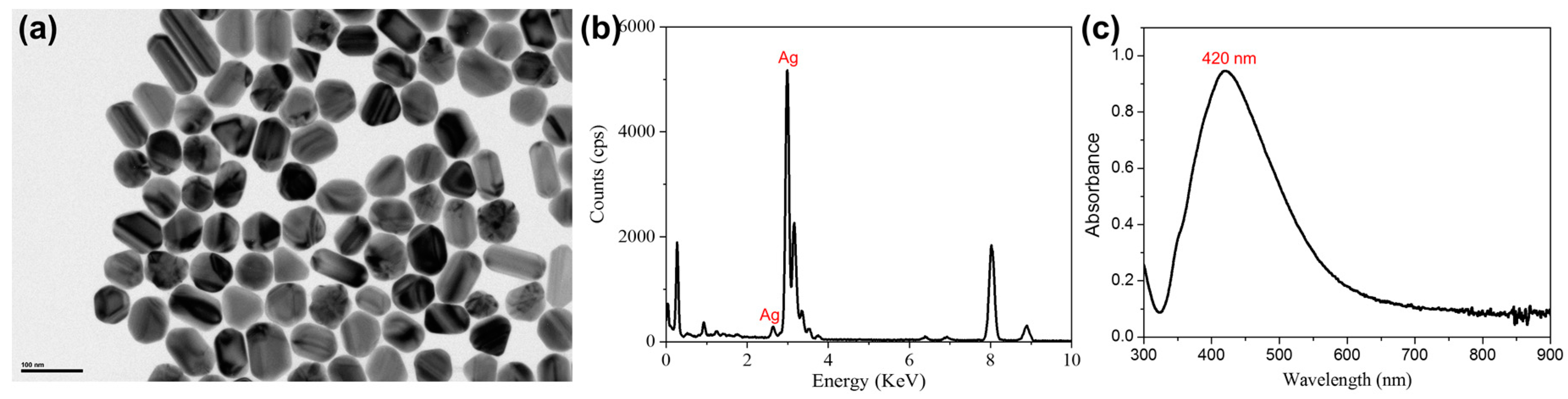
3.1. Characterization of the Synthesized AgNPs
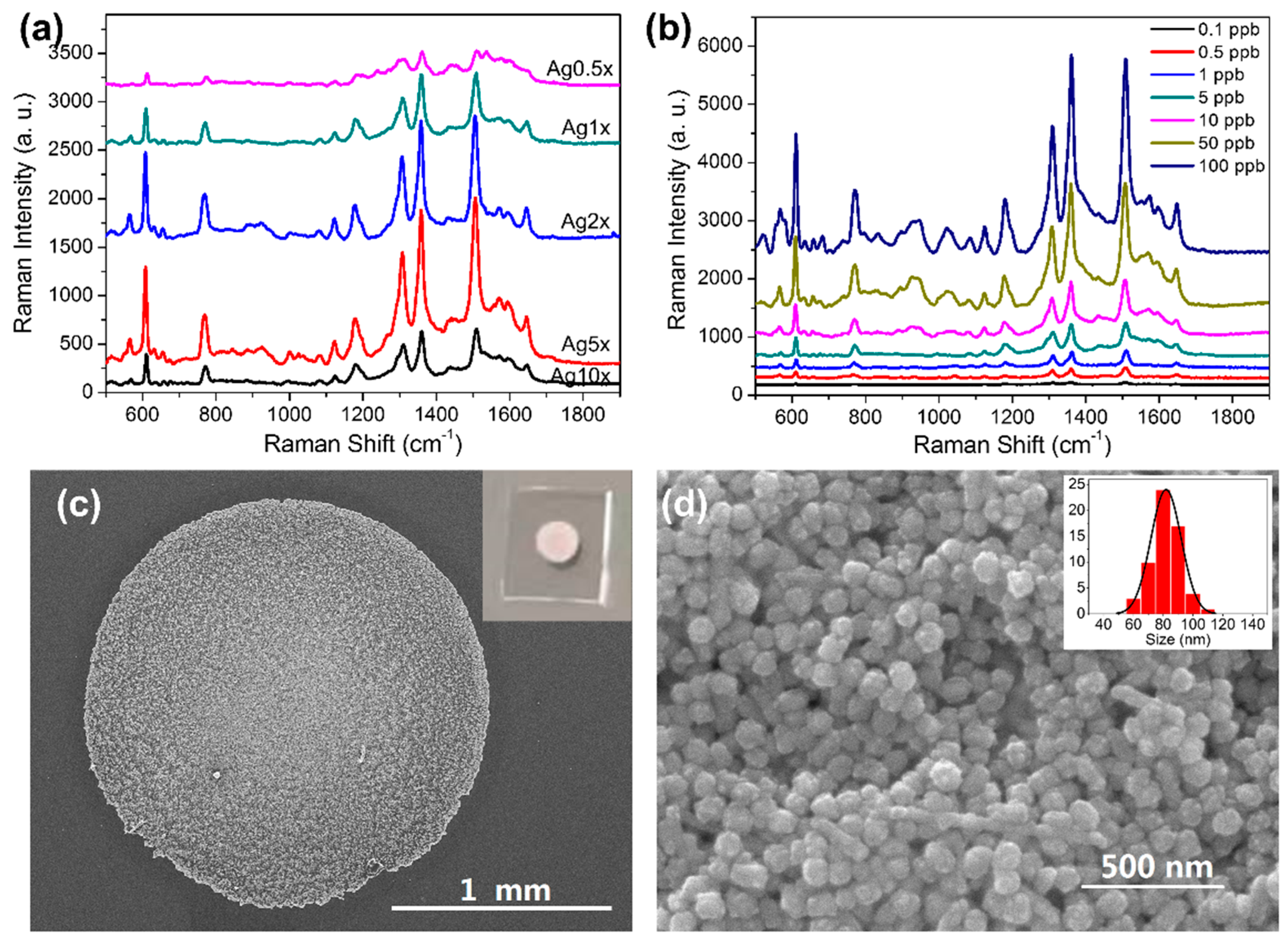
3.2. Optimization and Characterization of AgNPs-PDMS Substrate
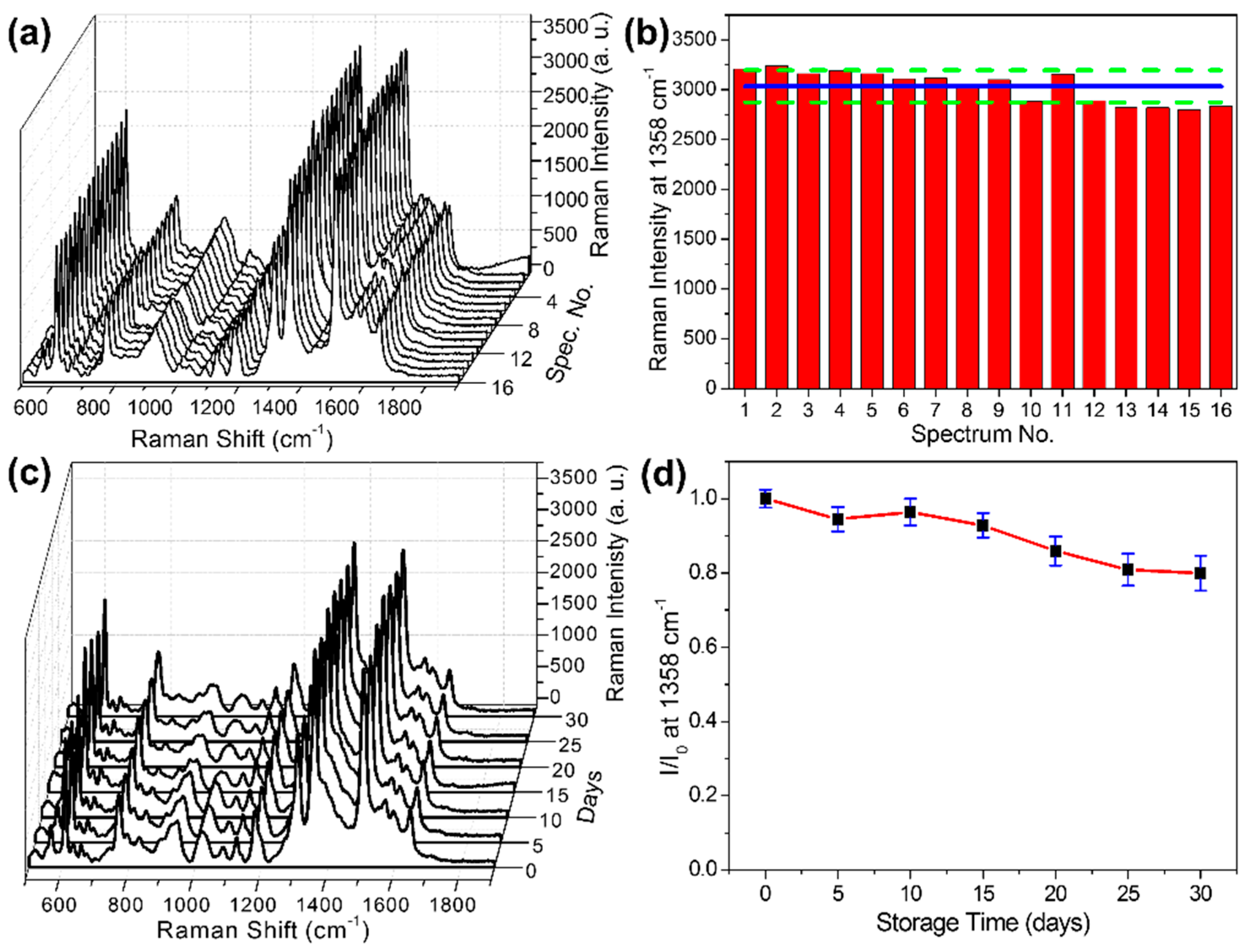
3.3. Evaluation of Signal Uniformity and Stability
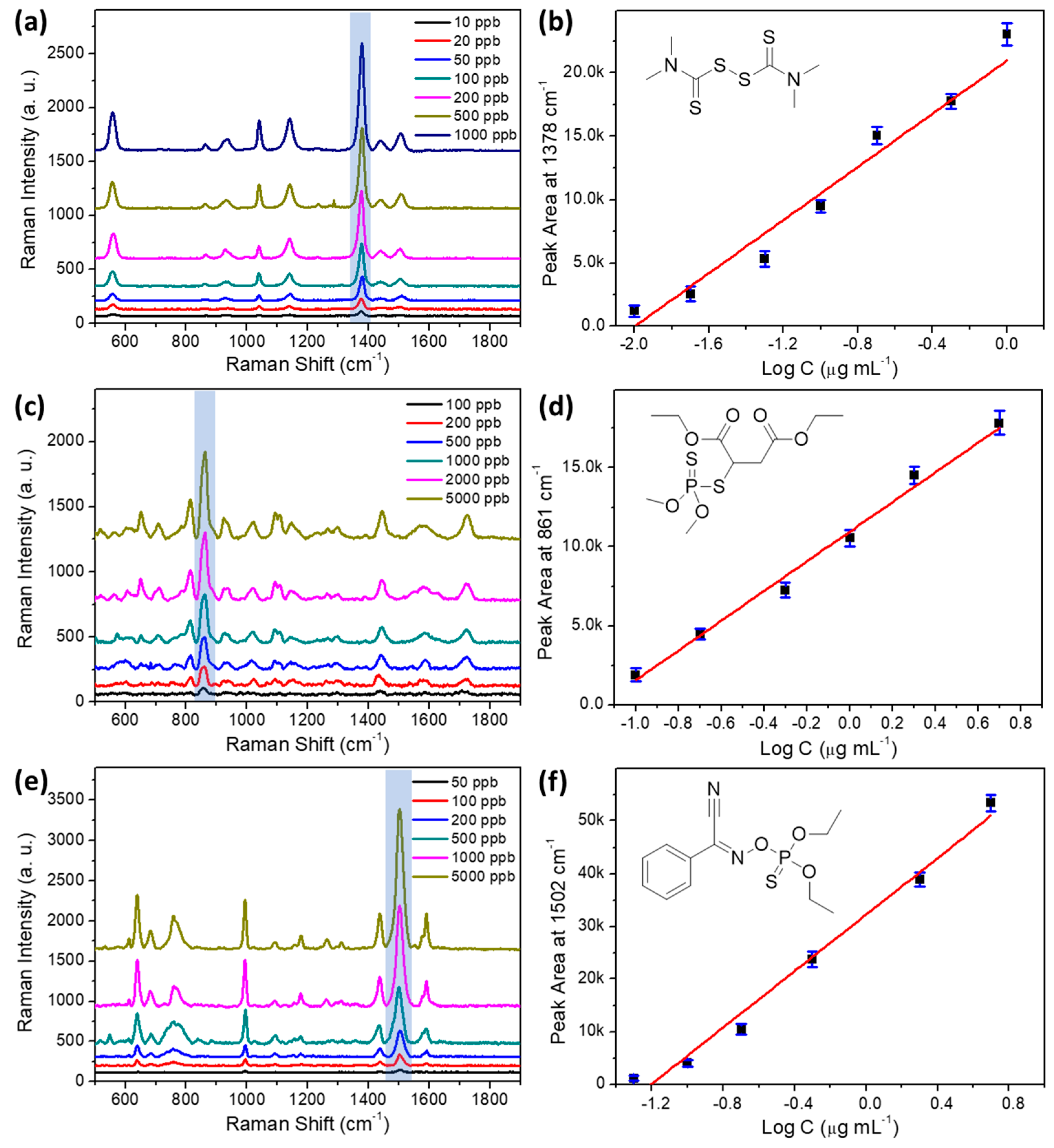
3.4. Detection of Individual Pesticide on AgNPs-PDMS Substrate
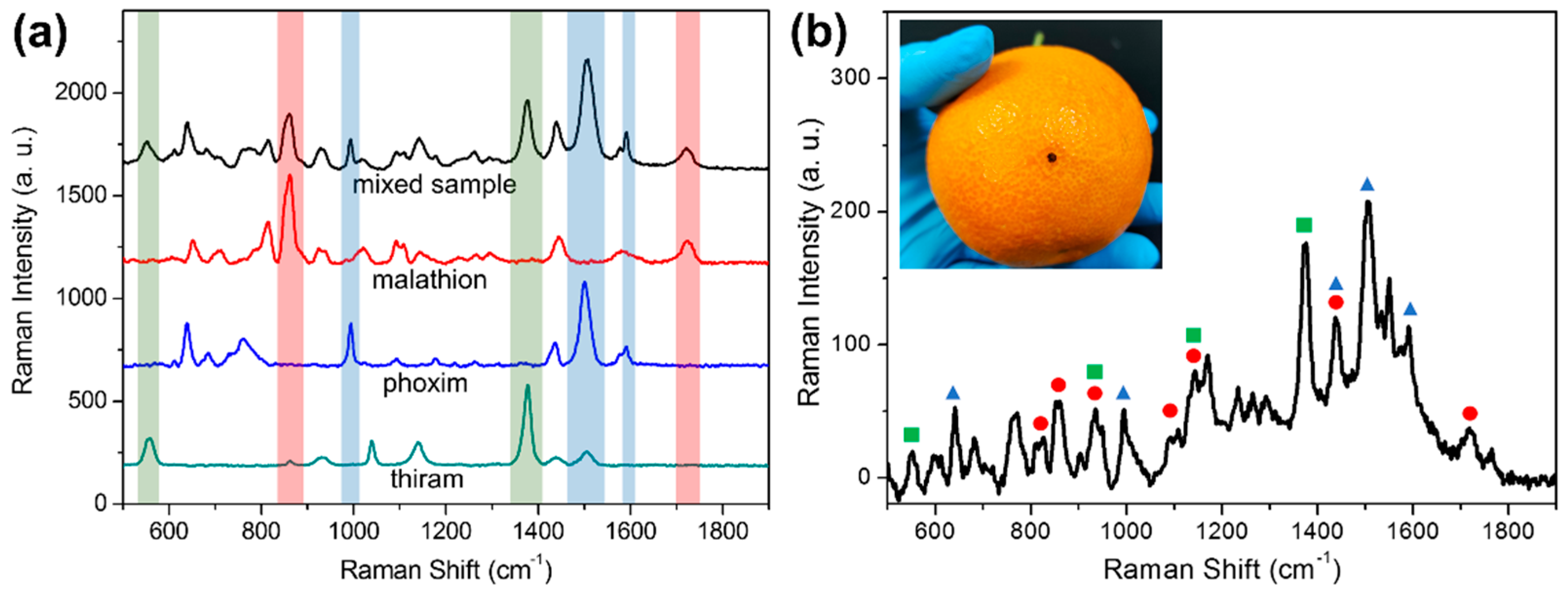
3.5. Detection of Mixed Pesticides on Orange Surface Using AgNPs-PDMS Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bajwa, U.; Sandhu, K.S. Effect of handling and processing on pesticide residues in food- a review. J. Food Sci. Technol. 2014, 51, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.-Y.; Chen, Y.; Wang, S.-Y.; Shi, X.-C.; Herrera-Balandrano, D.D.; Polo, V.; Laborda, P. Peel Diffusion and Antifungal Efficacy of Different Fungicides in Pear Fruit: Structure-Diffusion-Activity Relationships. J. Fungi 2022, 8, 547. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Chen, C.; Yang, G.; Li, Y.; Chen, Z.; Qian, Y. Combined cytotoxic effects of pesticide mixtures present in the Chinese diet on human hepatocarcinoma cell line. Chemosphere 2016, 159, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Mali, H.; Shah, C.; Raghunandan, B.H.; Prajapati, A.S.; Patel, D.H.; Trivedi, U.; Subramanian, R.B. Organophosphate pesticides an emerging environmental contaminant: Pollution, toxicity, bioremediation progress, and remaining challenges. J. Environ. Sci. 2023, 127, 234–250. [Google Scholar] [CrossRef]
- Dara, D.; Drabovich, A.P. Assessment of risks, implications, and opportunities of waterborne neurotoxic pesticides. J. Environ. Sci. 2023, 125, 735–741. [Google Scholar] [CrossRef]
- Bhanti, M.; Taneja, A. Contamination of vegetables of different seasons with organophosphorous pesticides and related health risk assessment in northern India. Chemosphere 2007, 69, 63–68. [Google Scholar] [CrossRef]
- Kulyar, M.F.; Yao, W.; Mo, Q.; Ding, Y.; Zhang, Y.; Gao, J.; Li, K.; Pan, H.; Nawaz, S.; Shahzad, M.; et al. Regulatory Role of Apoptotic and Inflammasome Related Proteins and Their Possible Functional Aspect in Thiram Associated Tibial Dyschondroplasia of Poultry. Animals 2022, 12, 2028. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Zhang, Y.; Qin, D.; Zheng, Z.; Zhu, G.; Lv, Y.; Liu, Z.; Dong, Z.; Liao, X.; et al. The degradation behaviour, residue distribution, and dietary risk assessment of malathion on vegetables and fruits in China by GC-FPD. Food Control 2020, 107, 106754. [Google Scholar] [CrossRef]
- Yang, N.; Zhou, X.; Yu, D.; Jiao, S.; Han, X.; Zhang, S.; Yin, H.; Mao, H. Pesticide residues identification by impedance time-sequence spectrum of enzyme inhibition on multilayer paper-based microfluidic chip. J. Food Process Eng. 2020, 43, e13544. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, Y.; Li, N.; Qian, Y.; Wang, Z.; Liu, Y. A national-scale cumulative exposure assessment of organophosphorus pesticides through dietary vegetable consumption in China. Food Control 2019, 104, 34–41. [Google Scholar] [CrossRef]
- Mirres, A.C.; Silva, B.E.; Tessaro, L.; Galvan, D.; Andrade, J.C.; Aquino, A.; Joshi, N.; Conte-Junior, C.A. Recent Advances in Nanomaterial-Based Biosensors for Pesticide Detection in Foods. Biosensors 2022, 12, 572. [Google Scholar] [CrossRef] [PubMed]
- Hejabri kandeh, S.; Amini, S.; Ebrahimzadeh, H. PVA/Stevia/MIL-88A@AuNPs composite nanofibers as a novel sorbent for simultaneous extraction of eight agricultural pesticides in food and vegetable samples followed by HPLC-UV analysis. Food Chem. 2022, 386, 132734. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-B.; Ying, G.-G.; Kookana, R.S. Rapid multiresidue determination for currently used pesticides in agricultural drainage waters and soils using gas chromatography–mass spectrometry. J. Environ. Sci. Health B 2010, 45, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhu, B.; Hu, X.-p.; Shi, X.-y.; Qi, L.-l.; Liang, P.; Gao, X.-W. Overexpression of PxαE14 Contributing to Detoxification of Multiple Insecticides in Plutella xylostella (L.). J. Agric. Food Chem. 2022, 70, 5794–5804. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kwak, S.-Y.; Sarker, A.; Moon, J.-K.; Kim, J.-E. Optimization of a Multi-Residue Analytical Method during Determination of Pesticides in Meat Products by GC-MS/MS. Foods 2022, 11, 2930. [Google Scholar] [CrossRef]
- Huo, D.; Li, Q.; Zhang, Y.; Hou, C.; Lei, Y. A highly efficient organophosphorus pesticides sensor based on CuO nanowires–SWCNTs hybrid nanocomposite. Sens. Actuators B Chem. 2014, 199, 410–417. [Google Scholar] [CrossRef]
- Luo, R.; Feng, Z.; Shen, G.; Xiu, Y.; Zhou, Y.; Niu, X.; Wang, H. Acetylcholinesterase Biosensor Based On Mesoporous Hollow Carbon Spheres/Core-Shell Magnetic Nanoparticles-Modified Electrode for the Detection of Organophosphorus Pesticides. Sensors 2018, 18, 4429. [Google Scholar] [CrossRef]
- Bedair, H.; Rady, H.A.; Hussien, A.M.; Pandey, M.; Apollon, W.; AlKafaas, S.S.; Ghosh, S. Pesticide Detection in Vegetable Crops Using Enzyme Inhibition Methods: A Comprehensive Review. Food Anal. Methods 2022, 15, 1979–2000. [Google Scholar] [CrossRef]
- Fang, L.; Liao, X.; Jia, B.; Shi, L.; Kang, L.; Zhou, L.; Kong, W. Recent progress in immunosensors for pesticides. Biosens. Bioelectron. 2020, 164, 112255. [Google Scholar] [CrossRef]
- Cheng, N.; Song, Y.; Fu, Q.; Du, D.; Luo, Y.; Wang, Y.; Xu, W.; Lin, Y. Aptasensor based on fluorophore-quencher nano-pair and smartphone spectrum reader for on-site quantification of multi-pesticides. Biosens. Bioelectron. 2018, 117, 75–83. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, B.; Zhu, G.; Zhou, X. Synthesis of silver nanowires as a SERS substrate for the detection of pesticide thiram. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S.; Wu, G.; Aghvami, S.A.; Zhang, Y.; Lin, M. Optimisation using the finite element method of a filter-based microfluidic SERS sensor for detection of multiple pesticides in strawberry. Food Addit. Contam. Part A 2021, 38, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Pu, H.; Sun, D.-W. Recent advances in nanofabrication techniques for SERS substrates and their applications in food safety analysis. Crit. Rev. Food Sci. Nutr. 2018, 58, 2800–2813. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, Y.; Wang, L.; Li, X.; Liu, M.; Li, G. Facile Detection and Quantification of Acetamiprid Using a Portable Raman Spectrometer Combined with Self-Assembled Gold Nanoparticle Array. Chemosensors 2021, 9, 327. [Google Scholar] [CrossRef]
- Ali, A.; Nettey-Oppong, E.E.; Effah, E.; Yu, C.Y.; Muhammad, R.; Soomro, T.A.; Byun, K.M.; Choi, S.H. Miniaturized Raman Instruments for SERS-Based Point-of-Care Testing on Respiratory Viruses. Biosensors 2022, 12, 590. [Google Scholar] [CrossRef]
- Yu, H.; Wang, M.; Cao, J.; She, Y.; Zhu, Y.; Ye, J.; Wang, J.; Lao, S.; Abd El-Aty, A.M. Determination of Dichlorvos in Pears by Surface-Enhanced Raman Scattering (SERS) with Catalysis by Platinum Coated Gold Nanoparticles. Anal. Lett. 2022, 55, 427–437. [Google Scholar] [CrossRef]
- Zhai, W.; You, T.; Ouyang, X.; Wang, M. Recent progress in mycotoxins detection based on surface-enhanced Raman spectroscopy. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1887–1909. [Google Scholar] [CrossRef]
- Han, C.; Zhai, W.; Wang, Y.; Cao, J.; Wang, M. A SERS aptasensor for rapid detection of aflatoxin B1 in coix seed using satellite structured Fe3O4@Au nanocomposites. Food Control 2022, 142, 109228. [Google Scholar] [CrossRef]
- Yan, M.; Chen, G.; She, Y.; Ma, J.; Hong, S.; Shao, Y.; Abd El-Aty, A.M.; Wang, M.; Wang, S.; Wang, J. Sensitive and Simple Competitive Biomimetic Nanozyme-Linked Immunosorbent Assay for Colorimetric and Surface-Enhanced Raman Scattering Sensing of Triazophos. J. Agric. Food Chem. 2019, 67, 9658–9666. [Google Scholar] [CrossRef]
- Li, D.-W.; Zhai, W.-L.; Li, Y.-T.; Long, Y.-T. Recent progress in surface enhanced Raman spectroscopy for the detection of environmental pollutants. Microchim. Acta 2014, 181, 23–43. [Google Scholar] [CrossRef]
- Wu, L.; Dias, A.; Diéguez, L. Surface enhanced Raman spectroscopy for tumor nucleic acid: Towards cancer diagnosis and precision medicine. Biosens. Bioelectron. 2022, 204, 114075. [Google Scholar] [CrossRef] [PubMed]
- Hakonen, A.; Andersson, P.O.; Stenbæk Schmidt, M.; Rindzevicius, T.; Käll, M. Explosive and chemical threat detection by surface-enhanced Raman scattering: A review. Anal. Chim. Acta 2015, 893, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, P.; Su, X.-O. Surface-enhanced Raman spectroscopy for polychlorinated biphenyl detection: Recent developments and future prospects. TrAC Trends Analyt. Chem. 2020, 125, 115836. [Google Scholar] [CrossRef]
- Prikhozhdenko, E.S.; Bratashov, D.N.; Gorin, D.A.; Yashchenok, A.M. Flexible surface-enhanced Raman scattering-active substrates based on nanofibrous membranes. Nano Res. 2018, 11, 4468–4488. [Google Scholar] [CrossRef]
- Xu, K.; Zhou, R.; Takei, K.; Hong, M. Toward Flexible Surface-Enhanced Raman Scattering (SERS) Sensors for Point-of-Care Diagnostics. Adv. Sci. 2019, 6, 1900925. [Google Scholar] [CrossRef] [PubMed]
- Kalachyova, Y.; Erzina, M.; Postnikov, P.; Svorcik, V.; Lyutakov, O. Flexible SERS substrate for portable Raman analysis of biosamples. Appl. Surf. Sci. 2018, 458, 95–99. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Z.; Li, Y.; Wang, C.; Sun, J.; Ye, W.; Wang, X.; Shao, B. Durable and flexible Ag-nanowire-embedded PDMS films for the recyclable swabbing detection of malachite green residue in fruits and fingerprints. Sens. Actuators B Chem. 2021, 347, 130602. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Chang, Y.; Gao, C.; Chen, J.; Zhang, X.; Zhan, J. A 3D spongy flexible nanosheet array for on-site recyclable swabbing extraction and subsequent SERS analysis of thiram. Microchim. Acta 2019, 186, 458. [Google Scholar] [CrossRef]
- Wang, T.-J.; Barveen, N.R.; Liu, Z.-Y.; Chen, C.-H.; Chou, M.-H. Transparent, Flexible Plasmonic Ag NP/PMMA Substrates Using Chemically Patterned Ferroelectric Crystals for Detecting Pesticides on Curved Surfaces. ACS Appl. Mater. Interfaces 2021, 13, 34910–34922. [Google Scholar] [CrossRef]
- Bai, F.; Dong, J.; Qu, J.; Zhang, Z. Construction of flexible, transparent and mechanically robust SERS-active substrate with an efficient spin coating method for rapid in-situ target molecules detection. Nanotechnology 2021, 32, 385501. [Google Scholar] [CrossRef]
- Ma, X.; Xie, J.; Wang, Z.; Zhang, Y. Transparent and flexible AuNSs/PDMS-based SERS substrates for in-situ detection of pesticide residues. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120542. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, P.; Chen, H.; Chang, M.; Lu, P.; Chen, N.; Yuan, Y.; Chen, N.; Zhang, X. Rapid Determination of Mixed Pesticide Residues on Apple Surfaces by Surface-Enhanced Raman Spectroscopy. Foods 2022, 11, 1089. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhong, L.-B.; Liu, Q.; Zhou, X.; Zheng, Y.-M. Polymer induced one-step interfacial self-assembly method for the fabrication of flexible, robust and free-standing SERS substrates for rapid on-site detection of pesticide residues. Nanoscale 2019, 11, 12829–12836. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Cao, Z.; Li, Y.; Li, Z.; Zhang, F.-L.; Gu, Y.; Han, C.; Yang, G.; Qu, L. Highly sensitive SERS substrates with multi-hot spots for on-site detection of pesticide residues. Food Chem. 2022, 381, 132208. [Google Scholar] [CrossRef]
- Li, J.; Yan, H.; Tan, X.; Lu, Z.; Han, H. Cauliflower-Inspired 3D SERS Substrate for Multiple Mycotoxins Detection. Anal. Chem. 2019, 91, 3885–3892. [Google Scholar] [CrossRef]
- Li, D.; Qu, L.; Zhai, W.; Xue, J.; Fossey, J.S.; Long, Y. Facile On-Site Detection of Substituted Aromatic Pollutants in Water Using Thin Layer Chromatography Combined with Surface-Enhanced Raman Spectroscopy. Environ. Sci. Technol. 2011, 45, 4046–4052. [Google Scholar] [CrossRef]
- Sikder, M.; Lead, J.R.; Chandler, G.T.; Baalousha, M. A rapid approach for measuring silver nanoparticle concentration and dissolution in seawater by UV–Vis. Sci. Total Environ. 2018, 618, 597–607. [Google Scholar] [CrossRef]
- Moreno-Martin, G.; León-González, M.E.; Madrid, Y. Simultaneous determination of the size and concentration of AgNPs in water samples by UV–vis spectrophotometry and chemometrics tools. Talanta 2018, 188, 393–403. [Google Scholar] [CrossRef]
- Zhai, W.-L.; Li, D.-W.; Qu, L.-L.; Fossey, J.S.; Long, Y.-T. Multiple depositions of Ag nanoparticles on chemically modified agarose films for surface-enhanced Raman spectroscopy. Nanoscale 2012, 4, 137–142. [Google Scholar] [CrossRef]
- Qu, L.-L.; Li, D.-W.; Xue, J.-Q.; Zhai, W.-L.; Fossey, J.S.; Long, Y.-T. Batch fabrication of disposable screen printed SERS arrays. Lab Chip 2012, 12, 876–881. [Google Scholar] [CrossRef]
- Qu, L.-L.; Geng, Z.-Q.; Wang, W.; Yang, K.-C.; Wang, W.-P.; Han, C.-Q.; Yang, G.-H.; Vajtai, R.; Li, D.-W.; Ajayan, P.M. Recyclable three-dimensional Ag nanorod arrays decorated with O-g-C3N4 for highly sensitive SERS sensing of organic pollutants. J. Hazard. Mater. 2019, 379, 120823. [Google Scholar] [CrossRef] [PubMed]
- Banaee, M.G.; Crozier, K.B. Gold nanorings as substrates for surface-enhanced Raman scattering. Opt. Lett. 2010, 35, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.D.; Glembocki, O.J.; Bezares, F.J.; Kariniemi, M.I.; Niinistö, J.T.; Hatanpää, T.T.; Rendell, R.W.; Ukaegbu, M.; Ritala, M.K.; Prokes, S.M.; et al. Large-area plasmonic hot-spot arrays: Sub-2 nm interparticle separations with plasma-enhanced atomic layer deposition of Ag on periodic arrays of Si nanopillars. Opt. Express 2011, 19, 26056–26064. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Jiang, P.; Cai, Z.; Zhou, R.; Tu, B.; Gao, N.; Chang, G.; He, H.; He, Y. Ag nanocubes monolayer-modified PDMS as flexible SERS substrates for pesticides sensing. Microchim. Acta 2022, 189, 232. [Google Scholar] [CrossRef]
- Picone, A.L.; Rizzato, M.L.; Lusi, A.R.; Romano, R.M. Stamplike flexible SERS substrate for in-situ rapid detection of thiram residues in fruits and vegetables. Food Chem. 2022, 373, 131570. [Google Scholar] [CrossRef]
- Li, L.; Chin, W.S. Rapid fabrication of a flexible and transparent Ag nanocubes@PDMS film as a SERS substrate with high performance. ACS Appl. Mater. Interfaces 2020, 12, 37538–37548. [Google Scholar] [CrossRef]
| Analyte | Raman/cm−1 | SERS/cm−1 | Assignments |
|---|---|---|---|
| thiram | 557 973 | 558 (m) 925 (w) | ν(S−S) ν(CH3N); ν(C=S) |
| 1149 | 1147 (m) | ρ(CH3); ν(C−N) | |
| 1374 1396 1455 | 1384 (s) 1443 (w) 1513 (m) | δs(CH3); ν(C−N) δas(CH3) ν(C−N); δ(CH3); ρ(CH3) | |
| malathion | 716 824 858 1096 | 709 (b, w) 815 (m) 862 (s) 1094 (m) | ν(P=S); ν(P−O) γ(C−H) νs(C−O−C) ν(C−C) |
| 1164 1452 1736 | 1144 (w) 1444 (m) 1726 (m) | ν(P−S) νs(C−O) ν(C=C) | |
| phoxim | 750 999 | 758 (b, m) 995 (m) | ν(P=S) γ(C−H) |
| 1098 1182 | 1092 (w) 1178 (w) | γ(C−H) γ(C−H) | |
| 1302 1446 1555 1597 | 1264 (w) 1437 (m) 1502 (s) 1591 (m) | δs(CH3) νas(CH3) ν(C6H5) ν(C6H5) |
| Parameters | Thiram | Malathion | Phoxim |
|---|---|---|---|
| Characteristic peak for semi-quantitative analysis (cm−1) | 1378 | 861 | 1502 |
| LOD (μg L−1) | 3.62 | 41.46 | 15.69 |
| Linear range (μg L−1) | 10~1000 | 100~5000 | 50~5000 |
| Regression equation | y = 11,226.81x + 21,852.76 | y = 9556.87x + 11,017.77 | y = 26,790.12x + 32,245.99 |
| R2 | 0.9665 | 0.9891 | 0.9805 |
| Intra-batch precision (% RSD) | 4.65 | 3.74 | 3.18 |
| Inter-batch precision (% RSD) | 8.27 | 5.96 | 7.03 |
| Recovery rate (%) * | 96.5 ± 3.3 | 104.6 ± 1.9 | 118.9 ± 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, W.; Cao, M.; Xiao, Z.; Li, D.; Wang, M. Rapid Detection of Malathion, Phoxim and Thiram on Orange Surfaces Using Ag Nanoparticle Modified PDMS as Surface-Enhanced Raman Spectroscopy Substrate. Foods 2022, 11, 3597. https://doi.org/10.3390/foods11223597
Zhai W, Cao M, Xiao Z, Li D, Wang M. Rapid Detection of Malathion, Phoxim and Thiram on Orange Surfaces Using Ag Nanoparticle Modified PDMS as Surface-Enhanced Raman Spectroscopy Substrate. Foods. 2022; 11(22):3597. https://doi.org/10.3390/foods11223597
Chicago/Turabian StyleZhai, Wenlei, Mingshuo Cao, Zhiyong Xiao, Dan Li, and Meng Wang. 2022. "Rapid Detection of Malathion, Phoxim and Thiram on Orange Surfaces Using Ag Nanoparticle Modified PDMS as Surface-Enhanced Raman Spectroscopy Substrate" Foods 11, no. 22: 3597. https://doi.org/10.3390/foods11223597
APA StyleZhai, W., Cao, M., Xiao, Z., Li, D., & Wang, M. (2022). Rapid Detection of Malathion, Phoxim and Thiram on Orange Surfaces Using Ag Nanoparticle Modified PDMS as Surface-Enhanced Raman Spectroscopy Substrate. Foods, 11(22), 3597. https://doi.org/10.3390/foods11223597





