Sorption Isotherms, Glass Transition and Bioactive Compounds of Ingredients Enriched with Soluble Fibre from Orange Pomace
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sorption isotherm
2.3. Glass Transition Temperature
2.4. Colour Analysis
2.5. Texture Analysis
2.6. Bioactive Characterization
2.7. Statistical Analysis
3. Results
3.1. Sorption Behaviour and Glass Transition
3.2. Colour Analysis
3.3. Texture Analysis
3.4. Bioactive Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Keefe, S.J. The Association between Dietary Fibre Deficiency and High-Income Lifestyle-Associated Diseases: Burkitt’s Hypothesis Revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996. [Google Scholar] [CrossRef]
- Chau, C.F.; Huang, Y.L. Comparison of the Chemical Composition and Physicochemical Properties of Different Fibers Prepared from the Peel of Citrus sinensis, L. Cv. Liucheng. J. Agric. Food Chem. 2003, 51, 2615–2618. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary Fibre in Foods: A Review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [Green Version]
- de Moraes Crizel, T.; Jablonski, A.; de Oliveira Rios, A.; Rech, R.; Hickmann Flôres, S. Dietary Fiber from Orange Byproducts as a Potential Fat Replacer. LWT Food Sci. Technol. 2013, 53, 9–14. [Google Scholar] [CrossRef]
- Huang, L.; Liu, J.; Addy, M.; Ding, B.; Cheng, Y.; Peng, P.; Wang, Y.; Liu, Y.; Chen, P.; Ruan, R. Physicochemical and Emulsifying Properties of Orange Fibers Stabilized Oil-in-Water Emulsions. LWT Food Sci. Technol. 2020, 133, 110054. [Google Scholar] [CrossRef]
- Quiles, A.; Campbell, G.M.; Struck, S.; Rohm, H.; Hernando, I. Fiber from Fruit Pomace: A Review of Applications in Cereal-Based Products. Food Rev. Int. 2018, 34, 162–181. [Google Scholar] [CrossRef]
- Bussolo de Souza, C.; Jonathan, M.; Isay Saad, S.M.; Schols, H.A.; Venema, K. Characterization and in Vitro Digestibility of By-Products from Brazilian Food Industry: Cassava Bagasse, Orange Bagasse and Passion Fruit Peel. Bioact. Carbohydr. Diet. Fibre 2018, 16, 90–99. [Google Scholar] [CrossRef]
- Hervalejo, A.; Arjona-López, J.M.; Ordóñez-Díaz, J.L.; Romero-Rodríguez, E.; Calero-Velázquez, R.; Moreno-Rojas, J.M.; Arenas-Arenas, F.J. Influence of Harvesting Season on Morphological and Sensory Quality, Bioactive Compounds and Antioxidant Activity of Three Late-Season Orange Cultivars ‘Barberina’, ‘Valencia Midknight’ and ‘Valencia Delta Seedless’. Agronomy 2021, 11, 673. [Google Scholar] [CrossRef]
- Negro, V.; Ruggeri, B.; Fino, D.; Tonini, D. Life Cycle Assessment of Orange Peel Waste Management. Resour. Conserv. Recycl. 2017, 127, 148–158. [Google Scholar] [CrossRef]
- de Moura, F.A.; Macagnan, F.T.; dos Santos, L.R.; Bizzani, M.; de Oliveira Petkowicz, C.L.; da Silva, L.P. Characterization and Physicochemical Properties of Pectins Extracted from Agroindustrial By-Products. J. Food Sci. Technol. 2017, 54, 3111–3117. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejada-Ortigoza, V.; García-Amezquita, L.E.; Serna-Saldívar, S.O.; Welti-Chanes, J. The Dietary Fiber Profile of Fruit Peels and Functionality Modifications Induced by High Hydrostatic Pressure Treatments. Food Sci. Technol. Int. 2017, 23, 396–402. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Yuan, F.; Fan, R.; Gao, Y. Preparation and Physicochemical Properties of Soluble Dietary Fiber from Orange Peel Assisted by Steam Explosion and Dilute Acid Soaking. Food Chem. 2015, 185, 90–98. [Google Scholar] [CrossRef]
- Karaman, E.; Yılmaz, E.; Tuncel, N.B. Physicochemical, Microstructural and Functional Characterization of Dietary Fibers Extracted from Lemon, Orange and Grapefruit Seeds Press Meals. Bioact. Carbohydr. Diet. Fibre 2017, 11, 9–17. [Google Scholar] [CrossRef]
- Cameron, R.G.; Chau, H.K.; Manthey, J.A. Continuous Process for Enhanced Release and Recovery of Pectic Hydrocolloids and Phenolics from Citrus Biomass. J. Chem. Technol. Biotechnol. 2015, 91, 2597–2606. [Google Scholar] [CrossRef]
- Cameron, R.G.; Chau, H.K.; Hotchkiss, A.T.; Manthey, J.A. Release and Recovery of Pectic Hydrocolloids and Phenolics from Culled Citrus Fruits. Food Hydrocoll. 2017, 72, 52–61. [Google Scholar] [CrossRef]
- Huang, Y.L.; Ma, Y.S. The Effect of Extrusion Processing on the Physiochemical Properties of Extruded Orange Pomace. Food Chem. 2016, 192, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pirotto, C.; Cozzano, S.; Hernando, I.; Arcia, P. Different Green Extraction Technologies for Soluble Dietary Fibre Extraction from Orange By-Product. Int. J. Food Sci. Technol. 2022, 1–8. [Google Scholar] [CrossRef]
- Gutiérrez Barrutia, M.B.; Curutchet, A.; Arcia, P.; Cozzano, S. New Functional Ingredient from Orange Juice Byproduct through a Green Extraction Method. J. Food Process. Preserv. 2019, 43, e13934. [Google Scholar] [CrossRef]
- Mutlu, C.; Koç, A.; Erbaş, M. Some Physical Properties and Adsorption Isotherms of Vacuum-Dried Honey Powder with Different Carrier Materials. LWT 2020, 134, 110166. [Google Scholar] [CrossRef]
- Mosquera, L.H.; Moraga, G.; Martínez-Navarrete, N. Effect of Maltodextrin on the Stability of Freeze-Dried Borojó (Borojoa patinoi Cuatrec.) Powder. J. Food Eng. 2010, 97, 72–78. [Google Scholar] [CrossRef]
- Mosquera, L.H.; Moraga, G.; de Córdoba, P.F.; Martínez-Navarrete, N. Water Content-Water Activity-Glass Transition Temperature Relationships of Spray-Dried Borojó as Related to Changes in Color and Mechanical Properties. Food Biophys. 2011, 6, 397–406. [Google Scholar] [CrossRef]
- Moraga, G.; Igual, M.; García-Martínez, E.; Mosquera, L.H.; Martínez-Navarrete, N. Effect of Relative Humidity and Storage Time on the Bioactive Compounds and Functional Properties of Grapefruit Powder. J. Food Eng. 2012, 112, 191–199. [Google Scholar] [CrossRef]
- Telis, V.R.N.; Martínez-Navarrete, N. Collapse and Color Changes in Grapefruit Juice Powder as Affected by Water Activity, Glass Transition, and Addition of Carbohydrate Polymers. Food Biophys. 2009, 4, 83–93. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.; Hong, Y.K.; Qu, Z.; Sablani, S.S. State Diagram, Water Sorption Isotherms and Color Stability of Pumpkin (Cucurbita pepo L.). J. Food Eng. 2020, 273, 109820. [Google Scholar] [CrossRef]
- Gabas, A.L.; Telis, V.R.N.; Sobral, P.J.A.; Telis-Romero, J. Effect of Maltodextrin and Arabic Gum in Water Vapor Sorption Thermodynamic Properties of Vacuum Dried Pineapple Pulp Powder. J. Food Eng. 2007, 82, 246–252. [Google Scholar] [CrossRef]
- Hawa, L.C.; Ubaidillah, U.; Damayanti, R.; Hendrawan, Y. Moisture Sorption Isotherms of Modified Cassava Flour during Drying and Storage. Heat Mass Transf. Und Stoffuebertragung 2020, 56, 2389–2396. [Google Scholar] [CrossRef]
- Flores-Ramírez, A.; Grajales-Lagunes, A.; Abud-Archila, M.; Ruiz-Cabrera, M.A. Effect of Maltodextrin Weight Fraction on the Amorphous State and Critical Storage Conditions of Freeze-Dried Juices. Int. J. Food Sci. Technol. 2022, 57, 1–10. [Google Scholar] [CrossRef]
- González, C.M.; Llorca, E.; Quiles, A.; Hernando, I.; Moraga, G. Water Sorption and Glass Transition in Freeze-Dried Persimmon Slices. Effect on Physical Properties and Bioactive Compounds. LWT 2020, 130, 109633. [Google Scholar] [CrossRef]
- Cruz-Tirado, J.P.; Martins, J.P.; Olmos, B.D.F.; Condotta, R.; Kurozawa, L.E. Impact of Glass Transition on Chemical Properties, Caking and Flowability of Soymilk Powder during Storage. Powder Technol. 2021, 386, 20–29. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity Fixed Points of Binary Saturated Aqueous Solutions. J. Res. Natl. Bur. Stand. A Phys. Chem. 1977, 81, 89–96. [Google Scholar] [CrossRef]
- Shah, B.R.; Zhang, C.; Li, Y.; Li, B. Bioaccessibility and Antioxidant Activity of Curcumin after Encapsulated by Nano and Pickering Emulsion Based on Chitosan-Tripolyphosphate Nanoparticles. Food Res. Int. 2016, 89, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols as Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van Der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Kaderides, K.; Goula, A.M. Development and Characterization of a New Encapsulating Agent from Orange Juice By-Products. Food Res. Int. 2017, 100, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Roos, Y.H. Water and Phase Transitions. In Phase Transitions in Foods; Academic Press: Cambridge, MA, USA, 1995; pp. 73–107. [Google Scholar] [CrossRef]
- Telis, V.R.N.; Martínez-Navarrete, N. Application of Compression Test in Analysis of Mechanical and Color Changes in Grapefruit Juice Powder as Related to Glass Transition and Water Activity. LWT Food Sci. Technol. 2010, 43, 744–751. [Google Scholar] [CrossRef]
- Stępień, A.; Witczak, M.; Witczak, T. Moisture Sorption Characteristics of Food Powders Containing Freeze Dried Avocado, Maltodextrin and Inulin. Int. J. Biol. Macromol. 2020, 149, 256–261. [Google Scholar] [CrossRef]
- Roos, Y.H. Water activity and physical state effects on amorphous food stability. J. Food Process. Preserv. 1993, 16, 433–447. [Google Scholar] [CrossRef]
- Obón, J.M.; Castellar, M.R.; Alacid, M.; Fernández-López, J.A. Production of a Red-Purple Food Colorant from Opuntia Stricta Fruits by Spray Drying and Its Application in Food Model Systems. J. Food Eng. 2009, 90, 471–479. [Google Scholar] [CrossRef]
- Ling, H.I.; Birch, J.; Lim, M. The Glass Transition Approach to Determination of Drying Protocols for Colour Stability in Dehydrated Pear Slices. Int. J. Food Sci. Technol. 2005, 40, 921–927. [Google Scholar] [CrossRef]
- Venir, E.; Munari, M.; Tonizzo, A.; Maltini, E. Structure Related Changes during Moistening of Freeze Dried Apple Tissue. J. Food Eng. 2007, 81, 27–32. [Google Scholar] [CrossRef]
- Li, R.; Lin, D.; Roos, Y.H.; Miao, S. Glass Transition, Structural Relaxation and Stability of Spray-Dried Amorphous Food Solids: A Review. Dry. Technol. 2019, 37, 287–300. [Google Scholar] [CrossRef]
- Maldonado-Astudillo, Y.I.; Jiménez-Hernández, J.; Arámbula-Villa, G.; Flores-Casamayor, V.; Álvarez-Fitz, P.; Ramírez-Ruano, M.; Salazar, R. Effect of Water Activity on Extractable Polyphenols and Some Physical Properties of Hibiscus sabdariffa L. Calyces. J. Food Meas. Charact. 2019, 13, 687–696. [Google Scholar] [CrossRef]
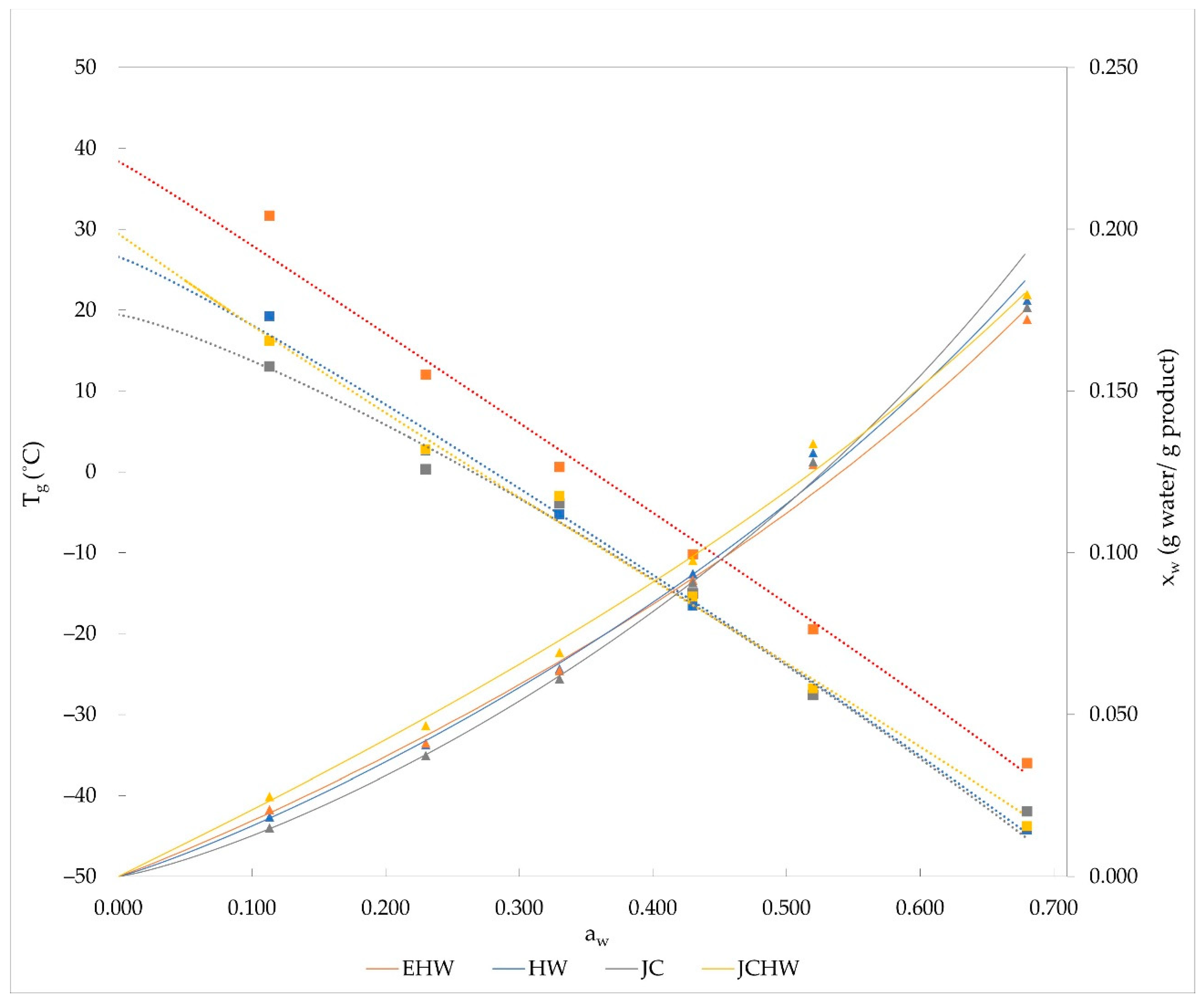
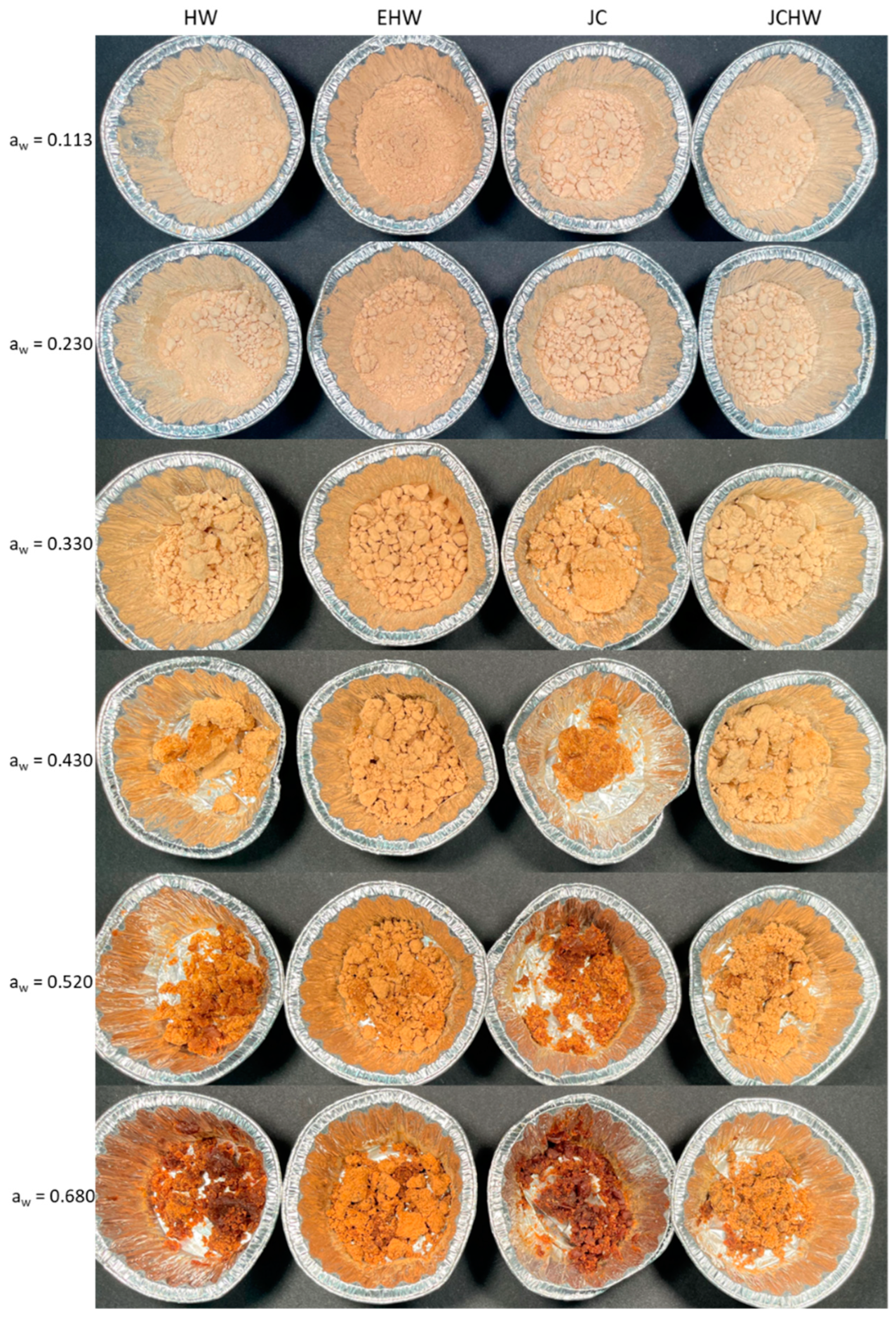
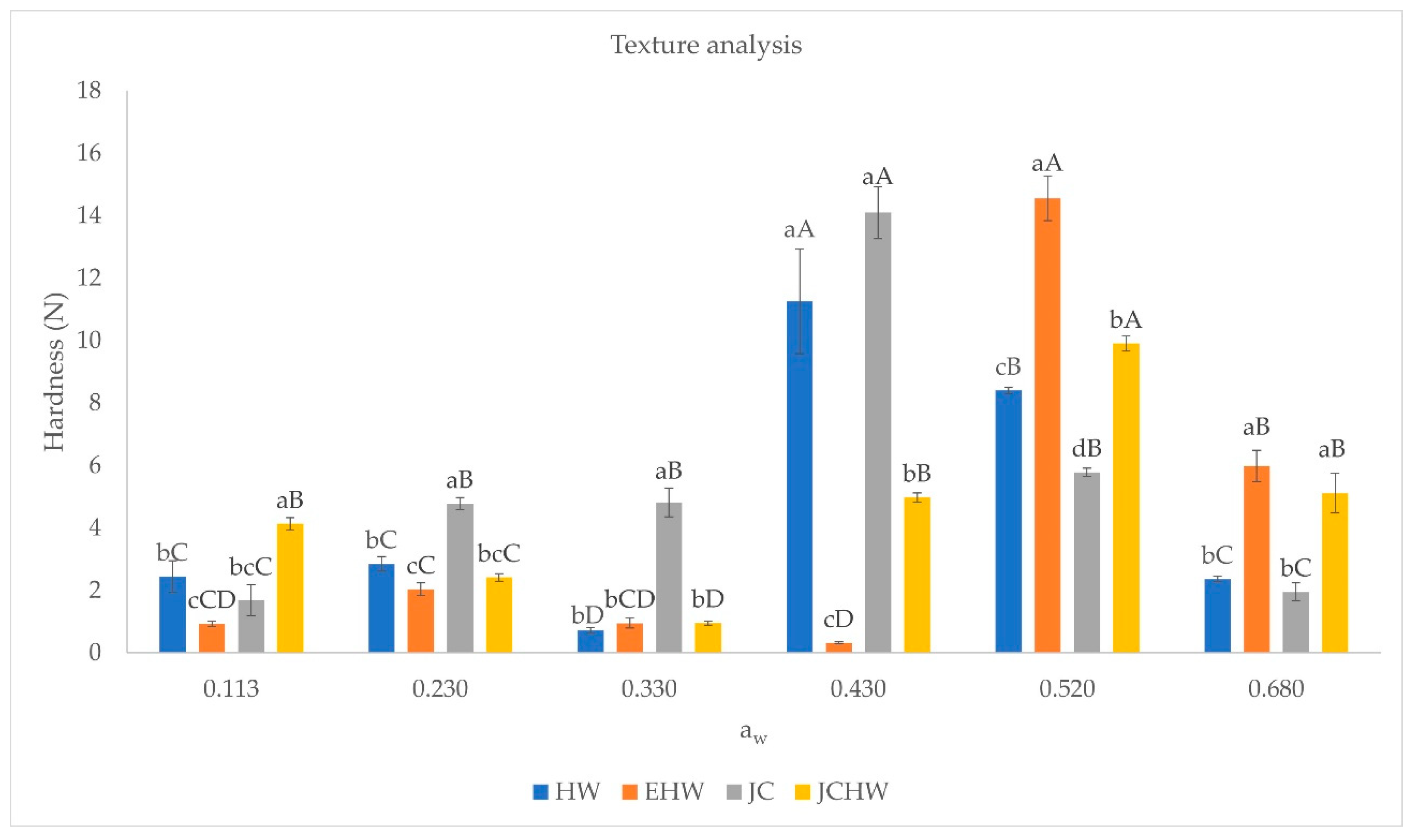

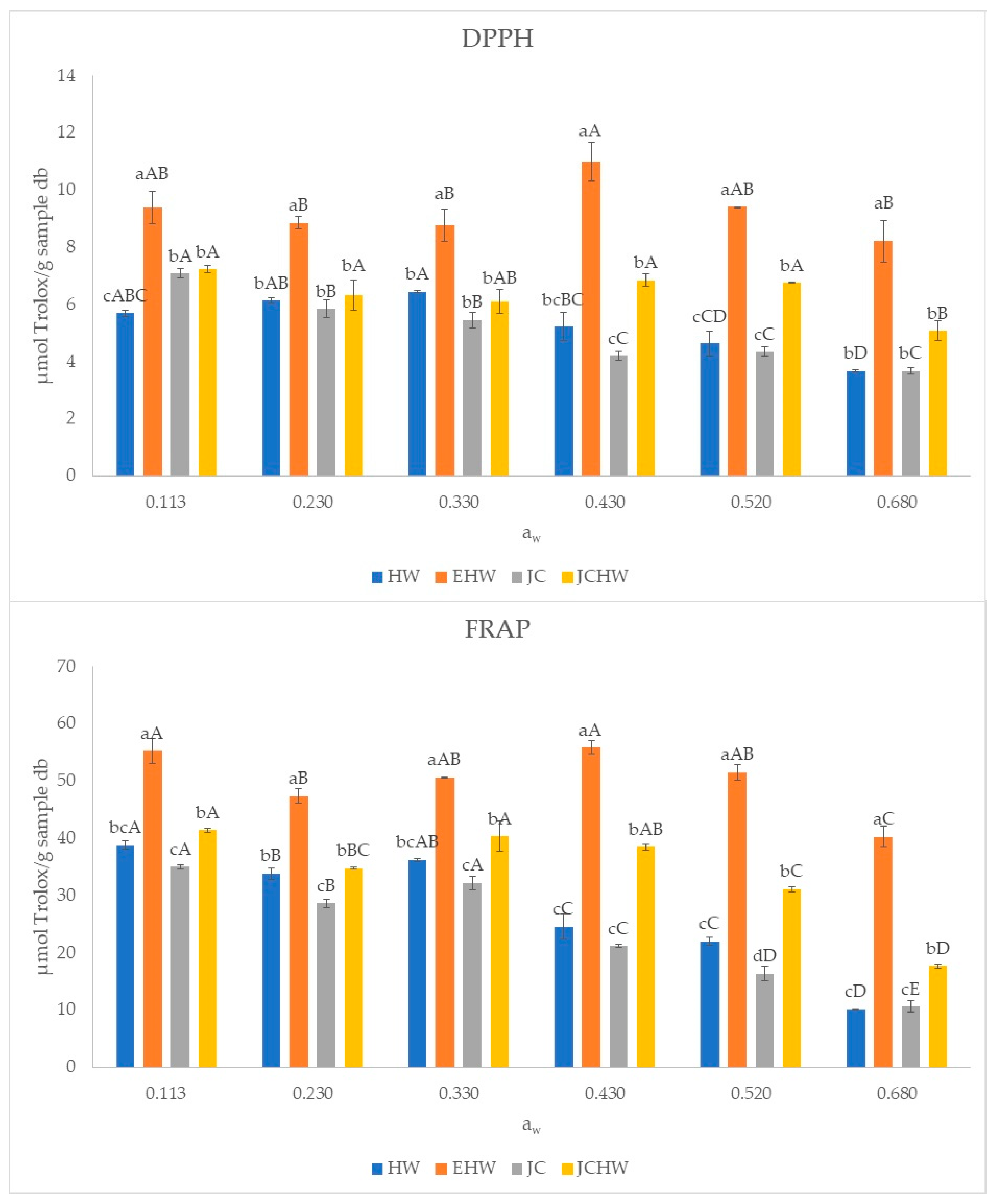
| HW | EHW | JC | JCHW | ||
|---|---|---|---|---|---|
| BET | wo (g water/g solids) | 0.121 | 0.098 | 0.158 | 0.094 |
| C | 1.240 | 1.785 | 0.756 | 2.356 | |
| R2 | 0.976 | 0.915 | 0.811 | 0.947 | |
| Henderson | f | −1.519 | −1.571 | −1.440 | −1.666 |
| n | 0.895 | 0.952 | 0.833 | 1.012 | |
| R2 | 0.997 | 0.996 | 0.997 | 0.995 | |
| Caurie | r | 72.509 | 56.746 | 97.413 | 45.045 |
| ws (g water/g solids) | 0.053 | 0.054 | 0.050 | 0.056 | |
| R2 | 0.962 | 0.970 | 0.953 | 0.975 |
| Gordon and Taylor Model | Critical Values at 20 °C | Critical Values at 5 °C | |||||
|---|---|---|---|---|---|---|---|
| Tg (as) | k | R2 | CWC | CWA | CWC | CWA | |
| HW | 26.57 | 3.48 | 0.986 | 0.0120 | 0.080 | 0.0424 | 0.232 |
| EHW | 38.36 | 3.64 | 0.970 | 0.0315 | 0.173 | 0.0614 | 0.309 |
| JC | 19.43 | 3.01 | 0.989 | - | - | 0.0331 | 0.208 |
| JCHW | 29.40 | 3.52 | 0.988 | 0.0169 | 0.082 | 0.0471 | 0.222 |
| 0.113 | 0.230 | 0.330 | 0.430 | 0.520 | 0.680 | ||
|---|---|---|---|---|---|---|---|
| L* | HW | 82.39 ± 0.14 Aa | 81.09 ± 0.21 Aa | 77.02 ± 0.20 Ba | 61.88 ± 0.84 Cb | 39.83 ± 0.27 Db | 34.73 ± 0.83 Eb |
| EHW | 74.74 ± 0.04 Ad | 72.98 ± 0.24 Bc | 69.33 ± 0.17 Cc | 61.81 ± 0.56 Db | 54.64 ± 0.76 Ea | 39.54 ± 0.71 Fa | |
| JC | 80.16 ± 0.05 Ac | 79.38 ± 0.14 Ab | 66.98 ± 0.67 Bd | 54.51 ± 0.64 Cc | 36.23 ± 0.45 Dc | 32.54 ± 0.41 Ec | |
| JCHW | 81.18 ± 0.12 Ab | 79.43 ± 0.20 Ab | 75.66 ± 0.30 Bb | 68.36 ± 0.31 Cd | 53.98 ± 1.69 Da | 35.57 ± 1.19 Eb | |
| C* | HW | 20.90 ± 0.14 Dc | 22.07 ± 0.13 Cc | 25.06 ± 0.03 Bb | 26.32 ± 0.18 Aa | 20.54 ± 0.18 Dc | 18.56 ± 0.45 Ea |
| EHW | 22.58 ± 0.05 Da | 23.43 ± 0.17 Ca | 25.45 ± 0.06 Ab | 24.97 ± 0.41 ABb | 24.61 ± 0.24 Ba | 17.24 ± 0.15 Eb | |
| JC | 22.13 ± 0.06 Cb | 22.78 ± 0.02 Cb | 26.35 ± 0.43 Aa | 24.59 ± 0.55 Bbc | 19.38 ± 0.32 Dd | 16.60 ± 0.55 Eb | |
| JCHW | 20.00 ± 0.10 Cd | 21.09 ± 0.10 Cd | 23.37 ± 0.06 ABc | 24.15 ± 0.16 Ac | 22.79 ± 0.54 Bb | 15.77 ± 0.70 Ec | |
| h* | HW | 91.61 ± 0.08 Ab | 90.25 ± 0.12 Ab | 87.87 ± 0.13 Ba | 82.09 ± 0.48 Cb | 70.95 ± 0.72 Dc | 68.79 ± 1.25 Ea |
| EHW | 81.82 ± 0.11 Ad | 83.71 ± 0.12 Bd | 81.61 ± 0.23 Cc | 78.44 ± 0.07 Dc | 74.47 ± 0.23 Eb | 66.27 ± 0.24 Fb | |
| JC | 90.64 ± 0.04 Ac | 89.73 ± 0.04 Ac | 83.96 ± 0.37 Bb | 77.98 ± 0.85 Cc | 68.13 ± 0.68 Dd | 66.84 ± 0.20 Eb | |
| JCHW | 91.81 ± 0.12 Aa | 90.63 ± 0.04 Ba | 88.10 ± 0.31 Ca | 84.26 ± 0.33 Da | 77.85 ± 0.13 Ea | 67.18 ± 0.43 Fb | |
| ΔE* | HW | - | 1.84 ± 0.24 Ea | 6.97 ± 0.15 Db | 21.57 ± 0.87 Cb | 43.21 ± 0.30 Ba | 48.35 ± 0.89 Ab |
| EHW | - | 2.02 ± 0.18 Ea | 6.28 ± 0.16 Dc | 13.42 ± 0.47 Cc | 20.65 ± 0.74 Bc | 36.17 ± 0.69 Ac | |
| JC | - | 1.08 ± 0.10 Eb | 14.10 ± 0.46 Da | 26.27 ± 0.63 Ca | 44.75 ± 0.49 Ba | 48.58 ± 0.44 Aa | |
| JCHW | - | 2.11 ± 0.22 Ea | 6.62 ± 0.30 Db | 13.72 ± 0.43 Cc | 27.84 ± 1.60 Bb | 46.50 ± 1.15 Ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Pirotto, C.; Moraga, G.; Hernando, I.; Cozzano, S.; Arcia, P. Sorption Isotherms, Glass Transition and Bioactive Compounds of Ingredients Enriched with Soluble Fibre from Orange Pomace. Foods 2022, 11, 3615. https://doi.org/10.3390/foods11223615
Perez-Pirotto C, Moraga G, Hernando I, Cozzano S, Arcia P. Sorption Isotherms, Glass Transition and Bioactive Compounds of Ingredients Enriched with Soluble Fibre from Orange Pomace. Foods. 2022; 11(22):3615. https://doi.org/10.3390/foods11223615
Chicago/Turabian StylePerez-Pirotto, Claudia, Gemma Moraga, Isabel Hernando, Sonia Cozzano, and Patricia Arcia. 2022. "Sorption Isotherms, Glass Transition and Bioactive Compounds of Ingredients Enriched with Soluble Fibre from Orange Pomace" Foods 11, no. 22: 3615. https://doi.org/10.3390/foods11223615
APA StylePerez-Pirotto, C., Moraga, G., Hernando, I., Cozzano, S., & Arcia, P. (2022). Sorption Isotherms, Glass Transition and Bioactive Compounds of Ingredients Enriched with Soluble Fibre from Orange Pomace. Foods, 11(22), 3615. https://doi.org/10.3390/foods11223615






