Simultaneous Determination of Pyrethroid Insecticides in Foods of Animal Origins Using the Modified QuEChERS Method and Gas Chromatography-Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumental Conditions
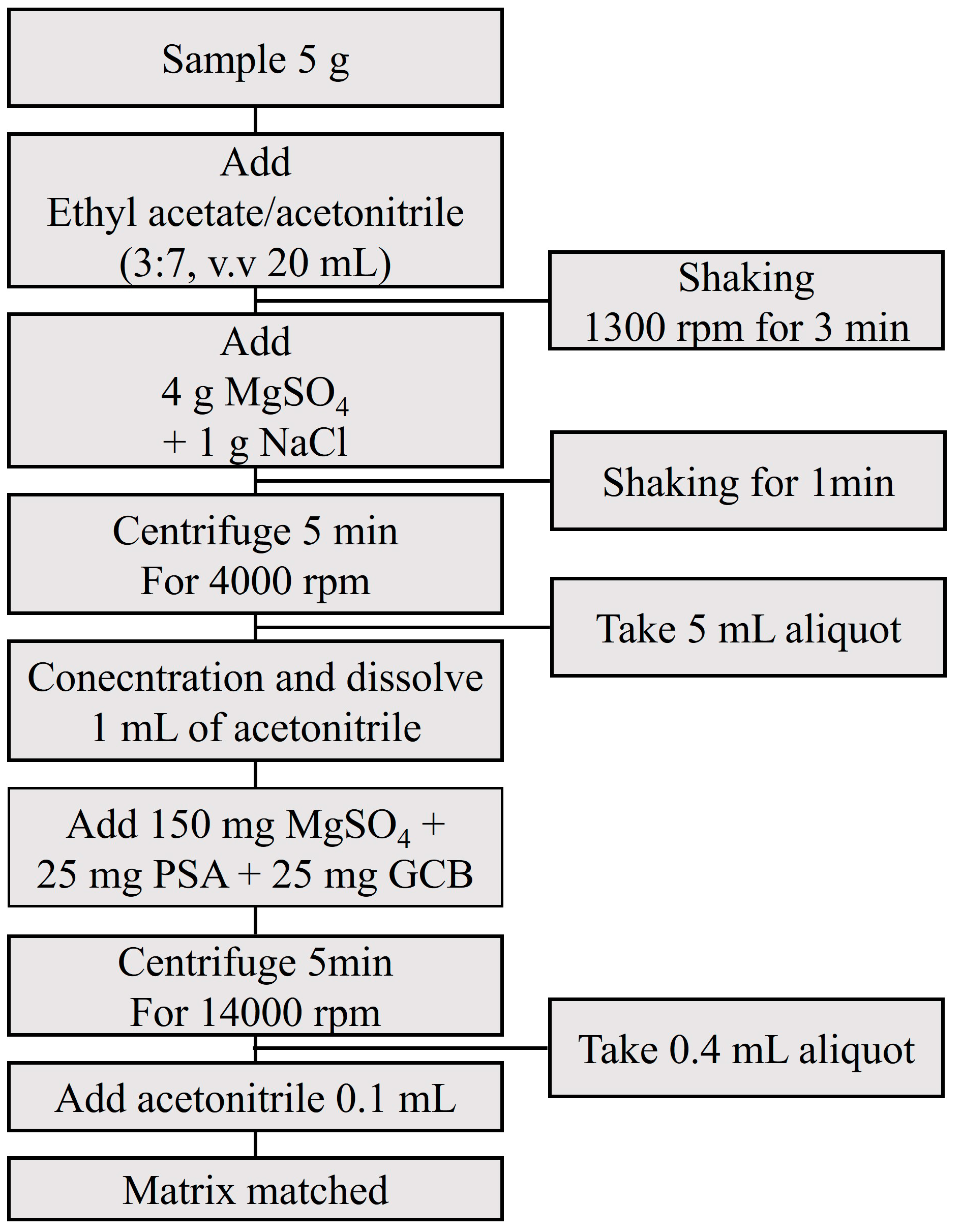
2.3. Sample Preparation: Extraction & Purification
2.4. Method Validation
3. Results and Discussion
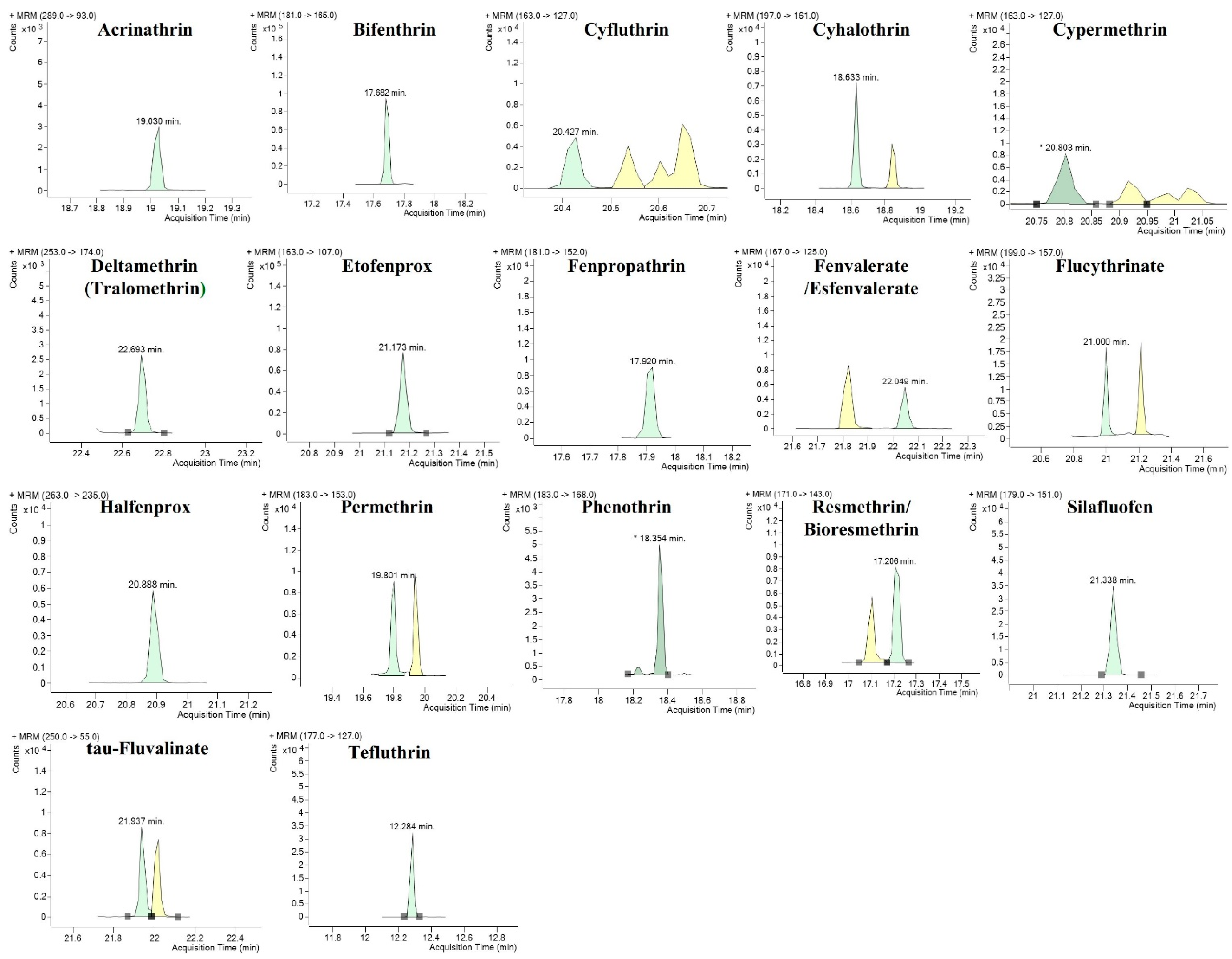
3.1. Multiple Reaction Monitoring Optimization
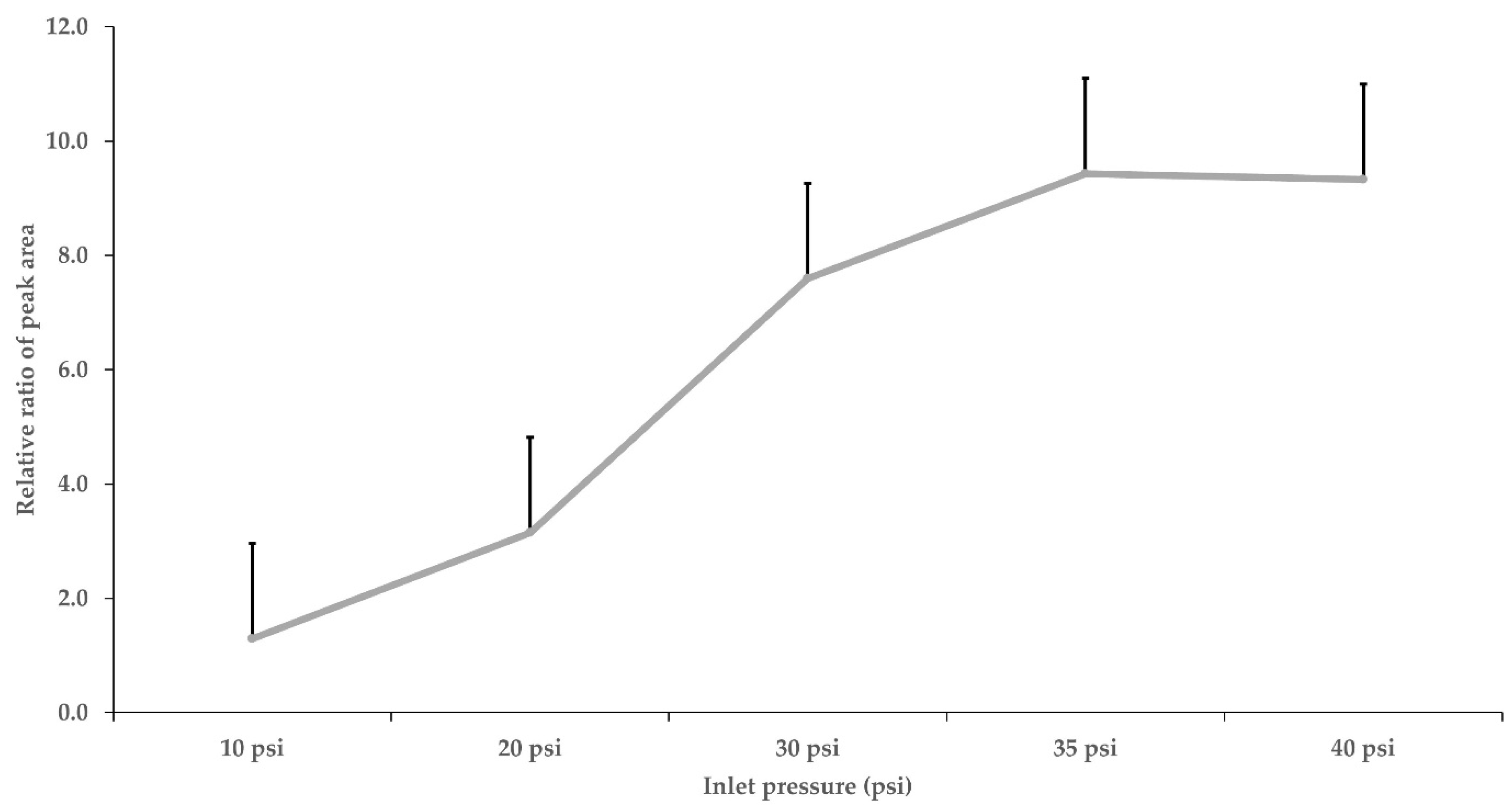
3.2. Instrumental Conditions
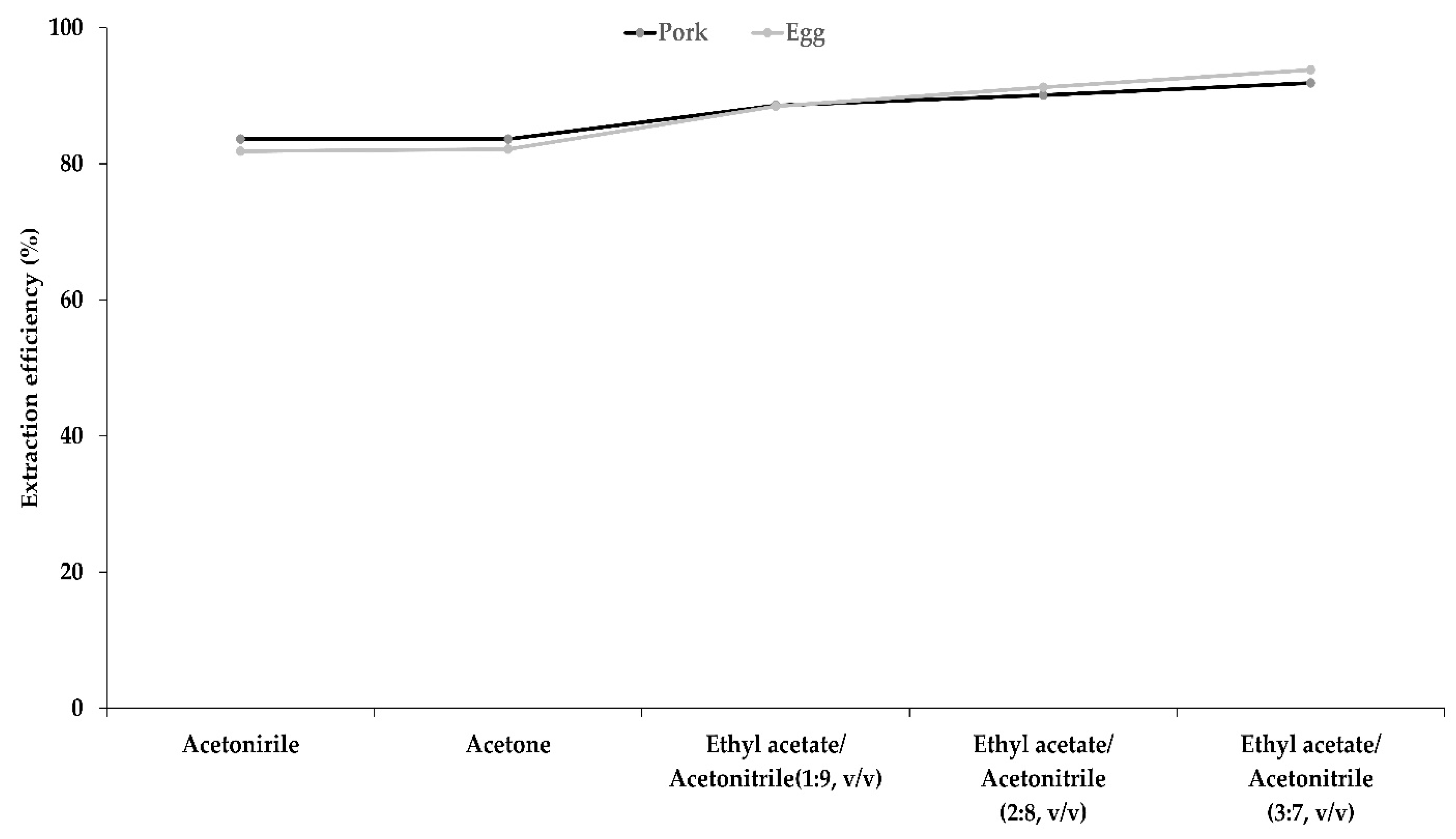
3.3. Optimization of the Extraction Solution and Salts
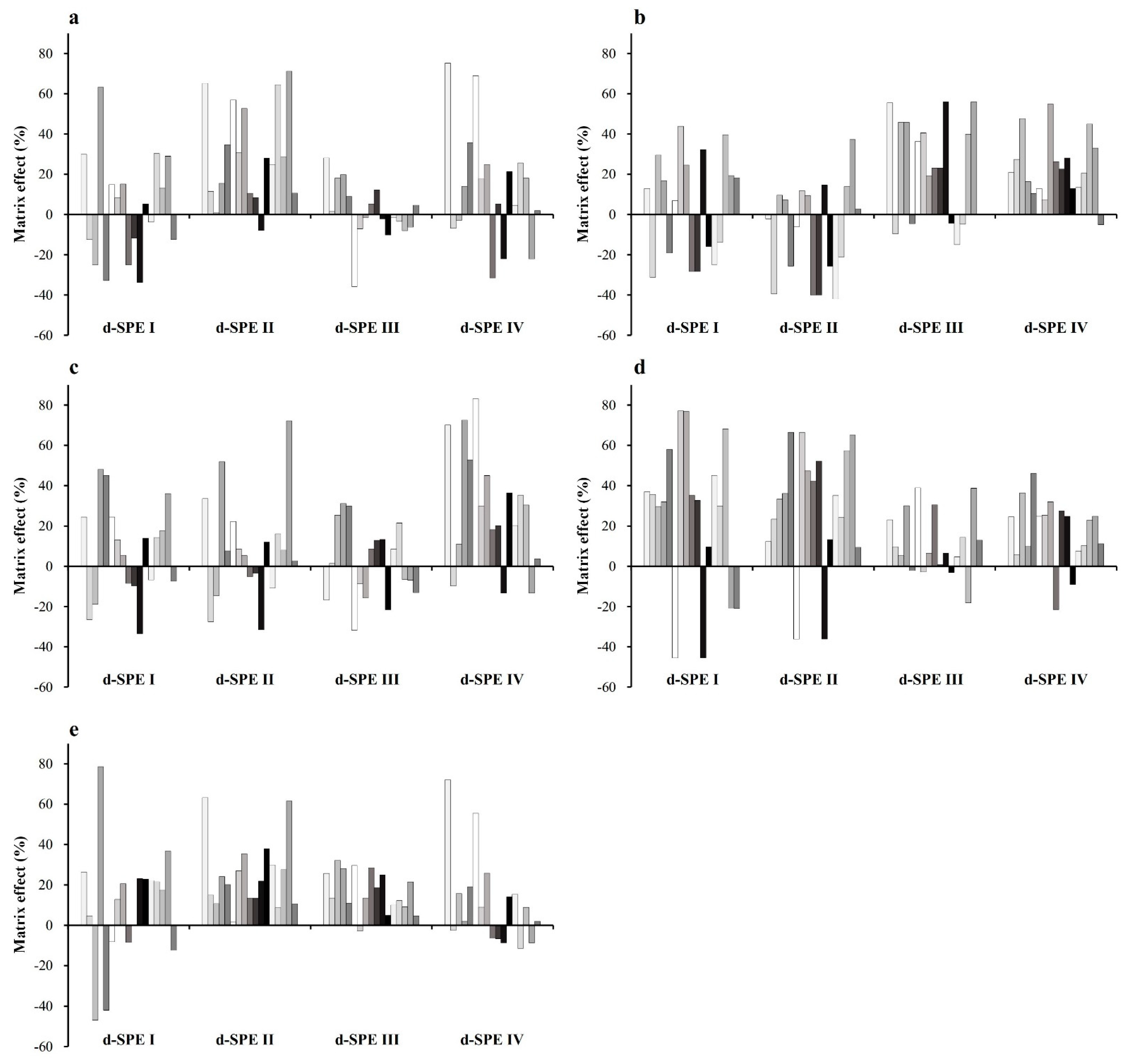
3.4. Optimization of d-SPE for Purification
3.5. Limit of Quantitation (LOQ) and Calibration Curve of the Optimized Method
3.6. Method Validation of the Established Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.M.; Wang, Y.H. Chromatographic methods for the determination of pyrethrin and pyrethroid pesticide residues in crops, foods and environmental samples. J. Chromatogr. A 1996, 754, 367–395. [Google Scholar] [CrossRef]
- Moloney, M.; Tuck, S.; Ramkumar, A.; Furey, A.; Danaher, M. Determination of pyrethrin and pyrethroid residues in animal fat using liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1077–1078, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Zanchetti Meneghini, L.; Rübensam, G.; Claudino Bica, V.; Ceccon, A.; Barreto, F.; Flores Ferrão, M.; Bergold, A.M. Multivariate optimization for extraction of pyrethroids in milk and validation for GC–ECD and CG–MS/MS analysis. Int. J. Environ. Res. Public Health 2014, 11, 11421–11437. [Google Scholar] [CrossRef]
- Anadón, A.; Martínez-Larrañaga, M.R.; Martínez, M.A. Use and abuse of pyrethrins and synthetic pyrethroids in veterinary medicine. Vet. J. 2009, 182, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Godara, R.; Katoch, R.; Rafiqi, S.I.; Yadav, A.; Nazim, K.; Sharma, R.; Singh, N.K.; Katoch, M. Synthetic pyrethroid resistance in Rhipicephalus (Boophilus) microplus ticks from north-western Himalayas, India. Trop. Anim. Health Prod. 2019, 51, 1203–1208. [Google Scholar] [CrossRef]
- Lin, X.-Y.; Mou, R.-X.; Cao, Z.-Y.; Cao, Z.-Z.; Chen, M.-X. Analysis of pyrethroid pesticides in Chinese vegetables and fruits by GC–MS/MS. Chem. Pap. 2018, 72, 1953–1962. [Google Scholar] [CrossRef]
- Jenkins, D.W.; Hensens, A.; Lloyd, J.; Payne, M.; Cizmarik, P.; Hamel, S. Development and validation of a ‘universal’ HPLC method for pyrethroid quantification in long-lasting insecticidal mosquito nets for malaria control and prevention. Trop. Med. Int. Health 2013, 18, 2–11. [Google Scholar] [CrossRef]
- de Oliveira, L.G.; Kurz, M.H.; Guimarães, M.C.; Martins, M.L.; Prestes, O.D.; Zanella, R.; da Silva Ribeiro, J.N.; Gonçalves, F.F. Development and validation of a method for the analysis of pyrethroid residues in fish using GC–MS. Food Chem. 2019, 297, 124944. [Google Scholar] [CrossRef]
- Stanfill, S.B.; Jia, L.T.; Ashley, D.J.; Watson, C.H. Rapid and chemically selective nicotine quantification in smokeless tobacco products using GC–MS. J. Chromatogr. Sci. 2009, 47, 902–909. [Google Scholar] [CrossRef]
- David, F.; Devos, C.; Dumont, E.; Yang, Z.; Sandra, P.; Huertas-Pérez, J.F. Determination of pesticides in fatty matrices using gel permeation clean-up followed by GC–MS/MS and LC–MS/MS analysis: A comparison of low- and high-pressure gel permeation columns. Talanta 2017, 165, 201–210. [Google Scholar] [CrossRef]
- Kang, H.S.; Kim, M.; Kim, E.J.; Choe, W.J. Determination of 66 pesticide residues in livestock products using QuEChERS and GC-MS/MS. Food Sci. Biotechnol. 2020, 29, 1573–1586. [Google Scholar] [CrossRef]
- Luo, P.; Liu, X.; Kong, F.; Tang, L.; Wang, Q.; Li, W.; Xu, W.; Wen, S.; Chen, L.; Li, Y. Multi-residue determination of 325 pesticides in chicken eggs with EMR-Lipid clean-up by UHPLC–MS/MS and GC–MS/MS. Chromatographia 2020, 83, 593–599. [Google Scholar] [CrossRef]
- Lichtmannegger, K.; Fischer, R.; Steemann, F.X.; Unterluggauer, H.; Masselter, S. Alternative QuEChERS-based modular approach for pesticide residue analysis in food of animal origin. Anal. Bioanal. Chem. 2015, 407, 3727–3742. [Google Scholar] [CrossRef]
- Oliveira, F.; Pereira, E.N.C.; Gobbi, J.M.; Soto-Blanco, B.; Melo, M.M. Multiresidue method for detection of pesticides in beef meat using liquid chromatography coupled to mass spectrometry detection (LC–MS) after QuEChERS extraction. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 94–109. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Esteve-Turrillas, F.A.; Pastor, A.; de la Guardia, M. Determination of pyrethroid insecticide residues in vegetable oils by using combined solid-phases extraction and tandem mass spectrometry detection. Anal. Chim. Acta 2005, 553, 50–57. [Google Scholar] [CrossRef]
- Sheridan, R.S.; Meola, J.R. Analysis of pesticide residues in fruits, vegetables, and milk by gas chromatography/tandem mass spectrometry. J. AOAC Int. 1999, 82, 982–990. [Google Scholar] [CrossRef]
- Wei, H.; Tao, Y.; Chen, D.; Xie, S.; Pan, Y.; Liu, Z.; Huang, L.; Yuan, Z. Development and validation of a multi-residue screening method for veterinary drugs, their metabolites and pesticides in meat using liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 686–701. [Google Scholar] [CrossRef]
- Hamamoto, K.; Iwatsuki, K.; Akama, R.; Koike, R. Rapid multiresidue determination of pesticides in livestock muscle and liver tissue via modified QuEChERS sample preparation and LC–MS/MS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 1162–1171. [Google Scholar] [CrossRef]
- SANTE/11945/2015; Guidance Document on Analytical Quality Control and Validation Procedures for Pesticide Residues and Anlysis in Food and Feed. European Commission: Brussels, Belgium, 2015.
- Feo, M.; Eljarrat, E.; Barceló, D. Performance of gas chromatography/tandem mass spectrometry in the analysis of pyrethroid insecticides in environmental and food samples. Rapid Commun. Mass Spectrom. 2011, 25, 869–876. [Google Scholar] [CrossRef]
- Zawiyah, S.; Che Man, Y.B.; Nazimah, S.A.H.; Chin, C.K.; Tsukamoto, I.; Hamanyza, A.H.; Norhaizan, I. Determination of organochlorine and pyrethroid pesticides in fruit and vegetables using SAX/PSA clean-up column. Food Chem. 2007, 102, 98–103. [Google Scholar] [CrossRef]
- You, J.; Wang, D.; Lydy, M.J. Determination of pyrethroid insecticides in sediment by gas chromatography–Ion trap tandem mass spectrometry. Talanta 2010, 81, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Xiaodong, M.; Chongjiu, L. Application of Gas Chromatography-Tandem Mass Spectrometry (GC–MS–MS) with Pulsed Splitless Injection for the Determination of Multiclass Pesticides in Vegetables. Anal. Lett. 2006, 39, 985–996. [Google Scholar] [CrossRef]
- Maštovská, K.; Lehotay, S.J. Evaluation of common organic solvents for gas chromatographic analysis and stability of multiclass pesticide residues. J. Chromatogr. A 2004, 1040, 259–272. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Cui, X.; Pan, C.; Zhang, A.; Chen, F. A review of sample preparation methods for the pesticide residue analysis in foods. Cent. Eur. J. Chem. 2012, 10, 900–925. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Son, K.A.; Kwon, H.; Koesukwiwat, U.; Fu, W.; Mastovska, K.; Hoh, E.; Leepipatpiboon, N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J. Chromatogr. A 2010, 1217, 2548–2560. [Google Scholar] [CrossRef]
- Lopes, R.P.; de Freitas Passos, É.E.; de Alkimim Filho, J.F.; Vargas, E.A.; Augusti, D.V.; Augusti, R. Development and validation of a method for the determination of sulfonamides in animal feed by modified QuEChERS and LC–MS/MS analysis. Food Control 2012, 28, 192–198. [Google Scholar] [CrossRef]
- Shahsavari, S.; Shariatifar, N.; Arabameri, M.; Mahmoudi, R.; Moazzen, M.; Ghajarbeygi, P. Analysis of polychlorinated biphenyls in cream and ice cream using modified QuEChERS extraction and GC–QqQ–MS/MS method: A risk assessment study. Int. J. Dairy Technol. 2022, 75, 448–459. [Google Scholar] [CrossRef]
- Park, K.H.; Choi, J.H.; Abd El-Aty, A.M.; Rahman, M.M.; Jang, J.; Ko, A.Y.; Kwon, K.S.; Park, H.R.; Kim, H.S.; Shim, J.H. Quantifying fenobucarb residue levels in beef muscles using liquid chromatography-tandem mass spectrometry and QuEChERS sample preparation. Food Chem. 2013, 138, 2306–2311. [Google Scholar] [CrossRef]
- Hildmann, F.; Gottert, C.; Frenzel, T.; Kempe, G.; Speer, K. Pesticide residues in chicken eggs—A sample preparation methodology for analysis by gas and liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2015, 1403, 1–20. [Google Scholar] [CrossRef]
- Koesukwiwat, U.; Sanguankaew, K.; Leepipatpiboon, N. Rapid determination of phenoxy acid residues in rice by modified QuEChERS extraction and liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2008, 626, 10–20. [Google Scholar] [CrossRef]
- Koesukwiwat, U.; Lehotay, S.J.; Mastovska, K.; Dorweiler, K.J.; Leepipatpiboon, N. Extension of the QuEChERS Method for Pesticide Residues in Cereals to Flaxseeds, Peanuts, and Doughs. J. Agric. Food Chem. 2010, 58, 5950–5958. [Google Scholar] [CrossRef] [PubMed]
- Niessen, W.M.; Manini, P.; Andreoli, R. Matrix effects in quantitative pesticide analysis using liquid chromatography-mass spectrometry. Mass Spectrom. Rev. 2006, 25, 881–899. [Google Scholar] [CrossRef] [PubMed]
| Extraction Solvent | Pretreatment | Sentence |
|---|---|---|
| MeCN + 0.1% formic acid | QuEChERS + SPE | [11] |
| MeCN + 5% formic acid | EMR liquid clean up | [12] |
| MeCN/water (1:1, v/v) | QuEChERS + SPE | [13] |
| MeCH + 0.1% formic acid/ethyl acetate (7:3, v/v) | QuEChERS + dSPE | [14] |
| MeCN | QuEChERS + dSPE | [15] |
| MeCN | SPE | [16] |
| MeCN/ethoanol (95:5, v/v) | SPE | [17] |
| MeCN/ethyl acetate (1/1, v/v) | QuEChERS | [18] |
| acetone | QuEChERS + SPE | [19] |
| Compound | Molecular Weight | RT 1 (min) | Precursor Ion > Product Ion (CE 2, V 3) | |
|---|---|---|---|---|
| Quantifier (m/z) | Qualifier (m/z) | |||
| Acrinathrin | 541.4 | 19.0 | 289 < 93 (5) | 289 < 77 (40) |
| Bifenthrin | 422.9 | 17.6 | 181 < 206 (30) | 181 < 115 (55) |
| Cyfluthrin | 461.1 | 20.4, 20.5, 20.6, 20.6 | 226 < 206 (20) | 163 < 127 (5) |
| Cyhalothrin | 449.9 | 18.6, 18.8 | 197 < 161 (5) | 197 < 141 (5) |
| Cypermethrin | 416.3 | 20.8, 20.9, 20.9, 21.2 | 163 < 127 (5) | 163 < 109 (30) |
| Deltamethrin(Tralomethrin) | 381.1 | 22.7 | 253 < 174 (5) | 253 < 172 (10) |
| Etofenprox | 376.5 | 21.1 | 163 < 135 (10) | 163 < 107 (20) |
| Fenpropathrin | 349.4 | 17.9 | 265 < 210 (30) | 181 < 152 (30) |
| Fenvalerate/Esfenvalerate | 419.9 | 21.8, 22.0 | 225 < 119 (15) | 167 < 125 (15) |
| Flucythrinate | 451.5 | 21.0, 21.2 | 199 < 157 (5) | 199 < 107 (15) |
| Halfenprox | 477.3 | 20.9 | 263 < 235 (10) | 263 < 169 (30) |
| Permethrin | 391.3 | 19.8, 19.9 | 183 < 165 (20) | 183 < 153 (20) |
| Phenothrin | 350.5 | 18.3 | 183 < 168 (20) | 183 < 153 (20) |
| Resmethrin/Bioresmethrin | 338.4 | 17.1, 17.2 | 177 < 157 (10) | 177 < 127 (25) |
| Silafluofen | 408.6 | 21.3 | 286 < 258 (15) | 179 < 151 (15) |
| tau-Fluvalinate | 502.9 | 21.9, 22.0 | 250 < 200 (20) | 250 < 55 (20) |
| Tefluthrin | 418.7 | 12.3 | 177 < 157 (10) | 177 < 127 (25) |
| Insecticide | Fortification | Recovery, % | ||||
|---|---|---|---|---|---|---|
| Beef | Pork | Chicken | Milk | Eggs | ||
| Acrinathrin | LOQ | 105.8 ± 3.0 | 94.0 ± 1.3 | 87.1 ± 5.0 | 109.8 ± 5.0 | 98.6 ± 3.6 |
| 10LOQ | 100.6 ± 6.1 | 83.8 ± 1.8 | 98.0 ± 1.3 | 92.0 ± 6.6 | 90.5 ± 4.5 | |
| 50LOQ | 106.4 ± 4.5 | 94.9 ± 2.0 | 93.5 ± 2.0 | 101.9 ± 3.2 | 85.5 ± 1.2 | |
| Bifenthrin | LOQ | 99.1 ± 1.9 | 101.3 ± 4.5 | 82.1 ± 1.6 | 100.1 ± 2.9 | 83.8 ± 5.7 |
| 10LOQ | 99.7 ± 4.0 | 89.8 ± 5.5 | 85.0 ± 1.5 | 90.3 ± 4.3 | 85.0 ± 1.0 | |
| 50LOQ | 109.0 ± 2.0 | 82.7 ± 1.9 | 83.2 ± 0.8 | 90.9 ± 6.6 | 87.8 ± 2.5 | |
| Cyfluthrin | LOQ | 105.3 ± 4.8 | 91.2 ± 2.6 | 90.8 ± 1.3 | 109.8 ± 3.3 | 90.8 ± 5.6 |
| 10LOQ | 100.9 ± 8.0 | 85.0 ± 1.2 | 90.6 ± 1.2 | 101.3 ± 4.8 | 90.9 ± 0.7 | |
| 50LOQ | 104.1 ± 5.0 | 93.1 ± 1.7 | 88.5 ± 1.1 | 95.5 ± 4.4 | 84.8 ± 2.7 | |
| Cyhalothrin | LOQ | 103.1 ± 2.8 | 93.3 ± 1.6 | 97.8 ± 3.2 | 107.5 ± 3.0 | 89.0 ± 5.9 |
| 10LOQ | 99.7 ± 7.4 | 86.1 ± 2.2 | 95.0 ± 1.6 | 91.6 ± 4.4 | 92.8 ± 1.2 | |
| 50LOQ | 104.7 ± 5.4 | 94.5 ± 3.8 | 93.3 ± 2.0 | 84.5 ± 1.1 | 84.4 ± 4.4 | |
| Cypermethrin | LOQ | 100.9 ± 4.9 | 88.9 ± 1.9 | 96.2 ± 3.9 | 104.5 ± 4.2 | 91.7 ± 0.6 |
| 10LOQ | 98.5 ± 6.8 | 81.8 ± 1.5 | 88.6 ± 0.5 | 93.0 ± 4.5 | 87.4 ± 1.6 | |
| 50LOQ | 104.2 ± 3.4 | 90.4 ± 1.5 | 84.4 ± 0.8 | 91.8 ± 3.6 | 87.0 ± 1.2 | |
| Deltamethrin (Tralomethrin) | LOQ | 90.6 ± 3.6 | 91.1 ± 3.0 | 90.4 ± 3.6 | 106.7 ± 1.2 | 93.2 ± 2.7 |
| LOQ | 88.7 ± 1.6 | 82.1 ± 2.0 | 88.7 ± 1.6 | 88.6 ± 5.6 | 93.0 ± 2.3 | |
| 10LOQ | 98.7 ± 1.8 | 91.3 ± 1.6 | 98.7 ± 1.8 | 94.5 ± 4.4 | 89.4 ± 4.9 | |
| Etofenprox | LOQ | 98.9 ± 2.8 | 84.6 ± 3.3 | 80.4 ± 2.6 | 92.7 ± 1.4 | 88.0 ± 3.0 |
| 10LOQ | 97.5 ± 7.9 | 87.0 ± 5.1 | 94.8 ± 1.0 | 83.2 ± 3.3 | 82.2 ± 1.0 | |
| 50LOQ | 104.1 ± 2.7 | 89.4 ± 3.7 | 94.3 ± 2.6 | 91.0 ± 1.9 | 85.0 ± 3.2 | |
| Fenpropathrin | LOQ | 106.9 ± 3.9 | 88.9 ± 0.4 | 90.0 ± 2.3 | 105.2 ± 5.9 | 94.5 ± 3.1 |
| 10LOQ | 102.2 ± 5.8 | 81.7 ± 0.9 | 90.6 ± 3.6 | 91.3 ± 3.9 | 88.4 ± 1,0 | |
| 50LOQ | 108.1 ± 3.1 | 90.3 ± 1.7 | 86.4 ± 2.9 | 91.6 ± 4.2 | 87.8 ± 3.5 | |
| Fenvalerate/ Esfenvalerate | LOQ | 100.9 ± 1.2 | 91.6 ± 1.2 | 82.3 ± 4.0 | 106.1 ± 3.2 | 93.4 ± 6.6 |
| 10LOQ | 100.0 ± 6.3 | 84.7 ± 3.9 | 92.9 ± 3.0 | 92.5 ± 3.3 | 84.1 ± 3.9 | |
| 50LOQ | 108.6 ± 2.0 | 88.8 ± 2.2 | 86.1 ± 1.8 | 96.8 ± 1.9 | 83.1 ± 2.1 | |
| Flucythrinate | LOQ | 98.0 ± 3.4 | 94.5 ± 3.7 | 97.4 ± 4.9 | 101.2 ± 2.4 | 101.7 ± 6.4 |
| 10LOQ | 104.8 ± 7.8 | 87.1 ± 1.7 | 94.9 ± 2.7 | 97.2 ± 4.5 | 96.4 ± 4.8 | |
| 50LOQ | 104.2 ± 3.1 | 89.4 ± 3.3 | 100.9 ± 4.2 | 102.0 ± 2.3 | 84.9 ± 2.6 | |
| Halfenprox | LOQ | 101.0 ± 4.2 | 104.8 ± 2.5 | 84.5 ± 2.7 | 92.7 ± 4.7 | 83.4 ± 3.7 |
| 10LOQ | 96.9 ± 3.4 | 88.6 ± 4.5 | 83.4 ± 1.8 | 84.5 ± 1.7 | 83.0 ± 2.6 | |
| 50LOQ | 101.7 ± 3.9 | 99.9 ± 2.2 | 87.0 ± 3.6 | 92.0 ± 4.1 | 88.5 ± 2.2 | |
| Permethrin | LOQ | 98.8 ± 4.5 | 83.7 ± 3.0 | 84.5 ± 5.2 | 98.9 ± 4.4 | 85.9 ± 3.0 |
| 10LOQ | 100.1 ± 4.6 | 84.3 ± 3.1 | 78.0 ± 4.9 | 88.8 ± 2.8 | 89.4 ± 3.1 | |
| 50LOQ | 95.2 ± 6.4 | 87.6 ± 3.8 | 75.4 ± 1.0 | 83.0 ± 0.9 | 80.9 ± 1.5 | |
| Phenothrin | LOQ | 102.2 ± 1.9 | 104.8 ± 2.2 | 108.8 ± 5.1 | 108.8 ± 5.1 | 87.3 ± 2.1 |
| 10LOQ | 100.3 ± 5.6 | 82.8 ± 2.3 | 93.0 ± 3.8 | 93.0 ± 3.8 | 84.4 ± 2.6 | |
| 50LOQ | 101.8 ± 3.5 | 85.3 ± 3.8 | 89.6 ± 4.3 | 89.6 ± 4.3 | 88.9 ± 1.6 | |
| Resmethrin/ Bioresmethrin | LOQ | 94.8 ± 3.7 | 87.8 ± 3.4 | 106.4 ± 4.3 | 97.6 ± 2.2 | 92.4 ± 5.3 |
| 10LOQ | 91.4 ± 5.8 | 92.0 ± 0.9 | 102.2 ± 2.9 | 80.5 ± 1.7 | 96.3 ± 6.7 | |
| 50LOQ | 107.1 ± 3.4 | 89.0 ± 2.5 | 98.0 ± 2.5 | 92.8 ± 3.3 | 81.1 ± 2.0 | |
| Silafluofen | LOQ | 93.9 ± 1.7 | 106.3 ± 1.4 | 83.3 ± 3.5 | 93.9 ± 3.1 | 84.5 ± 5.0 |
| 10LOQ | 93.9 ± 4.3 | 80.9 ± 3.7 | 81.1 ± 3.4 | 80.4 ± 2.1 | 88.1 ± 3.7 | |
| 50LOQ | 100.9 ± 2.5 | 93.9 ± 6.9 | 82.2 ± 2.3 | 87.8 ± 2.3 | 85.8 ± 4.0 | |
| tau-Fluvalinate | LOQ | 103.5 ± 2.2 | 92.4 ± 1.7 | 83.9 ± 2.3 | 109.3 ± 3.5 | 106.2 ± 3.2 |
| 10LOQ | 101.3 ± 5.4 | 83.7 ± 0.9 | 87.5 ± 1.6 | 92.8 ± 3.7 | 94.1 ± 1.9 | |
| 50LOQ | 105.9 ± 5.3 | 97.6 ± 2.3 | 84.3 ± 1.1 | 90.6 ± 1.6 | 92.5 ± 4.5 | |
| Tefluthrin | LOQ | 105.2 ± 2.0 | 88.9 ± 1.1 | 91.5 ± 1.7 | 103.7 ± 2.1 | 87.0 ± 2.0 |
| 10LOQ | 104.0 ± 2.3 | 82.8 ± 0.6 | 88.1 ± 1.6 | 100.2 ± 3.9 | 89.0 ± 0.7 | |
| 50LOQ | 109.2 ± 2.1 | 87.9 ± 2.8 | 86.9 ± 1.3 | 101.5 ± 4.7 | 75.2 ± 1.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.J.; Yang, S.-H.; Choi, H. Simultaneous Determination of Pyrethroid Insecticides in Foods of Animal Origins Using the Modified QuEChERS Method and Gas Chromatography-Mass Spectrometry. Foods 2022, 11, 3634. https://doi.org/10.3390/foods11223634
Kim BJ, Yang S-H, Choi H. Simultaneous Determination of Pyrethroid Insecticides in Foods of Animal Origins Using the Modified QuEChERS Method and Gas Chromatography-Mass Spectrometry. Foods. 2022; 11(22):3634. https://doi.org/10.3390/foods11223634
Chicago/Turabian StyleKim, Byung Joon, Seung-Hyun Yang, and Hoon Choi. 2022. "Simultaneous Determination of Pyrethroid Insecticides in Foods of Animal Origins Using the Modified QuEChERS Method and Gas Chromatography-Mass Spectrometry" Foods 11, no. 22: 3634. https://doi.org/10.3390/foods11223634
APA StyleKim, B. J., Yang, S.-H., & Choi, H. (2022). Simultaneous Determination of Pyrethroid Insecticides in Foods of Animal Origins Using the Modified QuEChERS Method and Gas Chromatography-Mass Spectrometry. Foods, 11(22), 3634. https://doi.org/10.3390/foods11223634






