Encapsulation of Marjoram Phenolic Compounds Using Chitosan to Improve Its Colon Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Plant Material and Ultrasound Assisted Extraction (UAE)
2.2.2. Preparation of Chitosan Micro/Nanoparticles Containing Marjoram Extract
Ionic Gelation
Spray Drying
2.2.3. Analysis of Phenolic Composition and Quantification by HPLC-PAD
2.2.4. Characterization of the Chitosan Micro/Nanoparticles Containing Marjoram Extract
Determination of Particle Size Distribution, Zeta Potential, and Polydispersity Index (PDI)
Determination of Yield and Encapsulation Efficiency (EE)
2.2.5. Marjoram Phenolic Compounds Release from Micro/Nanoparticles
2.2.6. In Vitro Simulated Gastrointestinal Digestion
2.3. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Characterization of UAE Marjoram Extract
3.2. Formulation and Characterization of Micro/Nanoparticles Obtained by Ionic Gelation
3.2.1. Formulation of Particles
3.2.2. Yields
3.2.3. Encapsulation Efficiency (EE)
3.2.4. Particle Size, Zeta Potential, and Polydispersity Index (PDI)
3.3. Formulation and Characterization of Microparticles Obtained by Spray Drying
3.3.1. The Formulation of Microparticles
3.3.2. Yields
3.3.3. Encapsulation Efficiency (EE), Particle Size, Zeta Potential, and the Polydispersity Index (PDI)
3.4. EE of Marjoram Extract Phenolic Compounds
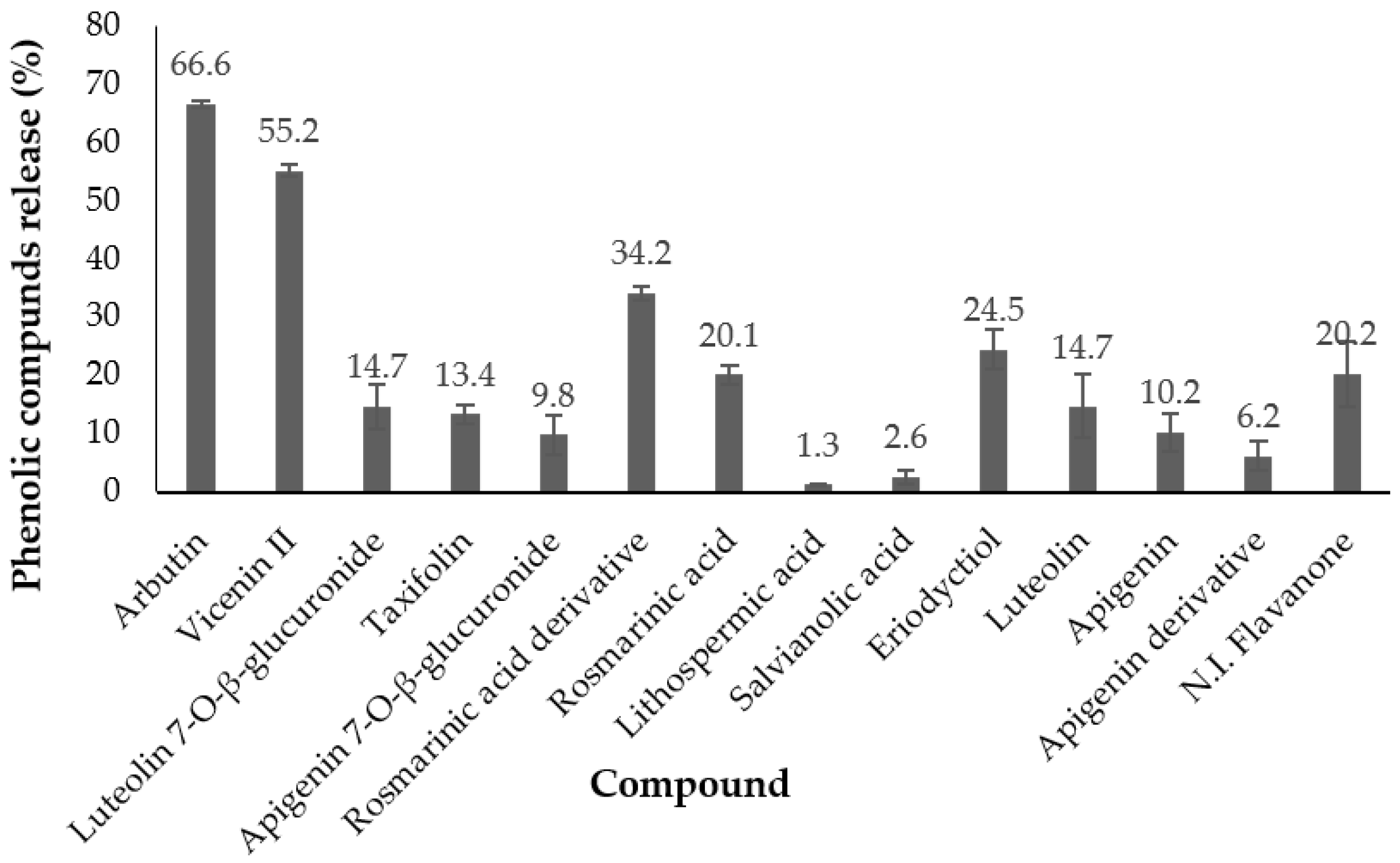
3.5. Controlled Release Studies
3.6. In Vitro Simulated Gastrointestinal Digestion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, S. Pectin based multi-Particulate for colon-Specific delivery of therapeutics agents. Int. J. Pharm. 2021, 605, 120814. [Google Scholar] [CrossRef] [PubMed]
- Oladzadabbasabadi, N.; Mohammadi, N.A.; Ariffin, F.; Wijekoon, M.M.J.O.; Al-Hassan, A.A.; Dheyab, M.A.; Ghasemlou, M. Recent advances in extraction, modification, and application of chitosan in packaging industry. Carbohydr. Polym. 2022, 277, 118876. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Gorityala, S.; Moharir, K. Recent trends in design and evaluation of chitosan-based colon targeted drug delivery systems. J. Drug Deliv. Sci. Technol. 2021, 64, 102579. [Google Scholar] [CrossRef]
- Arévalo-Pérez, R.; Maderuelo, C.; Lanao, J.M. Recent advances in colon drug delivery systems. J. Control. Release 2020, 327, 703–724. [Google Scholar] [CrossRef]
- Samprasit, W.; Opanasopit, P.; Chamsai, B. Alpha-Mangostin and resveratrol, dual-Drugs-Loaded mucoadhesive thiolated chitosan-Based nanoparticles for synergistic activity against colon cancer cells. J. Biomed. Mater. Res. Part B 2022, 110, 1221–1233. [Google Scholar] [CrossRef]
- Sabra, R.; Billa, N.; Roberts, C.J. An augmented delivery of the anticancer agent, curcumin, to the colon. React. Funct. Polym. 2018, 123, 54–60. [Google Scholar] [CrossRef]
- García-Couce, J.; Bada-Rivero, N.; López-Hernández, O.D.; Nogueira, A.; Caracciolo, P.C.; Abraham, G.A.; Hernández, J.A.R.; Peniche, C. Dexamethasone-Loaded chitosan beads coated with a pH-Dependent interpolymer complex for colon-specific drug delivery. Int. J. Polym. Sci. 2019, 9, 4204375. [Google Scholar] [CrossRef] [Green Version]
- Bouyahya, A.; Chamkhi, I.; Benali, T.; Guaouguaou, F.E.; Balahbib, A.; El Omari, N.; Taha, D.; Belmehdi, O.; Ghokhan, Z.; El Menyiy, N. Traditional use, phytochemistry, toxicology, and pharmacology of Origanum majorana L. J. Ethnopharmacol. 2021, 65, 113318. [Google Scholar] [CrossRef]
- Kasala, E.R.; Bodduluru, L.N.; Barua, C.C.; Gogoi, R. Antioxidant and antitumorefficacy of luteolin, a dietary flavone on benzo(a)pyrene-Induced experimental lung carcinogenesis. Biomed. Pharmacother. 2016, 82, 568–577. [Google Scholar] [CrossRef]
- Villalva, M.; Jaime, L.; Aguado, E.; Nieto, J.A.; Reglero, G.; Santoyo, S. Anti-inflammatory and antioxidant activities from the basolateral fraction of Caco-2 cells exposed to a rosmarinic acid enriched extract. J. Agric. Food Chem. 2018, 66, 1167–1174. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Parama, D.; Daimari, E.; Girisa, S.; Banik, K.; Harsha, C.; Dutta, U.; Kunnumakkara, A.B. Rationalizing the therapeutic potential of apigenin against cancer. Life Sci. 2021, 267, 118814. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Gunasekaran, S.; Namasivayam, N. Biochemical and molecular mechanisms underlying the chemopreventive efficacy of rosmarinic acid in a rat colon cancer. Eur. J. Pharmacol. 2016, 791, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Blanco, S.; Fernández, J.; Gutierrez-del-Rio, I.; Villar, C.J.; Lombó, F. New insights toward colorectal cancer chemotherapy using natural bioactive compounds. Front. Pharmacol. 2017, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Monti, E.; Marras, E.; Prini, P.; Gariboldi, M.B. Luteolin impairs hypoxia adaptation and progression in human breast and colon cancer cells. Eur. J. Pharmacol. 2020, 881, 173210. [Google Scholar] [CrossRef]
- Villalva, M.; Santoyo, S.; Salas-Pérez, L.; Siles-Sánchez, M.N.; García-Risco, M.R.; Fornari, T.; Reglero, G.; Jaime, L. Sustainable extraction techniques for obtaining antioxidant and anti-Inflammatory compounds from the Lamiaceae and Asteraceae Species. Foods 2021, 10, 2067. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.B.; Oliveira, A.; Ferreira, D.; Sarmento, B.; Pintado, M. Development and validation method for simultaneous quantification of phenolic compounds in natural extracts and nanosystems. Phytochem. Anal. 2013, 24, 638–644. [Google Scholar] [CrossRef]
- Cabral, B.R.P.; de Oliveira, P.M.; Gelfuso, G.M.; Quintão, T.d.S.C.; Chaker, J.A.; Karnikowski, M.G.d.O.; Gris, E.F. Improving stability of antioxidant compounds from Plinia cauliflora (jabuticaba) fruit peel extract by encapsulation in chitosan microparticles. J. Food Eng. 2018, 238, 195–201. [Google Scholar] [CrossRef]
- Lamien-Meda, A.; Lukas, B.; Schmiderer, C.; Franz, C.; Novak, J. Validation of a quantitative assay of arbutin using gas chromatography in Origanum majorana and Arctostaphylos uva-ursi extracts. Phytochem. Anal. 2009, 20, 416–420. [Google Scholar] [CrossRef]
- Cerchiara, T.; Abruzzo, A.; di Cagno, M.; Bigucci, F.; Bauer-Brandl, A.; Parolin, C.; Vitali, B.; Gallucci, M.C.; Luppi, B. Chitosan based micro- and nanoparticles for colon-targeted delivery of vancomycin prepared by alternative processing methods. Eur. J. Pharm. Biopharm. 2015, 92, 112–119. [Google Scholar] [CrossRef]
- Da Silva, S.B.; Amorim, M.; Fonte, P.; Madureira, R.; Ferreira, D.; Pintado, M.; Sarmento, B. Natural extracts into chitosan nanocarriers for rosmarinic acid drug delivery. Pharm. Biol. 2015, 53, 642–652. [Google Scholar] [CrossRef]
- Kunjachan, S.; Jose, S.; Lammers, T. Understanding the mechanism of ionic gelation for synthesis of chitosan nanoparticles using qualitative techniques. Asian J. Pharm. 2014, 4, 2. [Google Scholar] [CrossRef]
- Hesami, G.; Darvishi, S.; Zarei, M.; Hadidi, M. Fabrication of chitosan nanoparticles incorporated with Pistacia atlantica subsp. kurdica hulls’ essential oil as a potential antifungal preservative against strawberry grey mould. Int. J. Food Sci. Technol. 2021, 56, 4215–4223. [Google Scholar] [CrossRef]
- Da Silva, S.B.; Ferreira, D.; Pintado, M.; Sarmento, B. Chitosan-Based nanoparticles for rosmarinic acid ocular delivery-In vitro tests. Int. J. Biol. Macromol. 2016, 84, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Encapsulation of food bioactives and nutraceuticals by various chitosan-based nanocarriers. Food Hydrocoll. 2020, 105, 105774. [Google Scholar] [CrossRef]
- Endo, E.H.; Ueda-Nakamura, T.; Nakamura, C.V.; Filho, B.P.D. Activity of spray-dried microparticles containing pomegranate peel extract against Candida albicans. Molecules 2012, 17, 10094–10107. [Google Scholar] [CrossRef] [PubMed]
- Casanova, F.; Estevinho, B.N.; Santos, L. Preliminary studies of rosmarinic acid microencapsulation with chitosan and modified chitosan for topical delivery. Power Technol. 2016, 297, 44–49. [Google Scholar] [CrossRef]
- Gelfuso, G.M.; Gratieri, T.; Simao, P.S.; de Freitas, L.A.P.; Lopez, R.F.V. Chitosan microparticles for sustaining the topical delivery of minoxidil sulphate. J. Microencapsul. 2011, 28, 650–658. [Google Scholar] [CrossRef]
- Harris, H.; Lecumberri, E.; Mateos-Aparicio, I.; Mengibar, M.; Heras, A. Chitosan nanoparticles and microspheres for the encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydr. Polym. 2011, 84, 803–808. [Google Scholar] [CrossRef]
- Qin, W.; Ketnawa, S.; Ogawa, Y. Effect of digestive enzimes on variation of bioavailability of green tea during simulated in vitro gastrointestinal digestion. Food Sci. Hum. Wellness 2022, 11, 669–675. [Google Scholar] [CrossRef]
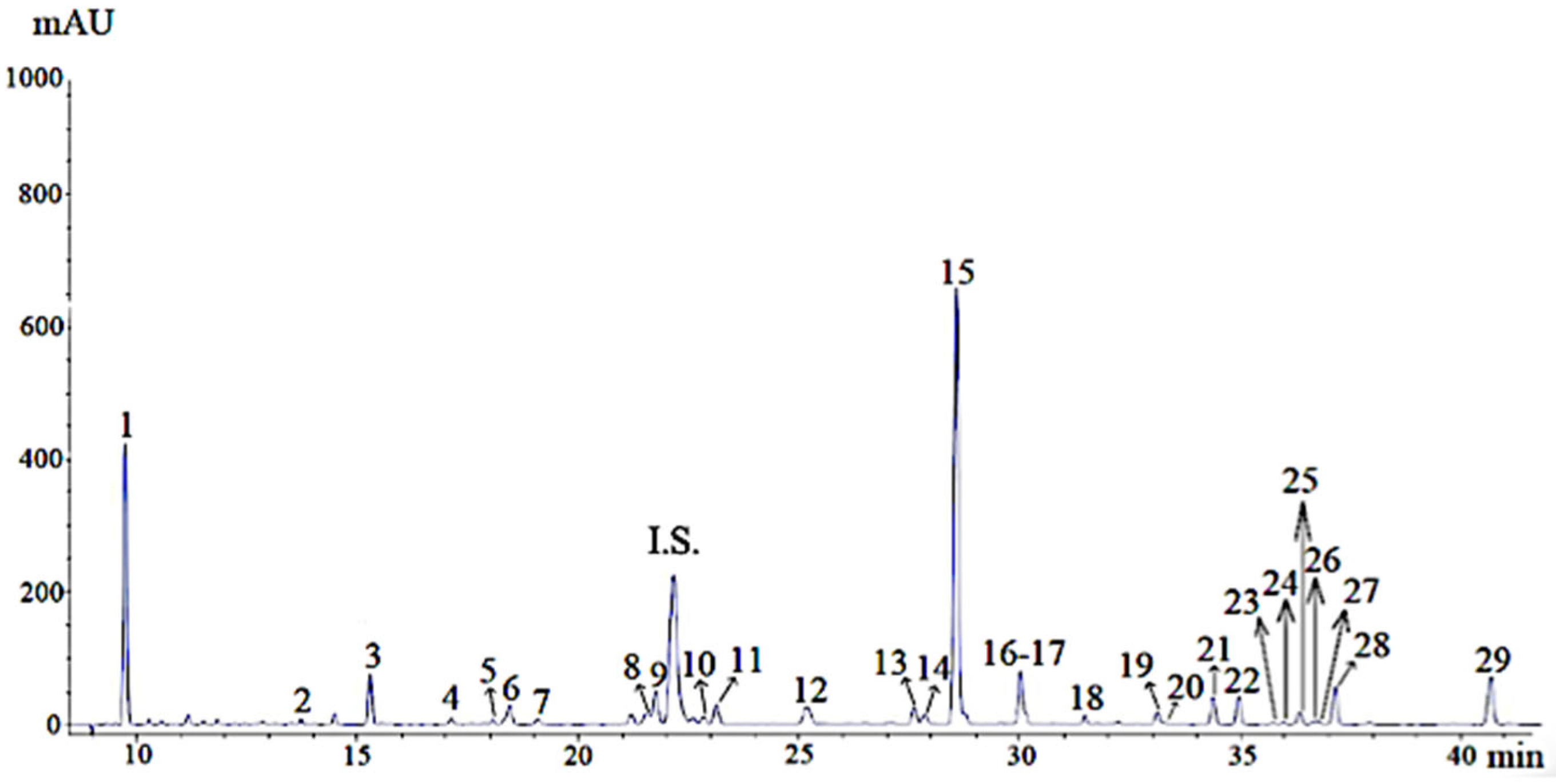
| Peak | Rt (min) | Compound | Concentration (mg/g) |
|---|---|---|---|
| 1 | 9.8 | Arbutin * | 78.10 ± 1.18 |
| 2 | 13.7 | Luteolin dihexoside | 0.20 ± 0.01 |
| 3 | 15.3 | Vicenin II * | 4.56 ± 0.10 |
| 4 | 17.1 | Caffeic acid * | 0.47 ± 0.01 |
| 5 | 18.1 | Luteolin monohexoside | 0.42 ± 0.01 |
| 6 | 18.4 | 2,5 Hydroxybenzoic acid | 3.23 ± 0.19 |
| 7 | 19.1 | 6-hydroxyluteolin-7-O-glucoside | 0.76 ± 0.01 |
| 8 | 21.6 | Apigenin derivative | 0.82 ± 0.02 |
| 9 | 22.0 | Caffeic acid derivative | 1.91 ± 0.03 |
| 10 | 22.8 | Luteolin 7-O-β-glucoside * | 1.06 ± 0.08 |
| 11 | 23.1 | Luteolin 7-O-β-glucuronide * | 3.27 ± 0.09 |
| 12 | 25.2 | Taxifolin * | 2.33 ± 0.03 |
| 13 | 27.6 | Apigenin 7-O-β-glucuronide * | 2.24 ± 0.04 |
| 14 | 27.8 | Rosmarinic acid derivative | 1.99 ± 0.01 |
| 15 | 28.0 | Rosmarinic acid * | 48.32 ± 0.70 |
| 16 | 30.0 | Lithospermic acid isomer | 8.48 ± 0.10 |
| 17 | 30.2 | Salvianolic acid isomer | 2.09 ± 0.08 |
| 18 | 31.5 | Rosmarinic acid derivative | 1.69 ± 0.03 |
| 19 | 33.1 | Eriodyctiol * | 1.26 ± 0.01 |
| 20 | 33.3 | Luteolin * | 0.21 ± 0.00 |
| 21 | 34.4 | N.I. Flavanone | 1.78 ± 0.01 |
| 22 | 35.0 | Apigenin methoxylated | 1.34 ± 0.02 |
| 23 | 35.8 | N. I. Flavanone | 0.24 ± 0.00 |
| 24 | 36.0 | Rosmarinic acid derivative | 1.07 ± 0.04 |
| 25 | 36.3 | N.I. Flavanone | 1.02 ± 0.01 |
| 26 | 36.7 | Apigenin * | 0.21 ± 0.00 |
| 27 | 36.8 | Naringenin * | 0.34 ± 0.00 |
| 28 | 37.2 | Apigenin derivative | 1.29 ± 0.03 |
| 29 | 40.7 | N.I. Flavanone | 3.51 ± 0.05 |
| Σ Phenolic compounds | 174.18 ± 2.89 |
| Ratio CH:TPP | Formulation Code | Extract (mg/mL) | Yield (%) | EE (%) | Particle Size (nm) | ζ-Potential (mV) | PDI |
|---|---|---|---|---|---|---|---|
| LCH 5:1 | IG1 | 0.25 | 32.0 ± 0.1 h | 59.3 ± 4.1 a | - | - | - |
| LCH 5:1 | IG2 | 0.5 | 36.8 ± 0.3 gh | 54.4 ± 0.6 a | 625.7 ± 54.7 b | 27.6 ± 0.7 a | 0.50 ± 0.02 bc |
| LCH 5:1 | IG3 | 1 | 39.1 ± 0.9 fgh | 25.3 ± 3.2 b | - | - | - |
| LCH 6:1 | IG4 | 0.25 | 42.1 ± 1.5 efg | 61.0 ± 7.2 a | - | - | - |
| LCH 6:1 | IG5 | 0.5 | 44.6 ± 6.2 def | 57.0 ± 2.8 a | 641.6 ± 53.6 b | 26.9 ± 0.9 a | 0.55 ± 0.04 b |
| LCH 6:1 | IG6 | 1 | 45.8 ± 2.9 de | 9.5 ± 0.8 d | - | - | - |
| MCH 5:1 | IG7 | 0.25 | 40.8 ± 2.6 efg | 58.3 ± 1.5 a | - | - | - |
| MCH 5:1 | IG8 | 0.5 | 49.2 ± 3.9 cd | 60.7 ± 5.5 a | 770.7 ± 30.1 a | 24.3 ± 1.7 | 0.69 ± 0.02 a |
| MCH 5:1 | IG9 | 1 | 55.1 ± 0.1 bc | 17.5 ± 2.6 c | - | - | - |
| MCH 6:1 | IG10 | 0.25 | 56.0 ± 1.4 bc | 60.8 ± 3.2 a | - | - | - |
| MCH 6:1 | IG11 | 0.5 | 60.4 ± 3.7 b | 57.7 ± 1.0 a | 780.1 ± 24.1 a | 28.9 ± 1.6 a | 0.48 ± 0.03 c |
| MCH 6:1 | IG12 | 1 | 70.1 ± 5.8 a | 9.3 ± 1.0 d | - | - | - |
| Code | CH (mg/mL) | Extract (mg/mL) | Relation CH:ext | Yield (%) | EE (%) | Particle Size (μm) | ζ-Potential (mV) | PDI |
|---|---|---|---|---|---|---|---|---|
| SP1 | 7.52 | 1.88 | 4:1 | 24.2 ± 1.7 b | 75.8 ± 5.3 a | 1.55 ± 0.16 a | 34.3 ± 0.7 a | 0.49 ± 0.04 a |
| SP2 | 7.52 | 1.25 | 6:1 | 26.9 ± 2.5 b | 73.2 ± 6.1 a | 1.68 ± 0.44 a | 29.2 ± 0.6 b | 0.56 ± 0.07 a |
| SP3 | 7.52 | 0.94 | 8:1 | 31.5 ± 0.7 a | 54.8 ± 0.9 b | 1.65 ± 0.11 a | 31.4 ± 0.6 b | 0.47 ± 0.03 a |
| Compounds | Ionic Gelation | Spray Drying | ||
|---|---|---|---|---|
| IG8 | IG11 | SP1 | SP2 | |
| Arbutin | 45.26 ± 7.35 a | 34.94 ± 6.75 b | 63.06 ± 2.04 | 58.95 ± 7.33 |
| Vicenin II | 43.77 ± 6.14 a | 32.81 ± 5.66 b | 63.50 ± 7.72 | 62.61 ± 10.94 |
| Luteolin 7-O-β-glucuronide | 49.93 ± 7.45 a | 37.25 ± 3.42 b | 100 ± 0.00 | 100 ± 0.00 |
| Taxifolin | 76.41 ± 8.52 | 73.59 ± 5.79 | 91.05 ± 7.57 | 93.44 ± 4.86 |
| Apigenin 7-O-β-glucuronide | 58.76 ± 6.37 | 53.24 ± 6.37 | 100 ± 0.00 | 100 ± 0.00 |
| Rosmarinic acid derivative | 60.63 ± 5.97 | 54.44 ± 5.85 | 83.07 ± 1.51 | 79.39 ± 2.28 |
| Rosmarinic acid | 55.84 ± 7.95 | 50.11 ± 5.80 | 96.40 ± 1.84 | 96.88 ± 1.85 |
| Lithospermic acid | 87.53 ± 4.89 | 86.93 ± 4.68 | 100 ± 0.00 | 100 ± 0.00 |
| Salvianolic acid | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| Eriodyctiol | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| Luteolin | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| Apigenin | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| Apigenin derivative | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| N. I. Flavanone | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| Compounds | Amount (%) Release at pH 2 | |||||
|---|---|---|---|---|---|---|
| SP1 | SP2 | |||||
| 1 h | 2 h | 3 h | 1 h | 2 h | 3 h | |
| Arbutin | 75.0 ± 5.6 | 84.1 ± 5.7 | 86.7 ± 9.9 | 75.4 ± 3.5 | 76.8 ± 4.5 | 84.4 ± 3.4 |
| Vicenin II | 53.9 ± 5.4 | 60.7 ± 7.6 | 70.2 ± 7.8 | 51.3 ± 2.8 | 59.9 ± 7.8 | 66.6 ± 2.2 |
| Luteolin 7-O-β-glucuronide | 35.1 ± 1.5 a | 46.2 ± 3.1 | 57.8 ± 3.6 | 30.5 ± 2.6 b | 46.3 ± 5.2 | 56.0 ± 2.4 |
| Taxifolin | 34.2 ± 6.4 | 34.8 ± 7.5 | 51.7 ± 9.9 | 25.4 ± 5.6 | 32.6 ± 9.9 | 41.1 ± 9.2 |
| Apigenin 7-O-β-glucuronide | 29.2 ± 0.3 | 31.6 ± 3.7 | 44.4 ± 10.8 | 25.8 ± 7.0 | 27.4 ± 3.9 | 32.7 ± 6.9 |
| Rosmarinic acid derivative | 36.0 ± 6.5 | 42.1 ± 7.3 | 55.4 ± 2.6 a | 28.5 ± 8.2 | 31.1 ± 5.8 | 34.4 ± 7.5 b |
| Rosmarinic acid | 32.7 ± 6.3 | 39.6 ± 4.7 | 54.8 ± 5.9 | 27.5 ± 9.6 | 38.7 ± 4.2 | 49.7 ± 5.6 |
| Lithospermic acid | 2.5 ± 1.3 | 2.8 ± 1.1 | 2.9 ± 1.2 | 2.9 ± 0.1 | 3.8 ± 1.4 | 5.1 ± 3.5 |
| Salvianolic acid | 0 ± 0 | 13.8 ± 1.7 | 14.8 ± 3.1 | 0 ± 0 | 12.1 ± 0.4 | 15.3 ± 5.1 |
| Eriodyctiol | 0 ± 0 | 3.5 ± 1.8 a | 6.8 ± 2.5 a | 0 ± 0 | 0 ± 0 b | 0 ± 0 b |
| Luteolin | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Apigenin | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Apigenin derivative | 0 ± 0 | 0 ± 0 | 4.2 ± 1.9 a | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| N. I. Flavanone | 0 ± 0 | 2.8 ± 1.1 a | 5.1 ± 3.2 a | 0 ± 0 | 0.1 ± 0 b | 1.7 ± 0.1 b |
| Compounds | Amount (%) Release at pH 7.4 | |||||
| SP1 | SP2 | |||||
| 1 h | 2 h | 3 h | 1 h | 2 h | 3 h | |
| Arbutin | 64.9 ± 8.3 | 80.9 ± 7.8 | 84.9 ± 4.4 | 53.6 ± 9.7 | 77.1 ± 1.8 | 79.7 ± 7.7 |
| Vicenin II | 44.9 ± 6.1 | 60.9 ± 6.2 | 72.7 ± 1.6 | 35.8 ± 8.2 | 58.5 ± 3.6 | 63.4 ± 7.4 |
| Luteolin 7-O-β-glucuronide | 23.9 ± 1.2 | 34.7 ± 1.6 a | 36.3 ± 2.8 | 23.5 ± 1.3 | 29.5 ± 0.7 b | 34.9 ± 5.6 |
| Taxifolin | 26.4 ± 2.9 | 29.7 ± 7.9 | 32.7 ± 7.6 | 26.0 ± 3.9 | 29.9 ± 3.1 | 31.8 ± 8.7 |
| Apigenin 7-O-β-glucuronide | 19.2 ± 1.8 | 22.6 ± 4.1 | 29.1 ± 6.9 | 16.9 ± 3.3 | 20.5 ± 1.3 | 26.6 ± 6.9 |
| Rosmarinic acid derivative | 29.8 ± 1.6 | 30.5 ± 2.5 | 39.4 ± 9.1 | 27.6 ± 4.6 | 32.8 ± 2.5 | 33.8 ± 6.2 |
| Rosmarinic acid | 30.7 ± 8.1 | 46.8 ± 2.2 | 49.8 ± 9.5 | 28.4 ± 9.4 | 35.8 ± 8.3 | 41.6 ± 8.1 |
| Lithospermic acid | 2.5 ± 0.9 a | 2.8 ± 1.1 | 2.9 ± 1.2 | 0 ± 0 b | 1.3 ± 0.7 | 1.9 ± 0.6 |
| Salvianolic acid | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Eriodyctiol | 0 ± 0 | 0 ± 0 | 3.0 ± 1.2 | 0 ± 0 | 0 ± 0 | 2.2 ± 1.4 |
| Luteolin | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Apigenin | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Apigenin derivative | 0 ± 0 | 0 ± 0 | 1.9 ± 0.6 | 0 ± 0 | 0 ± 0 | 1.4 ± 0.5 |
| N.I. Flavanone | 0 ± 0 | 1.4 ± 0.6 | 2.8 ± 1.1 | 0 ± 0 | 1.3 ± 0.5 | 2.5 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siles-Sánchez, M.d.l.N.; Jaime, L.; Villalva, M.; Santoyo, S. Encapsulation of Marjoram Phenolic Compounds Using Chitosan to Improve Its Colon Delivery. Foods 2022, 11, 3657. https://doi.org/10.3390/foods11223657
Siles-Sánchez MdlN, Jaime L, Villalva M, Santoyo S. Encapsulation of Marjoram Phenolic Compounds Using Chitosan to Improve Its Colon Delivery. Foods. 2022; 11(22):3657. https://doi.org/10.3390/foods11223657
Chicago/Turabian StyleSiles-Sánchez, María de las Nieves, Laura Jaime, Marisol Villalva, and Susana Santoyo. 2022. "Encapsulation of Marjoram Phenolic Compounds Using Chitosan to Improve Its Colon Delivery" Foods 11, no. 22: 3657. https://doi.org/10.3390/foods11223657
APA StyleSiles-Sánchez, M. d. l. N., Jaime, L., Villalva, M., & Santoyo, S. (2022). Encapsulation of Marjoram Phenolic Compounds Using Chitosan to Improve Its Colon Delivery. Foods, 11(22), 3657. https://doi.org/10.3390/foods11223657










