Abstract
Deep eutectic solvents represent an important alternative in the field of green solvents due to their low volatility, non-toxicity, and low synthesis cost. In the present investigation, we propose the production of enriched polyphenolic extracts from maritime pine forest residues via an ultrasound-assisted approach. A Box–Behnken experimental design with a response surface methodology was used with six variables to be optimized: solid-to-solvent ratio, water percentage, temperature and time of extraction, amplitude, and catalyst concentration. The mixture of levulinic and formic acids achieved the highest extraction yield of polyphenols from pine needle and bark biomass. In addition, the solid-to-solvent ratio was found to be the only influential variable in the extraction (p-value: 0.0000). The optimal conditions were established as: 0.1 g of sample in 10 mL of LA:FA (70:30%, v/v) with 0% water and 0 M H2SO4 heated to 30 °C and extracted during 40 min with an ultrasound amplitude of 80% at 37 kHz. The bioactive properties of polyphenol-enriched extracts have been proven with significant antioxidant (45.90 ± 2.10 and 66.96 ± 2.75 mg Trolox equivalents/g dw) and antimicrobial activities. The possibility to recycle and reuse the solvent was also demonstrated; levulinic acid was successfully recovered from the extracts and reused in novel extractions on pine residues. This research shows an important alternative to obtaining polyphenol-enriched extracts from forest residues that are commonly discarded without any clear application, thus opening an important window toward the valorization of such residues.
1. Introduction
Maritime pine (Pinus pinaster Aiton) forests account for approximately 23% of Portuguese forests, providing a significant amount of harvested wood as raw material for the wood-based industries [1]. Consequently, a huge amount of pine-based residues are annually produced. These have been explored as potential renewable energy sources, with several recent public policies launched to encourage the use of residual forest biomass in Portugal [2]. However, maritime pine biomass holds many different chemicals, with added-value potential suitable for several applications [3]. Being a rich source of polyphenols with antioxidant and antimicrobial properties, the maritime pine extracts offer great potential for therapeutical practices, food enrichment, and biomaterial applications [4,5]. However, in order to produce extracts suitable for such applications, suitable solvents and extraction methodologies must be employed, according to the matrix source and desired type of target molecules [6].
Different methods have been developed ranging from the conventional maceration, Soxhlet, and solid–liquid extractions, to the more advanced supercritical fluid, pressurized water, microwave, or ultrasound-based approaches, which have been extensively performed in many different types of biomass [3,7,8]. Associated with these, harsh organic solvents have always been used for the extraction of bioactive compounds [9,10]. However, most modern techniques, such as ultrasound-assisted extraction (USE), are more efficient than conventional ones, mainly by reducing solvent volume, extraction time, and associated costs.
USE is based on the propagation of ultrasound pressure waves, resulting in cavitation forces that induce the explosive collapse of bubbles. This, generates localized pressure regions causing plant tissue rupture and improving the release of intra-cellular compounds to the solvent [11,12]. Nonetheless, advanced techniques such as USE also require optimization, mainly regarding solvent selection.
Nowadays, with the increasing environmental concern, some newly developed solvents have successfully replaced the commonly used organic solvents, such as methanol or acetone, in the extraction of bioactive components used in the pharmaceutical, food, and cosmetic industries. This is the case of deep eutectic solvents (DES), systems that are formed by mixing an appropriate molar ratio of a hydrogen-bond acceptor (HBA) with a hydrogen-bond donor (HBD) [13,14,15]. Once formed, these homogeneous mixtures present a significantly lower melting point than the melting points of each component [16,17]. These new solvent systems display similar properties to ionic liquids (ILs). In addition to the low volatility, DES are also non-toxic and simple to prepare at a lower cost than the traditional ILs. Contrary to ILs, DES synthesis and regeneration does not imply any chemical reaction as it should mainly involve the formation and rupturing of the hydrogen bonding network [16,18]. In certain cases, DESs can also hydrate up to 50% (v/v) without compromising their dissolution performance [19]. In fact, water addition has proved to influence the DES properties, such as density, viscosity, conductivity, and polarity [20]. The addition of water is desirable since most DESs have high viscosity. Therefore, the efficiency of extraction can be enhanced by improving the mass transfer rate by lowering the viscosity [21].
DESs have been used in the biomass fractionation of different plant species, such as acacia, Thymus, palm, Lavandula, or rosemary; fruit waste from olive, orange, pear, and grape skin; forest biowastes as pine needles and bark, wood from different trees or even the industrially processed kraft pulp; adding to this animal-based wastes such as cod skin and wool [12,14,17,22,23,24,25]. These are only a few examples of biomass versatility where DES-assisted extractions have been successfully employed. The ability to obtain valuable phenolic compounds from the valorization of bioresources and their application, for instance, in medicine, food, and cosmetics, is a key factor for a bio-based and sustainable economy [26]. Following this motto, in this research, the ultrasonic-assisted extraction of polyphenols present in pine needle and bark residues was assessed using several solvent systems. Choline chloride-based DES, acid and alkali solvents, and also a biomass-derived binary solvent system composed of levulinic acid:formic acid (LA:FA), previously used for lignin extraction from pine sawdust [27], were compared.
Since there are many variables related to the USE or the DESs preparation that can affect the extraction of polyphenols, a reliable design of experiment is required to evaluate the influence of each variable, their possible interactions, and consequently, their optimization to achieve the highest yield of extracted polyphenols. In this regard, a demanding Box–Behnken design with response surface methodology was employed to evaluate the influence of the different variables related to the extraction methodology on the final polyphenols’ recovery and bioactive properties. To the best of our knowledge, this is the first time that DESs combined with the USE technique have been employed for the extraction of polyphenols from maritime pine residues.
2. Materials and Methods
2.1. Chemicals
The solvents levulinic acid (LA), lactic acid (Lact), formic acid (FA), and acetic acid (Act) were acquired from Merck (Darmstadt, Germany), whereas choline chloride (ChCl) and methanol (MeOH) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Sodium hydroxide (NaOH), potassium hydroxide (KOH), and sulfuric acid (H2SO4) were obtained from Panreac (Barcelona, Spain). All solvents and reagents were purchased in analytical grade. When required, distilled-grade water was used.
Different solutions were prepared to evaluate the efficiency of polyphenols extraction from P. pinaster residues: (i) LA:H2O (30:60, v/v); (ii) LA:FA (70:30, v/v); (iii) LA:ChCl (70:30, v/v); (iv) Lact:ChCl (70:30, v/v); and (v) a solution of MeOH:H2O (80:20, v/v), solutions of NaOH:H2O (1:99, v/v) and KOH:H2O (1:99, v/v), and solutions of Act:H2O (24:76, v/v) and Lact:H2O (36:64, v/v) were used as standard chemical solvents. Solutions (ii), (iii), and (iv) were subsequently diluted in 60% of water: LA/FA:H2O (30:60, v/v), LA/ChCl:H2O (30:60, v/v), Lact/ChCl:H2O (30:60, v/v). These were prepared by manual mixing, followed by stirring to ensure the complete homogenization of the final solution.
Gallic acid (95% purity, Sigma-Aldrich Chemical Co., St. Louis, MO, USA) was used as standard to quantify the total polyphenol content. For the antioxidant activity measurements, 2,2-diphenyl-1-picrylhydrazyl (DPPH) from Sigma-Aldrich (St. Louis, MO, USA), and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) from Thermo Fisher Scientific (Waltham, MA, USA) were used as standards. Cultures of S. aureus (ATCC 6538) were grown on Tryptic soy broth from Biokar Diagnostics (Paris, France).
2.2. Samples
Pine residues (needles and bark) were collected from P. pinaster trees in the vicinity of the Universidade do Algarve in the Campus of Gambelas (Gambelas, Faro, Portugal) in October of 2021. Dirt, insects, and possible chemical residues were removed by washing with deionized water. After that, residues were manually cut into small pieces and dried in an oven (Model ED23, Binder GmbH, Tuttlingen, Germany) at 40 °C until constant weight, achieving 5% of moisture. Finally, the biomass was pulverized in a grinder until obtaining a fine and uniform powder that was kept dry at room temperature until further analysis.
2.3. Polyphenols Extraction
2.3.1. Ultrasound-Assisted Extraction System
Ultrasound-assisted extractions were performed using a 5 mm two-cylinder ultrasound probe coupled to a Vibracell 75186 ultrasound (Sonics, Newton, UK) (20 kHz, 130 W). For each extraction assay, 1.5 g of the sample was weighed in an Erlenmeyer flask with a capacity of 100 mL, and 10 mL of the corresponding solvent was added. The sample was placed in a water bath at the desired controlled temperature with a thermometer control unit. The ultrasound probe was carefully introduced without touching the bottom of the Erlenmeyer, and the frequency was fixed at 37 kHz. The corresponding amplitude and time were established before performing the extractions. After that, extracts were filtered with a Buchner funnel and a vacuum pump, collected in 10 mL volumetric flasks and kept at 5 °C until further analysis.
2.3.2. Box–Behnken Design of Experiments
The design of experiments is a methodology that relies on a mathematical description of the system behavior (in this case, polyphenols extraction employing USE) using the minimal number of experiments [28]. There are many designs of experiments, such as full factorial design, central composite design, mixture design, etc. In this case, a Box–Behnken design with response surface methodology (BBD-RSM) was used for the optimization of polyphenols extraction from pine residues. In this kind of design, three levels are assigned to each factor: (−1) as the lowest level, (0) as the intermediate level, and (1) as the highest level, producing a cube that consists of a central point and middle points at the edges, avoiding axial points [29]. It should be highlighted that in this approach, the number of experiments required to achieve the same information is lower, reason why it is considered more efficient than many of the alternative three-level full factorial designs [30]. In addition, the experiments performed avoid extreme conditions, so experimental methods that may pose an excessive economic or power consumption demand or that might lead to the degradation of the polyphenols are discarded [31].
The BBD-RSM can be defined by the following mathematical relation:
where Y is the response variable (i.e., total polyphenols); and β0, βi, βii, βij are the regression coefficients of the intercept, linear, quadratic and interaction terms, respectively. Xi and Xj are the independent variables.
Six variables were selected to be optimized: solid-to-solvent ratio (0.50–2.50 g/10 mL of solvent), water percentage (0–60%), temperature of extraction (30–60 °C), time of extraction (1–2 h), amplitude (20–80%), and catalyst concentration (0–2 M H2SO4). These variables and the studied range were based on the previous experience of the research group [17,27,32,33]. It is important to remark that although DESs mixtures usually present high boiling points due to their inherent physicochemical characteristics, many authors have proved the degradation of polyphenols at temperatures higher than 60 °C [34]. Thus, in this work, the maximum temperature was limited to 60 °C.
2.3.3. Total Polyphenols Content
The optimization of the USE method was based on extracting the maximum total polyphenols from pine residues. The extracts were analyzed at 280 nm, using a Genesys 10uv spectrophotometer (Thermo Scientific, Waltham, MA, USA), a wavelength where maximum absorbance is observed in polyphenols [35]. A gallic acid calibration curve was prepared between 0 and 800 mg/L and used as standard to estimate the total concentration of polyphenols. The calibration curve obtained was y = 6.08x − 0.01, where y is the absorbance, and x is the gallic acid concentration in mg/L. The coefficient of regression was R2 = 0.9994. The total polyphenol concentration was expressed as mg/g dry weight (dw).
2.4. Antioxidant Activity
The DPPH method was selected to evaluate the antioxidant activity of the extracts [36]. The procedure consisted of mixing 300 µL of DPPH solution [90 µM DPPH in methanol:H2O (80:20% v/v)] with 30 µL of the extract and 570 µL of the MeOH:H2O solution (80:20% v/v). This mixture was kept at room temperature and in the dark for 30 min, and the absorbance was measured at 515 nm. Trolox was the selected standard, and a six-point regression curve was prepared with concentrations from 0 mM to 0.30 mM. The obtained equation was y = 221.57x + 2.51, with a coefficient of R2 = 0.9915. The antioxidant activity was expressed as mg of Trolox equivalents (TE) per gram of dry weight (mg TE/g dw).
2.5. Antimicrobial Activity
The antimicrobial activity of the extracts was evaluated following the S. aureus (ATCC 6538) growth in the presence of the extracted polyphenols. The initial inoculum had a concentration of 104–105 colony-forming units (CFU/mL: colony-forming units per milliliter), grown in TSB (Tryptic soy broth) medium. The following procedure considers 200 µL of the inoculum, which was loaded in microplate wells and supplemented with 10 µL of the polyphenol extract. All tests were performed in triplicate, and a non-supplemented bacterial culture was used as a control. After 24 h at 37 °C, the optical density of the culture was measured at 600 nm, and the bacterial growth was evaluated.
2.6. Solvent Reuse
As previously mentioned, DESs mixtures can be considered “green solvents” due to their inherent physicochemical characteristics, origin, and synthesis procedure. For this reason, the recycling and reuse of the DES mixture employed for the polyphenols’ extraction were evaluated. The extracts obtained from pine residues under optimal conditions were diluted 1:100 with deionized water and filtered using a Strata-X cartridge (Phenomenex, Madrid, Spain). The polyphenols were retained in the cartridge, and the diluted solvent was collected. Those extracts were analyzed by LC-MS to ensure that no LA and FA were present. The water and FA were evaporated using a rotary evaporator fixed at 100 °C. After 15 min, the recovered LA was collected, reused for the preparation of novel LA:FA (70%, 30% v/v) mixtures, and applied in subsequent pine needle and bark extractions under optimal conditions.
2.7. Data Analysis
The BBD-RSM design was performed using Statgraphic Centurion software (version XVII) (Statgraphics Technologies, Inc., The Plains, VA, USA). During the analysis of the results, data were compared and grouped according to the LSD (least significant difference), response surface regression techniques, and the Fisher test considering a significance of 95% (p-value ≤ 0.05).
3. Results and Discussion
3.1. Suitability of DESs Solvents
The first task of this research focused on the feasibility of using the DESs mixtures for the extraction of polyphenols from pine residues. To do so, different extractions were carried out using the solvent mixtures and experimental conditions introduced in the experimental section.
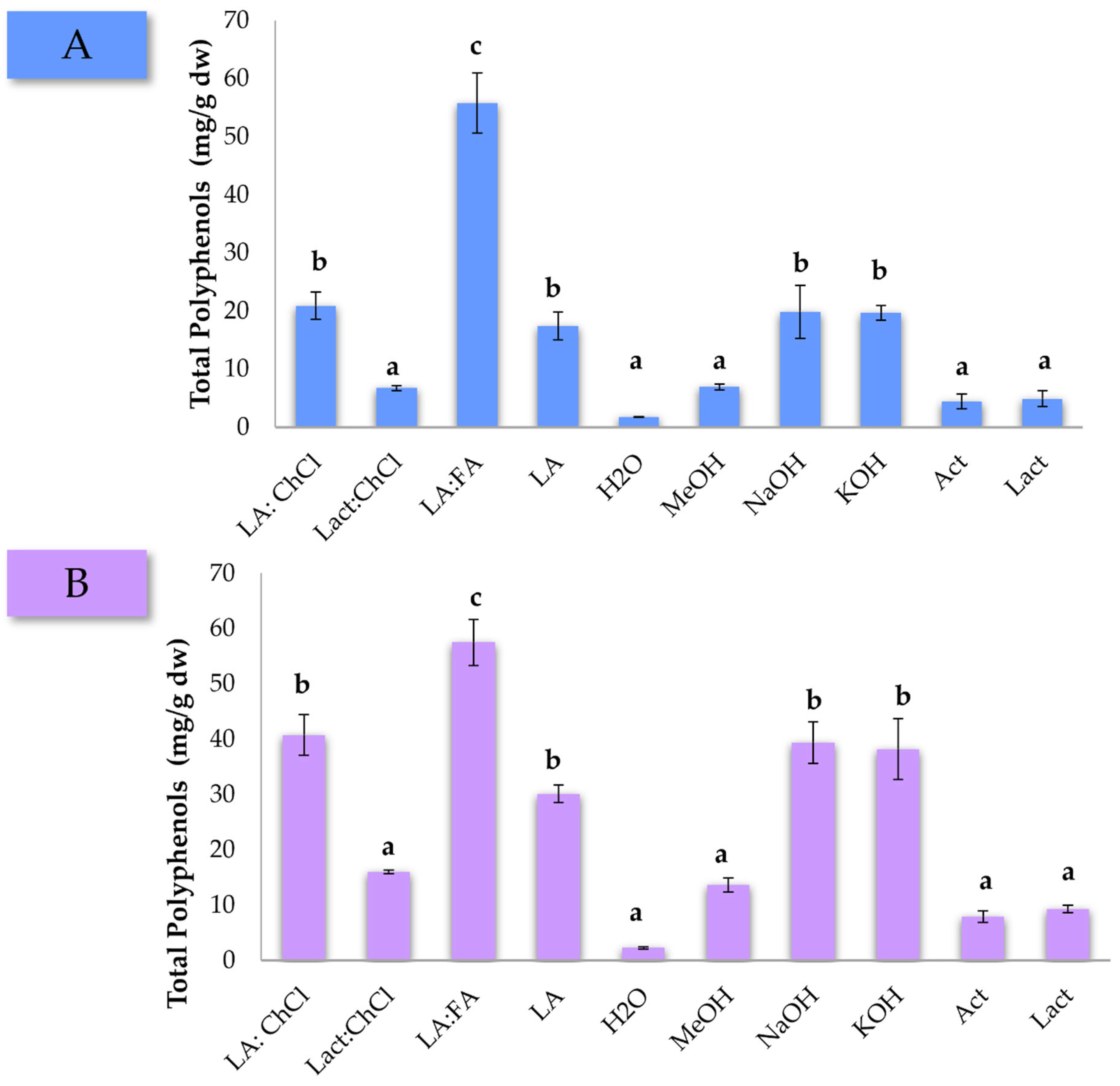
The extractions were carried out for 1 h in triplicate, and the followed procedure to obtain the final extracts can be found in the Section 2. The average of the total polyphenol concentration is represented in Figure 1. The first striking observation is that the LA:FA mixture allowed extracting the maximum concentration of polyphenols in both pine matrices. Moreover, the LA:ChCl mixture is regarded as an important alternative for the extraction of these compounds, reaching higher concentrations than those extracted with NaOH and KOH aqueous solutions. On top of the evident superior extraction performance, it should be added that recent advances in the preparation of DES compounds, such as FA, have allowed their sourcing from natural resources using eco-friendly approaches [37]. Extraction with H2O represented the lowest concentration of polyphenols recovered in both pine wastes. Finally, it is also observed that the concentration of polyphenols extracted from pine bark was higher than from the needles. This result is aligned with the fact that bark is known to have a higher native concentration of polyphenols [3]. Overall, data suggest that DESs mixtures are an interesting alternative to traditional chemical solvents for the extraction of polyphenols [38,39,40].
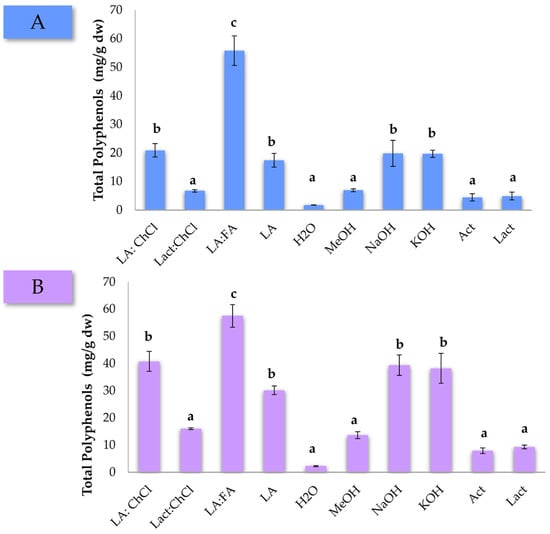
Figure 1.
Total polyphenols concentration (mg/g dw) for P. pinaster (A) needles and (B) bark according to the different DES mixtures and chemical solvents used for the extraction. Solvent acronyms have been defined in the experimental section. Different letters indicate significant differences in the ANOVA Duncan post hoc test with 95% of confidence.
One-way ANOVA tests were carried out, obtaining p-values of 5.0 × 10−6 and 1.3 × 10−5 for pine needles and pine bark, respectively, thus ensuring significant differences among the solvents used. After this initial screening, the LA:FA mixture was selected as the DESs mixture for the optimization using the USE-based method for the extraction of polyphenols from pine residues.
3.2. BBD-RSM Optimization
Once the DESs solvent was selected for the extraction, the USE method was optimized using a BBD-RSM design where six variables were selected as described in the experimental section. The selection of these variables as well as their range of study were based on the previous experience of the group. In addition, since it was previously observed that the concentration of polyphenols was lower in the pine needle residue than in the bark, it was decided to perform the optimization of the method using only the pine needles (poorer matrix) and later apply the optimized conditions to the pine bark.
This design resulted in a total of 54 experiments (Table 1) that were randomly performed. The extracts obtained were manipulated as previously described. The results were analyzed by means of the BBD-RSM design using the Statgraphics software.

Table 1.
Box–Behnken design conditions used for the optimization of the USE-based extraction of polyphenols from pine needles.
The total polyphenols concentration predicted by the response surface methodology using developed coefficients based on the conditions of each experiment and Equation (1), was compared with the concentration of total polyphenols observed, obtaining an average error of 5.11%. It should be mentioned that this error ranged from 0.11% to 18.29%, being less than 10% in most cases and, therefore, demonstrating the suitability of the developed method. In addition, the R-Squared statistic indicates that the developed model explains 80.73% of the variance of THE response variable, demonstrating its correct mathematical adjustment. The standard error of the estimate shows that the standard deviation of the residuals is 43.71. On the other hand, the Durbin–Watson statistic tests the residuals to determine if there is any significant connection based on the order in which the data is presented. Since the p-value is greater than 5.0%, there is no indication of serial autocorrelation in the residuals at the 95% level of confidence.
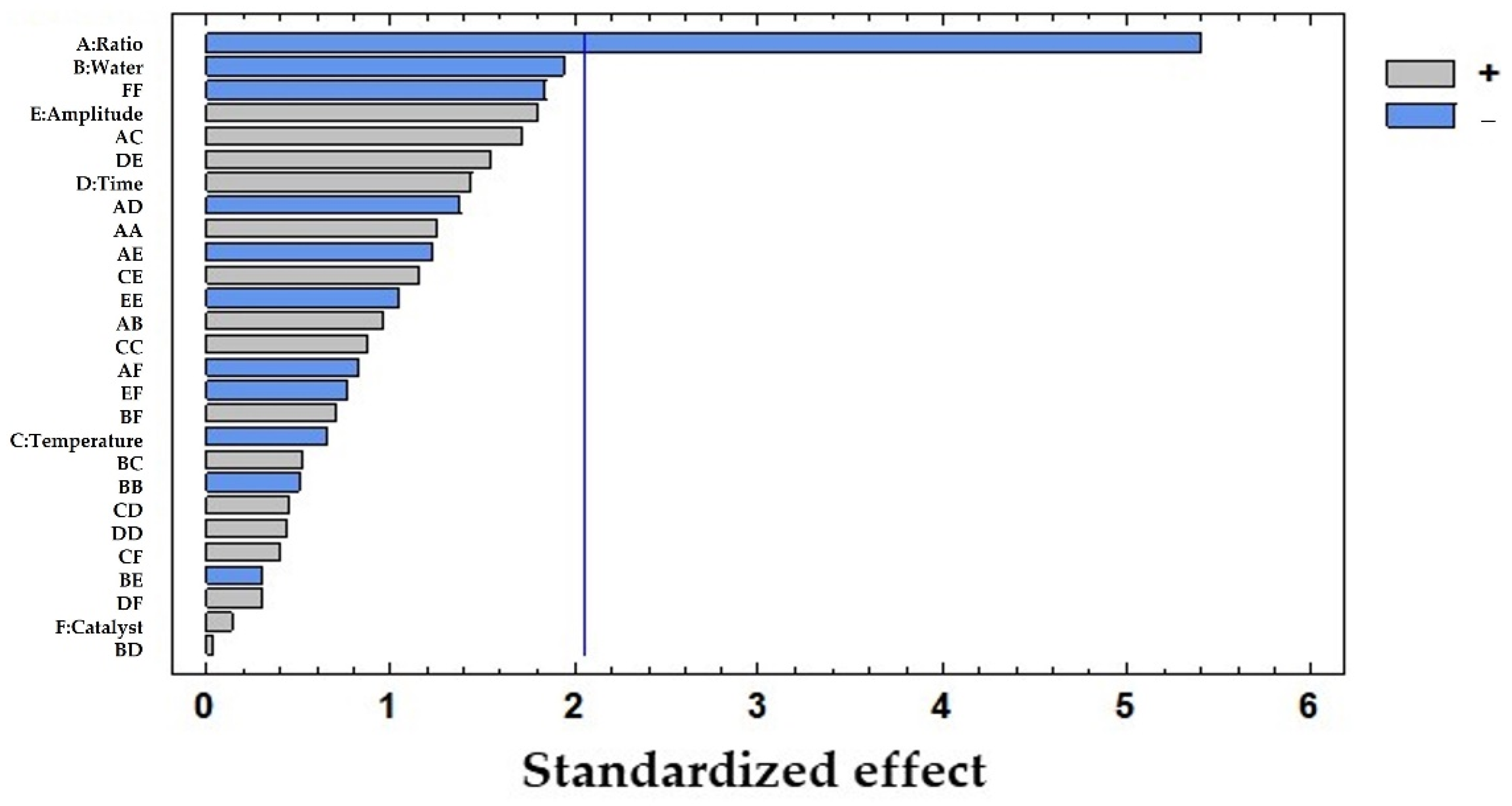
To evaluate the influence of each of the studied variables, an ANOVA test was carried out with LSD considering a 95% confidence level (Table 2). The solid-to-solvent ratio was found to be the only influential variable (p-value: 0.0000), while the rest of the variables showed p-values > 0.05, with the percentage of water being the closest to being influential (p-value: 0.0638). The results were graphically represented in a Pareto diagram as shown in Figure 2. In this chart, the negative influence of the solid-to-solvent ratio on the extraction of polyphenols from pine needles is observed which suggests that the lower the ratio, the higher the concentration of polyphenols extracted.

Table 2.
Variance analysis of the quadratic model stepwise adjusted to the USE-based extraction of polyphenols from pine needles.
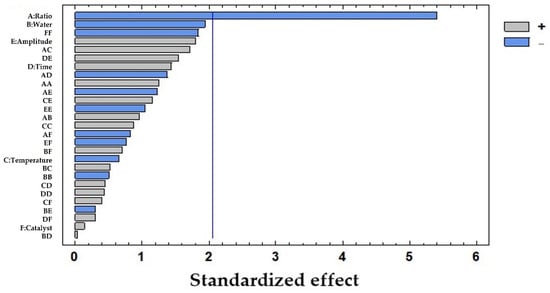
Figure 2.
Pareto chart results from the total polyphenols extracted (mg/g dw) from pine needles using USE: A: solid-to-solvent ratio (g sample in 10 mL solvent); B: water (%) in solvent; C: temperature of extraction (°C); D: time of extraction (h); E: amplitude (%); F: catalyst concentration of H2SO4 (M). The vertical line marks 95% of confidence.
Finally, since the β coefficients have been calculated using the least-squares method, the response surface methodology is used to obtain the values of the controllable parameters (Xi) that optimize the response or to discover what values will result in the maximum recovery of polyphenols. In this case, the optimal extraction conditions were established as 0.50 g of sample in 10 mL of LA:FA (70:30%, v/v) with 0% water and 0 M H2SO4 heated to 30 °C and extracted for 30 min with an amplitude of 80% at 37 kHz.
As can be observed, the amount of sample was found at the lower end of the studied range, and thus it was decided to further expand it. The optimum temperature value was also found at the lower end of the studied range. Nevertheless, to save energy and guarantee better control over the method and repeatability, the lowest temperature is preferred. Therefore, the subsequent trials were performed at 30 °C. On the other hand, the ultrasound amplitude was found at the upper end of the studied range, but given the system used, it was technically impossible to increase this value. Moreover, since it is not an influential variable, it was decided to proceed with the trials with 80% amplitude. Finally, the extraction time was also at the upper end of the studied range, and thus it was decided to further widen the range of this variable as well.
Two-Factorial Design
As indicated above, some variables were selected to be further optimized considering a wider range of studies. This was the case of the extraction time and solid-to-solvent ratio. For that, a two-factorial design with RSM was selected to evaluate the influence of the new ranges in the extraction of total polyphenols from pine needles by USE. The new solid-to-solvent ratio range was set from 0.1 to 0.5 g per 10 mL of solvent, while the extraction time was set from 5 to 60 min, implying a total of nine experiments (Table 3) that were randomly performed.

Table 3.
Experimental conditions and the resulting two-factorial design used to optimize: solid-to-solvent ratio and extraction time parameters.
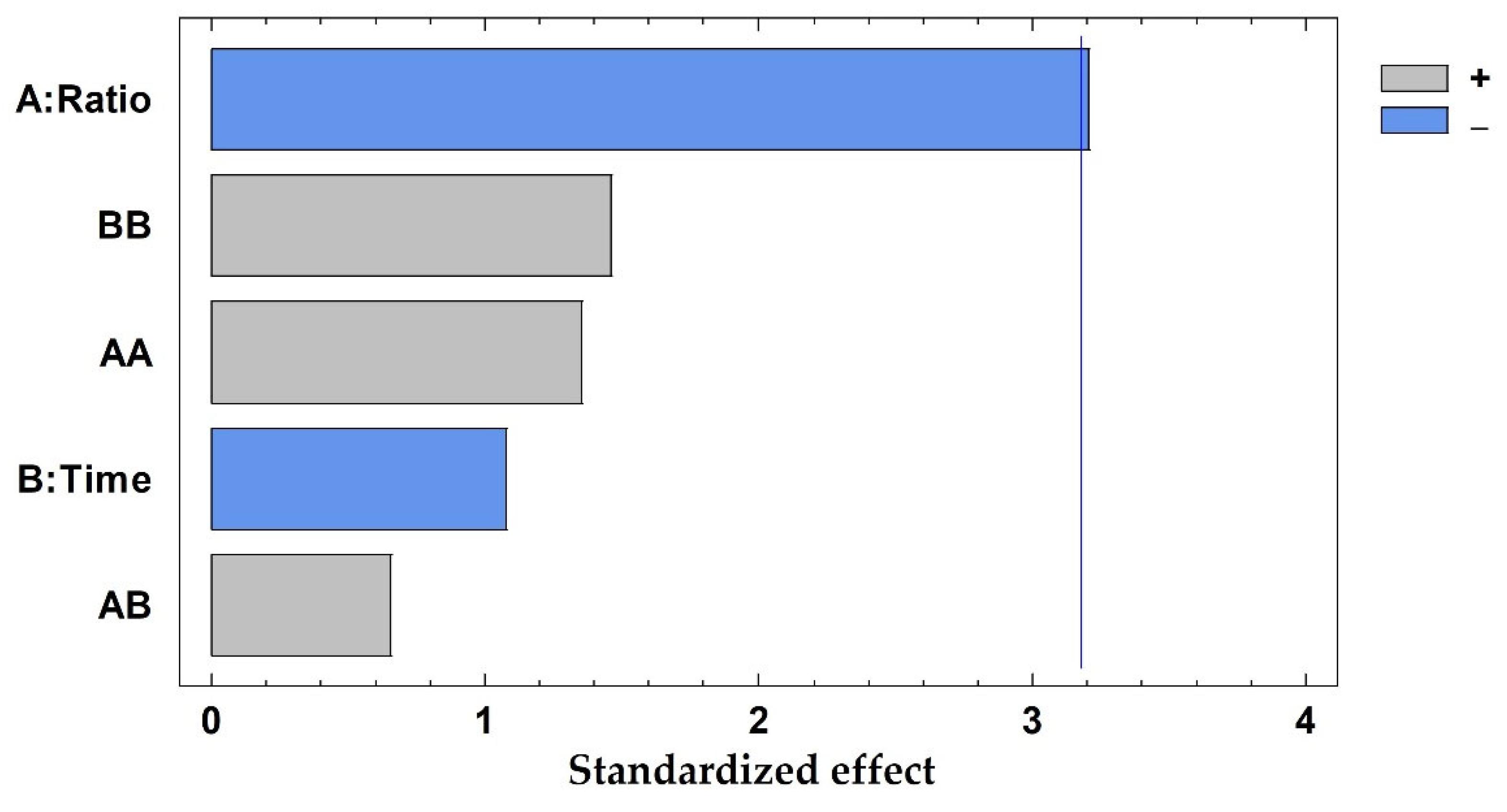
The average prediction error was 7.24%, ranging from 0.47% to 18.63%, being lower than 20% in all cases. In addition, the R2 coefficient was 0.9333, ensuring a good adjustment of the developed method. The solid-to-solvent ratio was the only influential variable considering a 95% of confidence (p-value: 0.0093), whereas the time of extraction and the interaction between variables exhibited p-values higher than 0.05, proving that these are not influential in the current method. Results have been graphically represented in a Pareto chart (Figure 3), where the negative influence of the solid-to-solvent ratio can be observed, which means that the highest concentration of polyphenols was achieved when the lowest value of this parameter was considered.
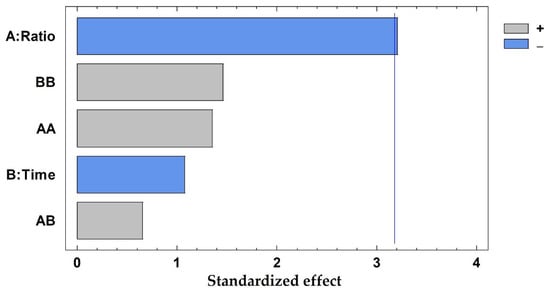
Figure 3.
Pareto chart results from the total polyphenols extracted (mg/g dw) from pine needles using factorial design, A: solid-to-solvent ratio (g sample in 10 mL solvent); B: time of extraction (minutes). The vertical line marks 95% of confidence.
After this extra optimization, the optimal extraction conditions were set at: 0.1 g of sample in 10 mL of LA:FA (70:30%, v/v) with 0% water and 0 M H2SO4 heated to 30 °C and extracted for 40 min with an amplitude of 80% at 37 kHz. As can be observed, the amount of sample continued to be in the lowest value of the studied range. However, it was considered that lower amounts of the sample could produce higher errors during the weighting procedure.
It should be highlighted that the optimal extraction conditions agreed with other studies in related residues. Meullemiestre et al. [8] optimized the application of USE for polyphenols extraction from pine sawdust and observed that an extraction performed at 40 °C and 40 min with 40 kHz of power maximizes the extraction yield, i.e., 224 mg/g. Aspé et al. [41] also evaluated the use of an ultrasonic bath in the extraction of polyphenols from P. radiata bark, obtaining the highest yield (12.7 mg/g) when 35 kHz at 85 W and 25 °C were used. Lastly, Nisca et al. [42] studied the use of ultrasound in the extraction of polyphenols from P. nigra and P. sylvestris bark, obtaining the best results (53.41 and 43.05 mg/g, respectively) when 1 g of sample was treated with an alcoholic solution and kept in a bath at 65 °C for 30 min. As can be observed, even if the conditions are not fully comparable, the optimal parameters reported in the literature agree with the conditions optimized in the current research. However, it should be highlighted that, in all cases, the total amount of obtained polyphenols is lower than the ones obtained in the present research. The innovative combination of DES and USE considerably boosts the total concentration of polyphenols recovered from pine biomass.
3.3. Repeatability and Intermediate Precision
The repeatability and intermediate precision as parameters were considered to access the suitability of the developed extraction method and the applicability in pine bark residues [43]. Six extractions were carried out under optimal conditions on the same day for the repeatability assay whereas 6 more extractions were performed on 2 consecutive days for the intermediate precision analysis, resulting in a total of 18 extractions. Note that these experiments were applied to both pine needle and bark residues.
Again, the extracts were analyzed at 280 nm to estimate the total concentration of polyphenols, and the coefficient of variation (CV) was the selected variable to infer on the suitability of the method (Table 4). In the repeatability assay, the CV was 1.65% and 1.64%, while in the case of the intermediate precision test, the CV was 3.92% and 3.36% for pine needles and bark residues, respectively. In all cases, the CV was lower than 5%, proving the suitability of the developed method for the superior extraction of polyphenols from pine needles and bark waste. In addition, the average total concentration of polyphenols extracted under optimal conditions from pine needles and bark were 274.38 and 314.62 mg/g, respectively, which are considerably higher than the concentrations previously reported in related studies [8,41,42].

Table 4.
Repeatability (n = 6) and intermediate precision (n = 18) tests for polyphenols recovery from pine needles and bark residues.
3.4. Bioactive Properties of the Extracts
Once polyphenol-enriched extracts from pine needle and bark were obtained under optimal conditions, the following step focused on evaluating their bioactive properties, particularly the antioxidant and antimicrobial activities. The antioxidant activity was evaluated according to the DPPH method [36,44]. For that, three extracts from pine needle and bark obtained using optimal conditions were selected, and the antioxidant activity was estimated following the procedure described in the methodology section. The antioxidant activity of the solvent was also assessed under the same conditions and its contribution subtracted.
The results showed average antioxidant activities of ca. 45.90 ± 2.10 and 66.96 ± 2.75 mg Trolox equivalents/g dw for pine needles and bark, respectively. These results are in perfect agreement with those reported by Nisca et al. [42] where polyphenols extracted with an alcoholic solution with USE exhibited 74.59% and 70.36% inhibition for P. nigra and P. sylvestris residues, respectively.
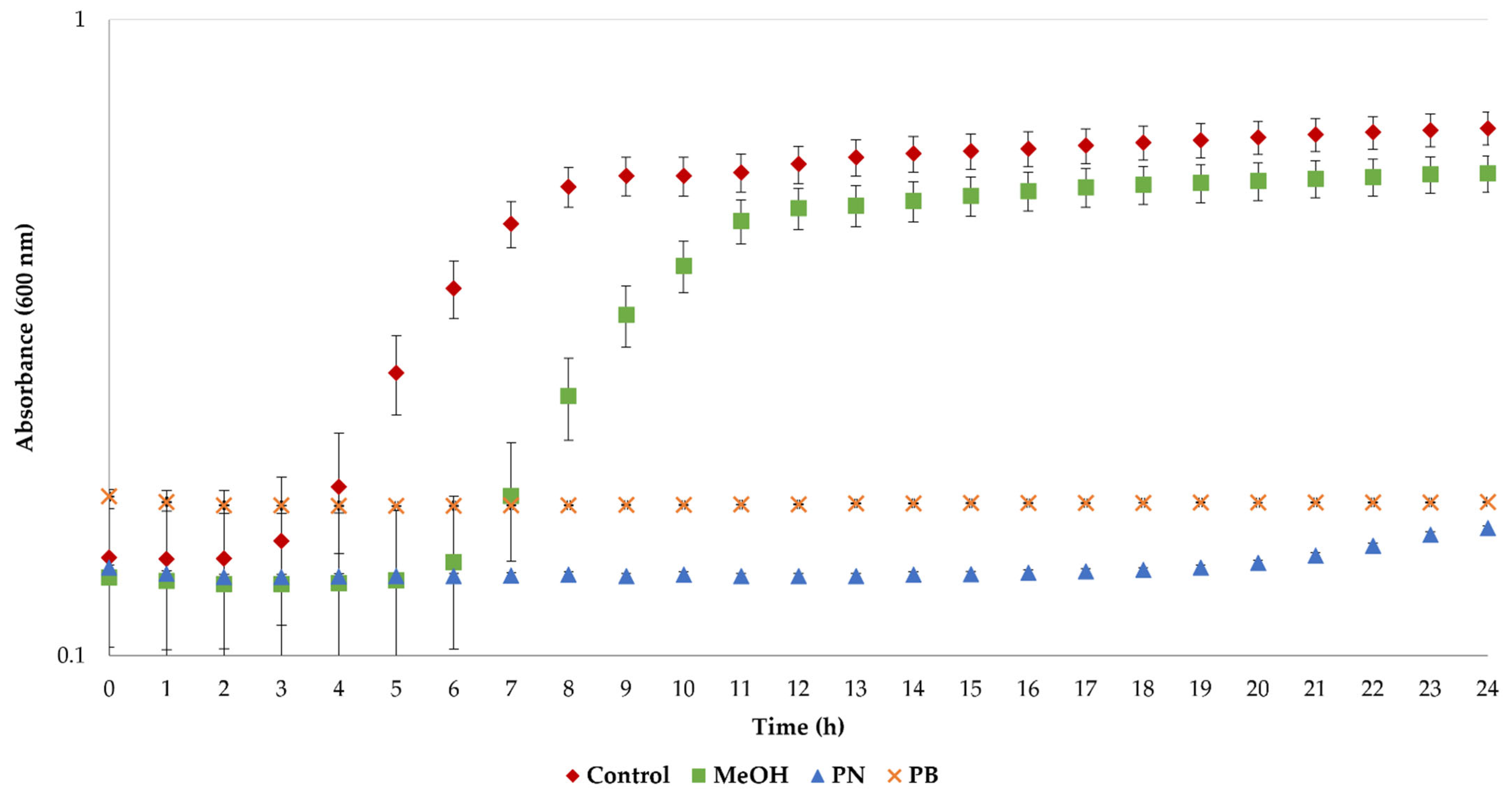
Bacterial cultures were also exposed to pine needle and bark extracts to evaluate their antimicrobial activity. As was previously mentioned, the antimicrobial properties of LA have been previously described [45], and therefore the extracted polyphenols were redissolved in MeOH to avoid any LA effect. As can be observed in Figure 4, the growth curves of S. aureus cells display typical sigmoidal profiles for the control cultures. In the case of the solvent (MeOH), the growth of cells was delayed, but exponential growth was achieved after 11 h. More importantly, no growth was observed when the bacteria were exposed to the polyphenols’ extracts, thus demonstrating their remarkable antimicrobial properties.
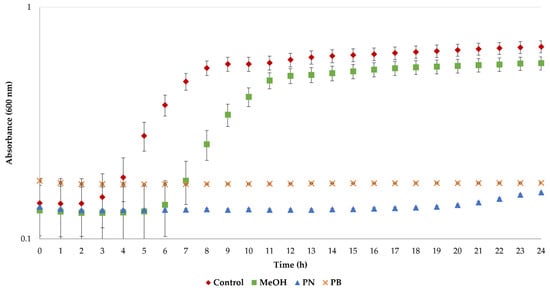
Figure 4.
Antimicrobial activity of the extracts from pine needle and bark residues obtained under optimal extraction conditions.
3.5. Solvent Recyclability and Reuse
From economic and environmental perspectives, solvent recyclability and reusability are highly desirable for sustainable large-scale applications. Therefore, the possibility of recycling and reusing LA from the polyphenols-enriched extracts was also evaluated. The extracts were treated as described in the Section 2, allowing the total separation of polyphenols from the solvent based on the similar polarity of Strata-X cartridges and polyphenols. Such favorable interactions induce the polyphenol adsorption and retention in the cartridges and consequently their separation from the solvent. After the evaporation, the recovery yield of LA was ca. 60%.
The recovered solvent was mixed with neat FA to prepare a new DES mixture (70:30%, LA:FA) which was subsequently employed for the extraction of polyphenols in both pine needle and bark under optimal conditions. Each extraction was performed in triplicate. Although the solvent recovery and purification protocols were not optimized and targeted in this work, it was observed that such simple proof of concept has allowed the preparation of novel DES with remarkable extraction capacity, i.e., the extraction performance with the recycled LA has only decreased 8% when compared with neat LA. These successful initial trials demonstrate the feasibility of recycling LA in an accessible way without significantly compromising the extraction performance.
4. Conclusions
In the present investigation, the use of novel deep eutectic solvents was evaluated as a sustainable alternative to the traditional chemical solvents commonly used for the extraction of bioactive compounds. Specifically, this study has focused on the extraction of polyphenols, bioactive compounds that have properties of great interest, such as antioxidant, antimicrobial, and anti-inflammatory activities. In addition, these compounds have been extracted from forest residues that are currently being discarded without any added value. Therefore, the possibility of using green solvents for the extraction of these compounds with similar or even higher yields than those observed in the literature has been demonstrated.
The optimization of an ultrasound-assisted extraction method was performed by targeting the recovery of polyphenols. It was found that the solid-to-solvent ratio (p-value: 0.000) is the only significantly influential variable in the process, and the following optimal conditions were established: 0.1 g of pine residue in 10 mL of LA:FA (70:30%, v/v) with 0% water and 0 M H2SO4 heated to 30 °C and extracted 40 min with ultrasound amplitude of 80% at 37 kHz. The suitability of the method was demonstrated by reaching coefficients of variation lower than 5% when the repeatability and intermediate precision assays were evaluated. Although not fully optimized, solvent recyclability and reusability were shown to be possible; separation of the bioactive compounds from the solvent used was achieved with a simple and affordable procedure, where more than 60% of levulinic acid was recovered and successfully reused in new extractions.
Finally, the remarkable antioxidant and antimicrobial properties of the polyphenol-enriched pine extracts were demonstrated. This represents an important scientific advance toward sustainable alternatives for the extraction of added-value bioactive compounds with potential applications in different fields, such as nutrition, medicine, or nutraceutical. We believe that the combination of DES with the USE approach described in this work results in a novel and attractive green extraction process promising for large-scale applications, thus strongly contributing to the valorization of forest-based residues into novel end-applications.
Author Contributions
H.D.: formal analysis; data curation; writing—original draft preparation; V.G.: formal analysis; investigation; writing—original draft preparation; M.J.A.-G.: conceptualization; methodology; supervision; writing—review and editing; B.M.: conceptualization; writing—review and editing; visualization; supervision; L.F.: formal analysis; methodology; writing—review and editing; A.R.: writing—review and editing; visualization; funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Funds through FCT—Foundation for Science and Technology under Project UIDB/05183/2020. This work was also supported by funding from the Portuguese Foundation for Science and Technology (FCT) through the projects PTDC/ASP-SIL/30619/2017, UIDB/05183/2020, and the researcher grant CEECIND/01014/2018.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
María José Aliaño-González would like to thank the University of Cádiz for the grant in the modality “Margarita Salas”, of the call for the Requalification of the Spanish University System for 2021–2023 (Resolution of the Rector of the University of Cadiz UCA/R155REC/2021, of 2 July), and financed by the Ministry of Universities of the Government of Spain through the European Recovery Instrument “Next Generation EU”, of the European Union (Order UNI/551/2021, of 26 May).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alegria, C.; Roque, N.; Albuquerque, T.; Fernandez, P.; Ribeiro, M.M. Modelling Maritime Pine (Pinus pinaster Aiton) Spatial Distribution and Productivity in Portugal: Tools for Forest Management. Forests 2021, 12, 368. [Google Scholar] [CrossRef]
- da Costa, T.P.; Quinteiro, P.; Arroja, L.; Dias, A.C. Environmental Comparison of Forest Biomass Residues Application in Portugal: Electricity, Heat and Biofuel. Renew. Sustain. Energy Rev. 2020, 134, 110302. [Google Scholar] [CrossRef]
- Santos, J.; Pereira, J.; Ferreira, N.; Paiva, N.; Ferra, J.; Magalhães, F.D.; Martins, J.M.; Dulyanska, Y.; Carvalho, L.H. Valorisation of Non-Timber by-Products from Maritime Pine (Pinus pinaster, Ait) for Particleboard Production. Ind. Crops Prod. 2021, 168, 113581. [Google Scholar] [CrossRef]
- Alonso-Esteban, J.I.; Carocho, M.; Barros, D.; Velho, M.; Heleno, S.; Barros, B. Chemical Composition and Industrial Applications of Maritime Pine (Pinus pinaster Ait.) Bark and Other Non-Wood Parts. Rev. Environ. Sci. Biotechnol. 2022, 21, 583–633. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Pereira, C.; Gomes, A.P.; Neto, R.T.; Almeida, A.; Santos, S.A.O.; Silva, A.M.S.; Silvestre, A.J.D. Chemical Characterisation, Antioxidant and Antibacterial Activities of Pinus pinaster Ait. and Pinus pinea L. Bark Polar Extracts: Prospecting Forestry By-Products as Renewable Sources of Bioactive Compounds. Appl. Sci. 2022, 12, 784. [Google Scholar] [CrossRef]
- Pagano, I.; Campone, L.; Celano, R.; Piccinelli, A.L.; Rastrelli, L. Green Non-Conventional Techniques for the Extraction of Polyphenols from Agricultural Food by-Products: A Review. J. Chromatogr. A 2021, 1651, 462295. [Google Scholar] [CrossRef]
- Chupin, L.; Maunu, S.L.; Reynaud, S.; Pizzi, A.; Charrier, B.; Charrier-EL Bouhtoury, F. Microwave Assisted Extraction of Maritime Pine (Pinus pinaster) Bark: Impact of Particle Size and Characterization. Ind. Crops Prod. 2015, 65, 142–149. [Google Scholar] [CrossRef]
- Meullemiestre, A.; Petitcolas, E.; Maache-Rezzoug, Z.; Chemat, F.; Rezzoug, S.A. Impact of Ultrasound on Solid–Liquid Extraction of Phenolic Compounds from Maritime Pine Sawdust Waste. Kinetics, Optimization and Large Scale Experiments. Ultrason. Sonochem. 2016, 28, 230–239. [Google Scholar] [CrossRef]
- de Hoyos-Martínez, P.L.; Merle, J.; Labidi, J.; Charrier-El Bouhtoury, F. Tannins Extraction: A Key Point for Their Valorization and Cleaner Production. J. Clean. Prod. 2019, 206, 1138–1155. [Google Scholar] [CrossRef]
- Rente, D.; Paiva, A.; Duarte, A.R. The Role of Hydrogen Bond Donor on the Extraction of Phenolic Compounds from Natural Matrices Using Deep Eutectic Systems. Molecules 2021, 26, 2336. [Google Scholar] [CrossRef]
- Naqvi, S.A.R.; Akbar, N.; Shah, S.M.A.; Ali, S.; Abbas, A. Chapter 14—Green Approaches for the Extraction of Bioactive from Natural Sources for Pharmaceutical Applications. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Boddula, R., Ahamed, M.I., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 269–291. ISBN 978-0-12-821885-3. [Google Scholar]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Ultrasonic-Assisted Extraction and Natural Deep Eutectic Solvents Combination: A Green Strategy to Improve the Recovery of Phenolic Compounds from Lavandula pedunculata Subsp. lusitanica (Chaytor) Franco. Antioxidants 2021, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Duarte, H.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Response of Thymus lotocephalus In Vitro Cultures to Drought Stress and Role of Green Extracts in Cosmetics. Antioxidants 2022, 11, 1475. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Sangoro, J.; Ragauskas, A.J. Natural Deep Eutectic Solvents for Lignocellulosic Biomass Pretreatment: Recent Developments, Challenges and Novel Opportunities. Biotechnol. Adv. 2018, 36, 2032–2050. [Google Scholar] [CrossRef]
- Fernandes, C.; Melro, E.; Magalhães, S.; Alves, L.; Craveiro, R.; Filipe, A.; Valente, A.J.M.; Martins, G.; Antunes, F.E.; Romano, A.; et al. New Deep Eutectic Solvent Assisted Extraction of Highly Pure Lignin from Maritime Pine Sawdust (Pinus pinaster Ait.). Int. J. Biol. Macromol. 2021, 177, 294–305. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yu, J.; Lu, Y.; Jiang, B.; Fan, Y.; Wang, Z. High-Purity Lignin Isolated from Poplar Wood Meal through Dissolving Treatment with Deep Eutectic Solvents. R. Soc. Open Sci. 2019, 6, 181757. [Google Scholar] [CrossRef]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The Effect of Water upon Deep Eutectic Solvent Nanostructure: An Unusual Transition from Ionic Mixture to Aqueous Solution. Angew. Chem. Int. Ed. 2017, 56, 9782–9785. [Google Scholar] [CrossRef]
- Yang, M. Efficient Extraction of Bioactive Flavonoids from Ginkgo Biloba Leaves Using Deep Eutectic Solvent/Water Mixture as Green Media. Chem. Biochem. Eng. Q. 2018, 32, 315–324. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring Properties of Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Malvis, A.; Šima, J. Extraction of Value-Added Components from Food Industry Based and Agro-Forest Biowastes by Deep Eutectic Solvents. J. Biotechnol. 2018, 282, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of Polyphenolic Antioxidants from Orange Peel Waste Using Deep Eutectic Solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of Apple Tree Wood Residues by Polyphenols Extraction: Comparison between Conventional and Microwave-Assisted Extraction. Ind. Crops Prod. 2017, 104, 210–220. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of Phenolic Compounds from Olive Pomace by Using Natural Deep Eutectic Solvents and Innovative Extraction Techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Alam, M.A.; Muhammad, G.; Khan, M.N.; Mofijur, M.; Lv, Y.; Xiong, W.; Xu, J. Choline Chloride-Based Deep Eutectic Solvents as Green Extractants for the Isolation of Phenolic Compounds from Biomass. J. Clean. Prod. 2021, 309, 127445. [Google Scholar] [CrossRef]
- Magalhães, S.; Filipe, A.; Melro, E.; Fernandes, C.; Vitorino, C.; Alves, L.; Romano, A.; Rasteiro, M.G.; Medronho, B. Lignin Extraction from Waste Pine Sawdust Using a Biomass Derived Binary Solvent System. Polymers 2021, 13, 1090. [Google Scholar] [CrossRef]
- Dejaegher, B.; Heyden, Y.V. Experimental Designs and Their Recent Advances in Set-up, Data Interpretation, and Analytical Applications. J. Pharm. Biomed. Anal. 2011, 56, 141–158. [Google Scholar] [CrossRef]
- Ayyubi, S.N.; Purbasari, A.; Kusmiyati. The Effect of Composition on Mechanical Properties of Biodegradable Plastic Based on Chitosan/Cassava Starch/PVA/Crude Glycerol: Optimization of the Composition Using Box Behnken Design. Mater. Today 2022, 63, S78–S83. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken Design: An Alternative for the Optimization of Analytical Methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Khatib, I.; Chow, M.Y.T.; Ruan, J.; Cipolla, D.; Chan, H.-K. Modeling of a Spray Drying Method to Produce Ciprofloxacin Nanocrystals inside the Liposomes Utilizing a Response Surface Methodology: Box-Behnken Experimental Design. Int. J. Pharm. 2021, 597, 120277. [Google Scholar] [CrossRef]
- Magalhães, S.; Moreira, A.; Almeida, R.; Cruz, P.F.; Alves, L.; Costa, C.; Mendes, C.; Medronho, B.; Romano, A.; Carvalho, M. da G.; et al. Acacia Wood Fractionation Using Deep Eutectic Solvents: Extraction, Recovery, and Characterization of the Different Fractions. ACS Omega 2022, 7, 26005–26014. [Google Scholar] [CrossRef] [PubMed]
- Medronho, B.; Duarte, H.; Magalhães, S.; Alves, L.; Valente, A.J.M.; Romano, A. From a New Cellulose Solvent to the Cyclodextrin Induced Formation of Hydrogels. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 548–555. [Google Scholar] [CrossRef]
- Diaconeasa, Z. Time-Dependent Degradation of Polyphenols from Thermally-Processed Berries and Their In Vitro Antiproliferative Effects against Melanoma. Molecules 2018, 23, 2534. [Google Scholar] [CrossRef] [PubMed]
- Kaeswurm, J.A.H.; Scharinger, A.; Teipel, J.; Buchweitz, M. Absorption Coefficients of Phenolic Structures in Different Solvents Routinely Used for Experiments. Molecules 2021, 26, 4656. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; van Beek, T.A. Screening of Radical Scavenging Activity of Some Medicinal and Aromatic Plant Extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Voß, D.; Kahl, M.; Albert, J. Continuous Production of Formic Acid from Biomass in a Three-Phase Liquid–Liquid–Gas Reaction Process. ACS Sustain. Chem. Eng. 2020, 8, 10444–10453. [Google Scholar] [CrossRef]
- Xue, H.; Li, J.; Wang, G.; Zuo, W.; Zeng, Y.; Liu, L. Ultrasound-Assisted Extraction of Flavonoids from Potentilla fruticosa L. Using Natural Deep Eutectic Solvents. Molecules 2022, 27, 5794. [Google Scholar] [CrossRef]
- Zhen, S.; Chen, S.; Geng, S.; Zhang, H.; Chen, Y.; Liu, B. Ultrasound-Assisted Natural Deep Eutectic Solvent Extraction and Bioactivities of Flavonoids in Ampelopsis Grossedentata Leaves. Foods 2022, 11, 668. [Google Scholar] [CrossRef]
- Braham, F.; Amaral, L.M.P.F.; Biernacki, K.; Carvalho, D.O.; Guido, L.F.; Magalhães, J.M.C.S.; Zaidi, F.; Souza, H.K.S.; Gonçalves, M.P. Phenolic Extraction of Moringa Oleifera Leaves in DES: Characterization of the Extracts and Their Application in Methylcellulose Films for Food Packaging. Foods 2022, 11, 2641. [Google Scholar] [CrossRef]
- Aspé, E.; Fernández, K. The Effect of Different Extraction Techniques on Extraction Yield, Total Phenolic, and Anti-Radical Capacity of Extracts from Pinus radiata Bark. Ind. Crops Prod. 2011, 34, 838–844. [Google Scholar] [CrossRef]
- Nisca, A.; Ștefănescu, R.; Stegăruș, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Comparative Study Regarding the Chemical Composition and Biological Activity of Pine (Pinus nigra and P. sylvestris) Bark Extracts. Antioxidants 2021, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Kotani, A.; Watanabe, R.; Hayashi, Y.; Hakamata, H. Chemometric Evaluations of Repeatability and Detection Limit in High-Performance Liquid Chromatography with Electrochemical Detection. J. Chromatogr. A 2022, 1673, 463075. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Zhao, L.; Li, H.; Wang, K.; Li, X.; Guo, C.; Yang, H. Effects of Electrolysed Water and Levulinic Acid Combination on Microbial Safety and Polysaccharide Nanostructure of Organic Strawberry. Food Chem. 2022, 394, 133533. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).