Carrageenan-Based Pickering Emulsion Gels Stabilized by Xanthan Gum/Lysozyme Nanoparticle: Microstructure, Rheological, and Texture Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Complex Solution of XG/Ly NPs and kC
2.3. Preparation of Pickering Emulsion Gel
2.4. Characterization of Pickering Emulsion Gels
2.4.1. Rheological Analysis
2.4.2. Gel Microstructure
2.4.3. Hardness
2.4.4. Water Holding Capacity (WHC)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Microstructure Observation of Pickering Emulsion Gel
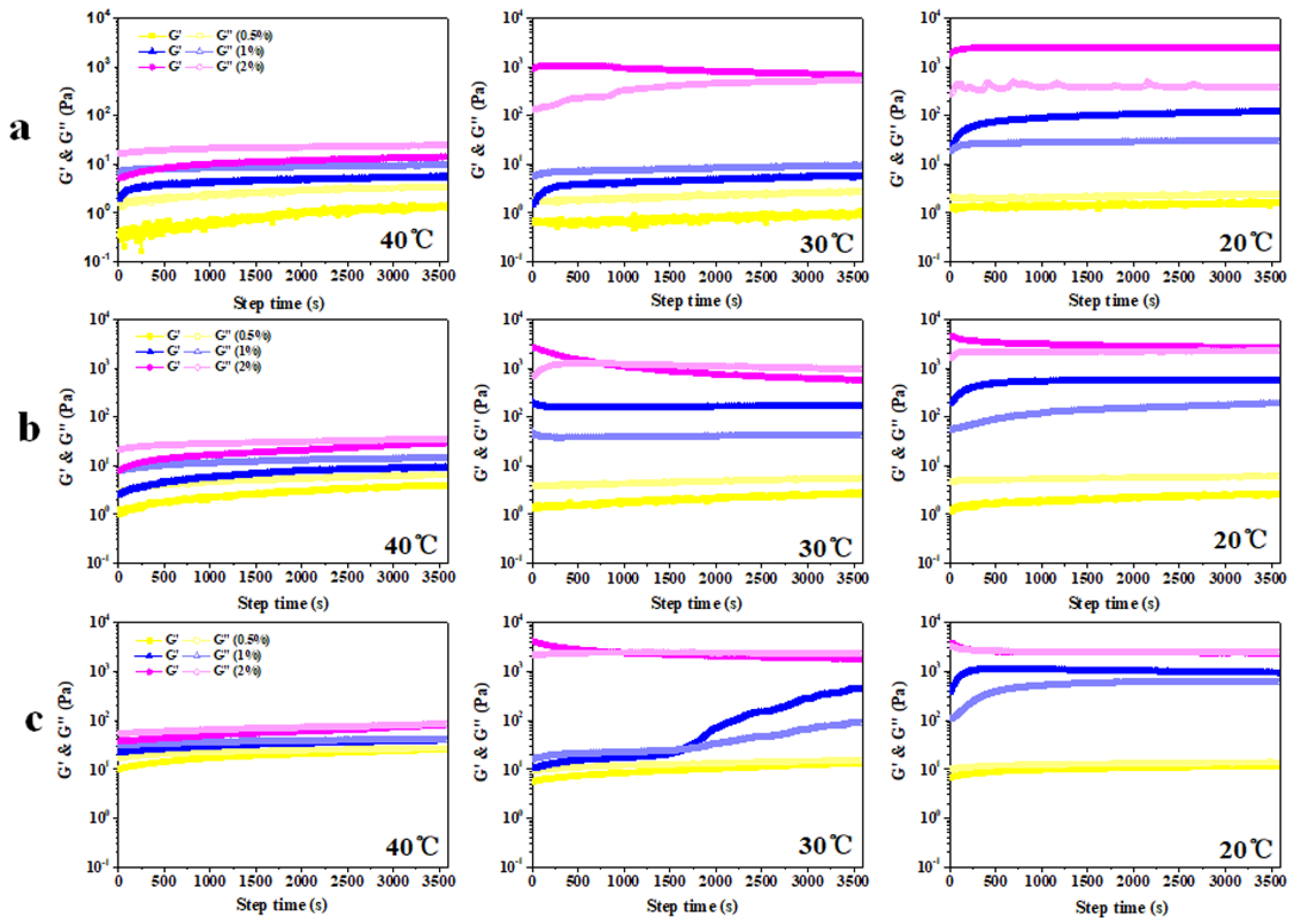
3.2. Rheological Analysis of Colloidal Sol
- (1)
- Time scanning
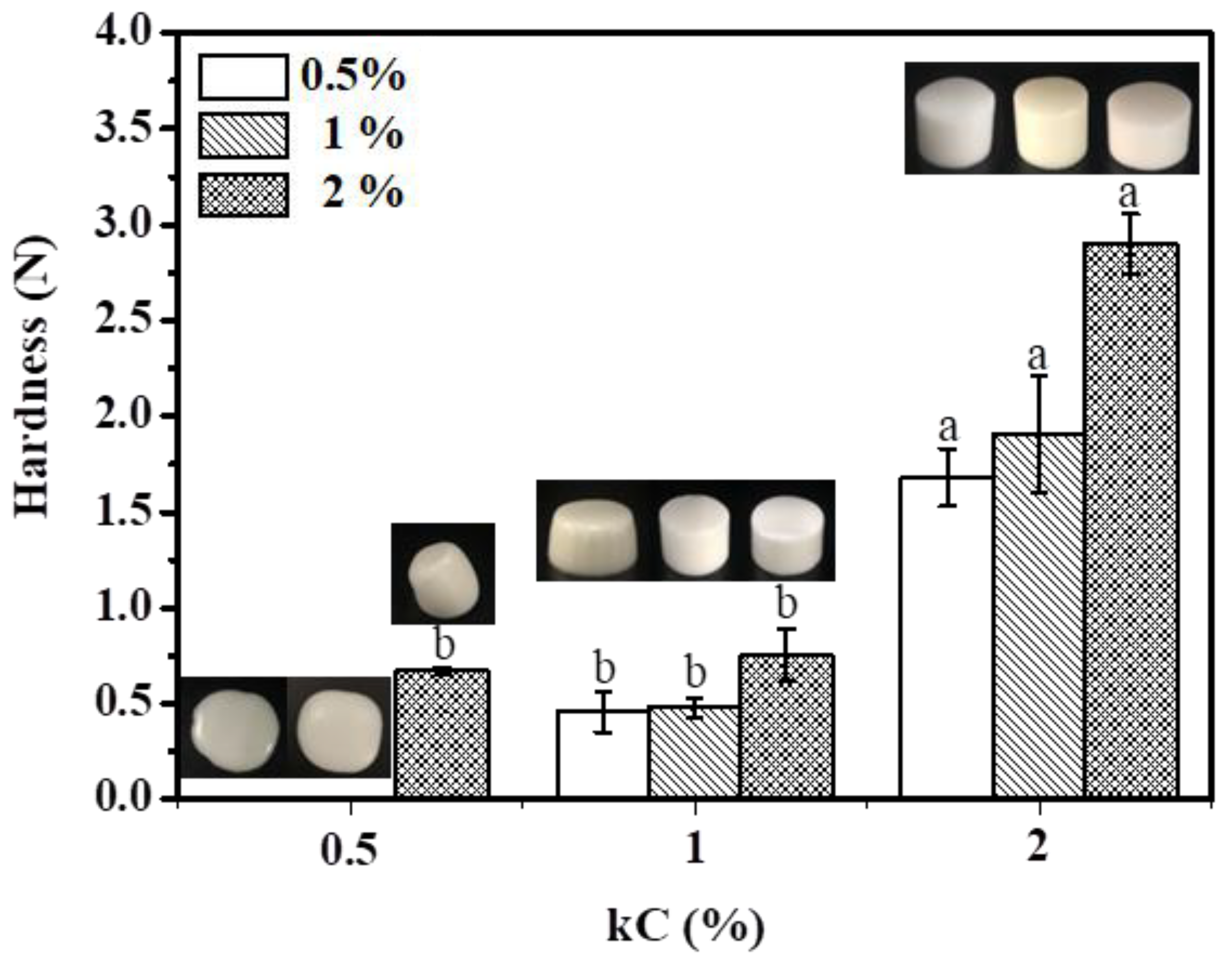
3.3. Hardness
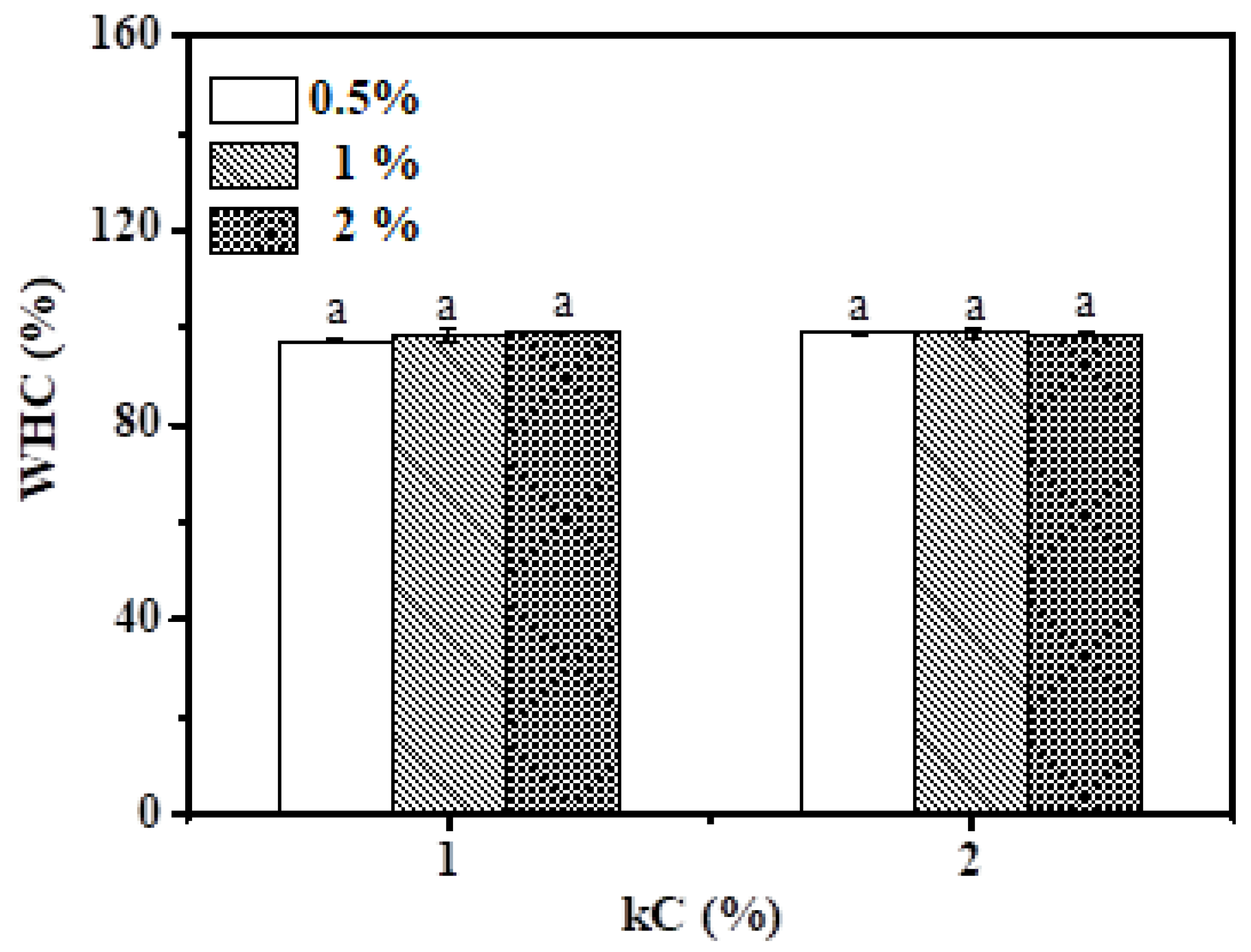
3.4. Water-Holding Capacity (WHC)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, S.; Wu, J.; Zhu, B.; Du, M.; Wu, C.; Yu, C.; Song, L.; Xu, X. Low oil emulsion gel stabilized by defatted Antarctic krill (Euphausia superba) protein using high-intensity ultrasound. Ultrason. Sonochem. 2021, 70, 105294. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Xue, C.; Wei, Z. Physicochemical characteristics, applications and research trends of edible Pickering emulsions. Trends Food Sci. Technol. 2021, 107, 1–15. [Google Scholar] [CrossRef]
- Xu, W.; Sun, H.; Kang, M.; Zheng, S.; Ning, Y.; Jia, Y.; Hu, Y.; Luo, D.; Zhang, C. Ethanol-tolerant pickering emulsion stabilized by gliadin nanoparticles. LWT-Food. Sci. Technol. 2022, 162, 113440. [Google Scholar] [CrossRef]
- Xu, W.; Li, Z.; Sun, H.; Zheng, S.; Li, H.; Luo, D.; Li, Y.; Wang, M.; Wang, Y. High Internal-Phase Pickering Emulsions Stabilized by Xanthan Gum/Lysozyme Nanoparticles: Rheological and Microstructural Perspective. Front. Nutr. 2022, 8, 744234. [Google Scholar] [CrossRef]
- Yang, F.; Yang, J.; Qiu, S.; Xu, W.; Wang, Y. Tannic acid enhanced the physical and oxidative stability of chitin particles stabilized oil in water emulsion. Food Chem. 2021, 346, 128762. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, S.; Zhao, C.; Liu, M.; Zhang, Z.; Xu, W.; Luo, D.; Shah, B.R. Stability, microstructural and rheological properties of Pickering emulsion stabilized by xanthan gum/lysozyme nanoparticles coupled with xanthan gum. Int. J. Biol. Macromol. 2020, 165, 2387–2394. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, L.; Wang, B.; Yang, X.; Chen, Z.; Zhong, Y.; Zhang, L.; Mao, Z.; Xu, H.; Sui, X. Polysaccharide-based edible emulsion gel stabilized by regenerated cellulose. Food Hydrocoll. 2019, 91, 232–237. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Miao, S. Preparation, structure-property relationships and applications of different emulsion gels: Bulk emulsion gels, emulsion gel particles, and fluid emulsion gels. Trends Food Sci. Technol. 2020, 102, 123–137. [Google Scholar] [CrossRef]
- Wu, B.; Yang, C.; Xin, Q.; Kong, L.; Eggersdorfer, M.; Ruan, J.; Zhao, P.; Shan, J.; Liu, K.; Chen, D.; et al. Attractive Pickering emulsion gels. Adv. Mater. 2021, 33, 2102362. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.N.; Chan, H.K.; Mohraz, A. Characteristics of pickering emulsion gels formed by droplet bridging. Langmuir 2012, 28, 3085–3091. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, C.; Yuan, J.; Wu, Y.; Li, F.; Waterhouse, G.I.; Li, D.; Huang, Q. Exploiting the robust network structure of zein/low-acyl gellan gum nanocomplexes to create Pickering emulsion gels with favorable properties. Food Chem. 2021, 349, 129112. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, S.; Du, L.; Liu, Y.; Meng, Z. Soft κ-carrageenan microgels stabilized pickering emulsion gels: Compact interfacial layer construction and particle-dominated emulsion gelation. J. Colloid Interface Sci. 2021, 602, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.; Jäger, H.; Ettelaie, R. Development of an Antioxidative Pickering Emulsion Gel through Polyphenol-Inspired Free-Radical Grafting of Microcrystalline Cellulose for 3D Food Printing. Biomacromolecules 2021, 22, 4592–4605. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Liu, X.; Ma, H.; Liu, B.; Liang, G. Multi-scale stabilization mechanism of pickering emulsion gels based on dihydromyricetin/high-amylose corn starch composite particles. Food Chem. 2021, 355, 129660. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S.; Liu, B. The fabrication and characterization of Pickering emulsion gels stabilized by sorghum flour. Foods 2022, 11, 2056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Meng, R.; Li, X.L.; Liu, W.J.; Cheng, J.S.; Wang, W. Preparation of Pickering emulsion gels based on κ-carrageenan and covalent crosslinking with EDC: Gelation mechanism and bioaccessibility of curcumin. Food Chem. 2021, 357, 129726. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cui, N.; Luo, J.; Zhang, H.; Guo, H.; Wen, P.; Ren, F. Stable water-in-oil emulsions formulated with polyglycerol polyricinoleate and glucono-δ-lactone-induced casein gels. Food Hydrocoll. 2016, 57, 217–220. [Google Scholar] [CrossRef]
- Li, X.M.; Meng, R.; Xu, B.C.; Zhang, B. Investigation of the fabrication, characterization, protective effect and digestive mechanism of a novel Pickering emulsion gels. Food Hydrocoll. 2021, 117, 106708. [Google Scholar] [CrossRef]
- Tang, M.; Lei, Y.; Wang, Y.; Li, D.; Wang, L. Rheological and structural properties of sodium caseinate as influenced by locust bean gum and κ-carrageenan. Food Hydrocoll. 2021, 112, 106251. [Google Scholar] [CrossRef]
- Elmarhoum, S.; Mathieu, S.; Ako, K.; Helbert, W. Sulfate groups position determines the ionic selectivity and syneresis properties of carrageenan systems. Carbohyd. Polym. 2023, 299, 120166. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, S.; Shen, M.; Jiang, L.; Ren, Y.; Luo, Y.; Xie, J. Effect of different Mesona chinensis polysaccharides on pasting, gelation, structural properties and in vitro digestibility of tapioca starch-Mesona chinensis polysaccharides gels. Food Hydrocoll. 2020, 99, 105327. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, H.; Ling, J.; Ouyang, X.; Song, X. Enhancing the stability of zein/fucoidan composite nanoparticles with calcium ions for quercetin delivery. Int. J. Biol. Macromol. 2021, 193, 2070–2078. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jin, W.; Li, Z.; Liang, H.; Wang, Y.; Shah, B.R.; Li, Y.; Li, B. Synthesis and characterization of nanoparticles based on negatively charged xanthan gum and lysozyme. Food Res. Int. 2015, 71, 83–90. [Google Scholar] [CrossRef]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of gellan—A review. Food Hydrocoll. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Ren, Z.; Li, Z.; Chen, Z.; Zhang, Y.; Lin, X.; Weng, W.; Yang, H.; Li, B. Characteristics and application of fish oil-in-water pickering emulsions structured with tea water-insoluble proteins/κ-carrageenan complexes. Food Hydrocoll. 2021, 114, 106562. [Google Scholar] [CrossRef]
- Chivero, P.; Gohtani, S.; Yoshii, H.; Nakamura, A. Effect of xanthan and guar gums on the formation and stability of soy soluble polysaccharide oil-in-water emulsions. Food Res. Int. 2015, 70, 7–14. [Google Scholar] [CrossRef]
- Yang, X.; Gong, T.; Li, D.; Li, A.; Sun, L.; Guo, Y. Preparation of high viscoelastic emulsion gels based on the synergistic gelation mechanism of xanthan and konjac glucomannan. Carbohyd. Polym. 2019, 226, 115278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liang, R.; Li, L. The interaction between anionic polysaccharides and legume protein and their influence mechanism on emulsion stability. Food Hydrocoll. 2022, 131, 107814. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B.; Li, C.; Fu, X.; Huang, Q. Pickering emulsion gel stabilized by octenylsuccinate quinoa starch granule as lutein carrier: Role of the gel network. Food Chem. 2020, 305, 125476. [Google Scholar] [CrossRef]
- Wu, D.; Yu, S.; Liang, H.; He, C.; Li, J.; Li, B. The influence of deacetylation degree of konjac glucomannan on rheological and gel properties of konjac glucomannan/κ-carrageenan mixed system. Food Hydrocoll. 2020, 101, 105523. [Google Scholar] [CrossRef]
- Shahbazi, M.; Jäger, H.; Ettelaie, R. A Promising Therapeutic Soy-Based Pickering Emulsion Gel Stabilized by a Multifunctional Microcrystalline Cellulose: Application in 3D Food Printing. J. Agric. Food Chem. 2022, 70, 2374–2388. [Google Scholar] [CrossRef]
- Alavi, F.; Emam-Djomeh, Z.; Salami, M.; Mohammadian, M. Effect of microbial transglutaminase on the mechanical properties and microstructure of acid-induced gels and emulsion gels produced from thermal denatured egg white proteins. Int. J. Biol. Macromol. 2020, 153, 523–532. [Google Scholar] [CrossRef]
- Wang, X.; Luo, K.; Liu, S.; Adhikari, B.; Chen, J. Improvement of gelation properties of soy protein isolate emulsion induced by calcium cooperated with magnesium. J. Food Eng. 2019, 244, 32–39. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, L.; Tian, Y.; Li, R.; Zhu, C.; Zhao, G.; Cheng, Y. A novel low-alkali konjac gel induced by ethanol to modulate sodium release. Food Hydrocoll. 2020, 103, 105653. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Maidannyk, V.; Miao, S. Effect of structuring emulsion gels by whey or soy protein isolate on the structure, mechanical properties, and in-vitro digestion of alginate-based emulsion gel beads. Food Hydrocoll. 2021, 110, 106165. [Google Scholar] [CrossRef]
- Omura, F.; Takahashi, K.; Okazaki, E.; Osako, K. A novel and simple non-thermal procedure for preparing low-pH-induced surimi gel from Alaska pollock (Theragra chalcogramma) using glucose oxidase. Food Chem. 2020, 321, 126722. [Google Scholar] [CrossRef] [PubMed]
- Qayum, A.; Hussain, M.; Li, M.; Li, J.; Shi, R.; Li, T.; Anwar, A.; Ahmed, Z.; Hou, J.; Jiang, Z. Gelling, microstructure and water-holding properties of alpha-lactalbumin emulsion gel: Impact of combined ultrasound pretreatment and laccase cross-linking. Food Hydrocoll. 2021, 110, 106122. [Google Scholar] [CrossRef]

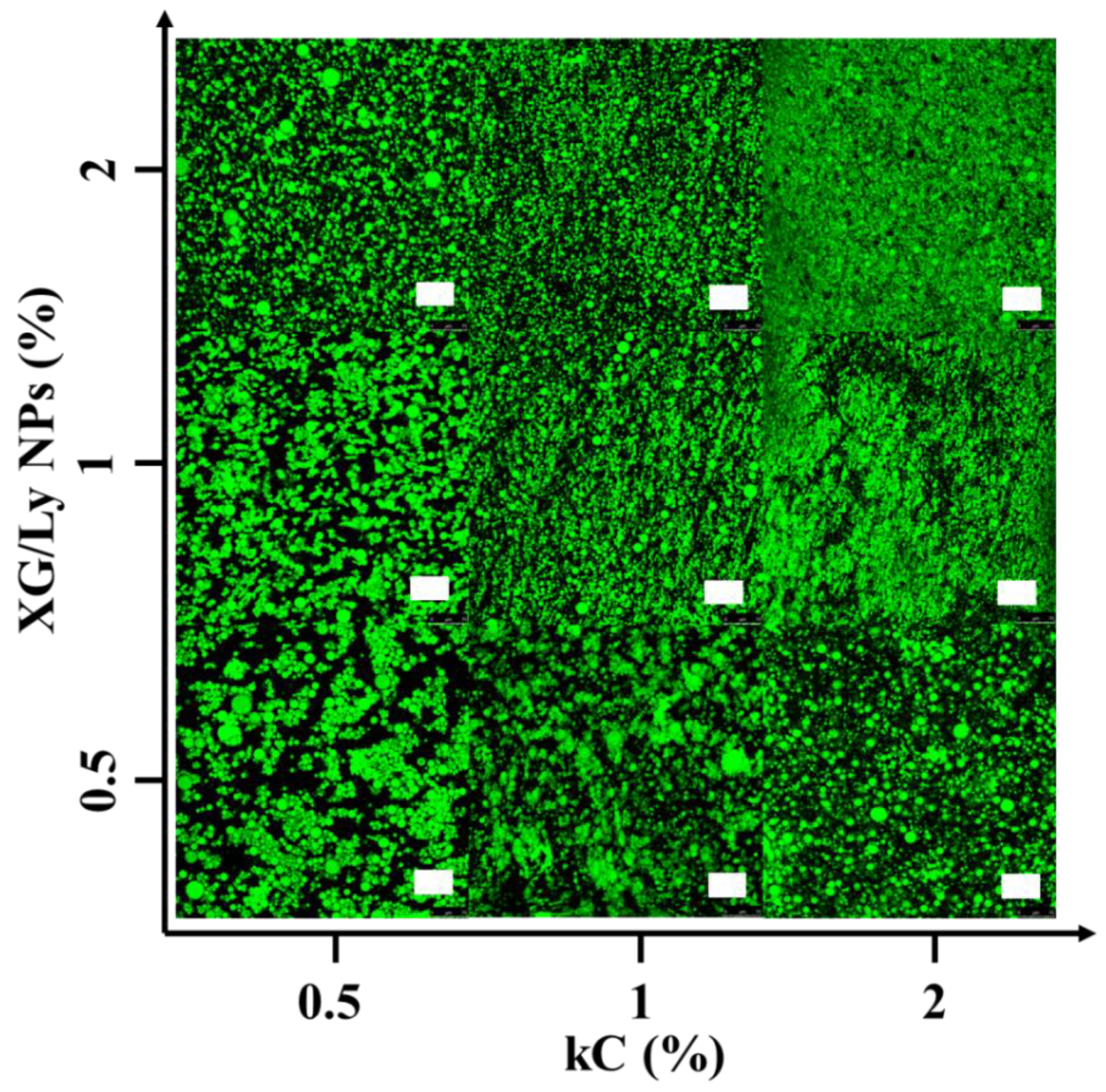
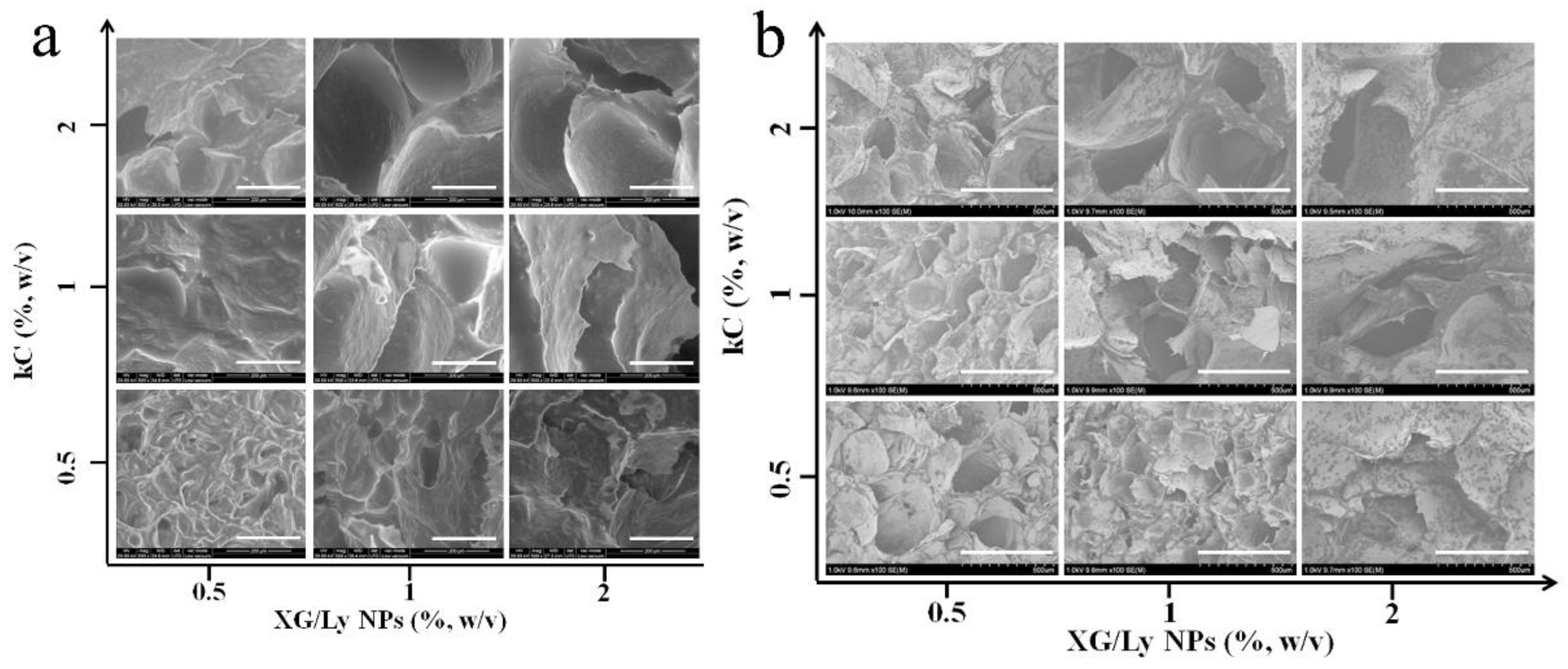

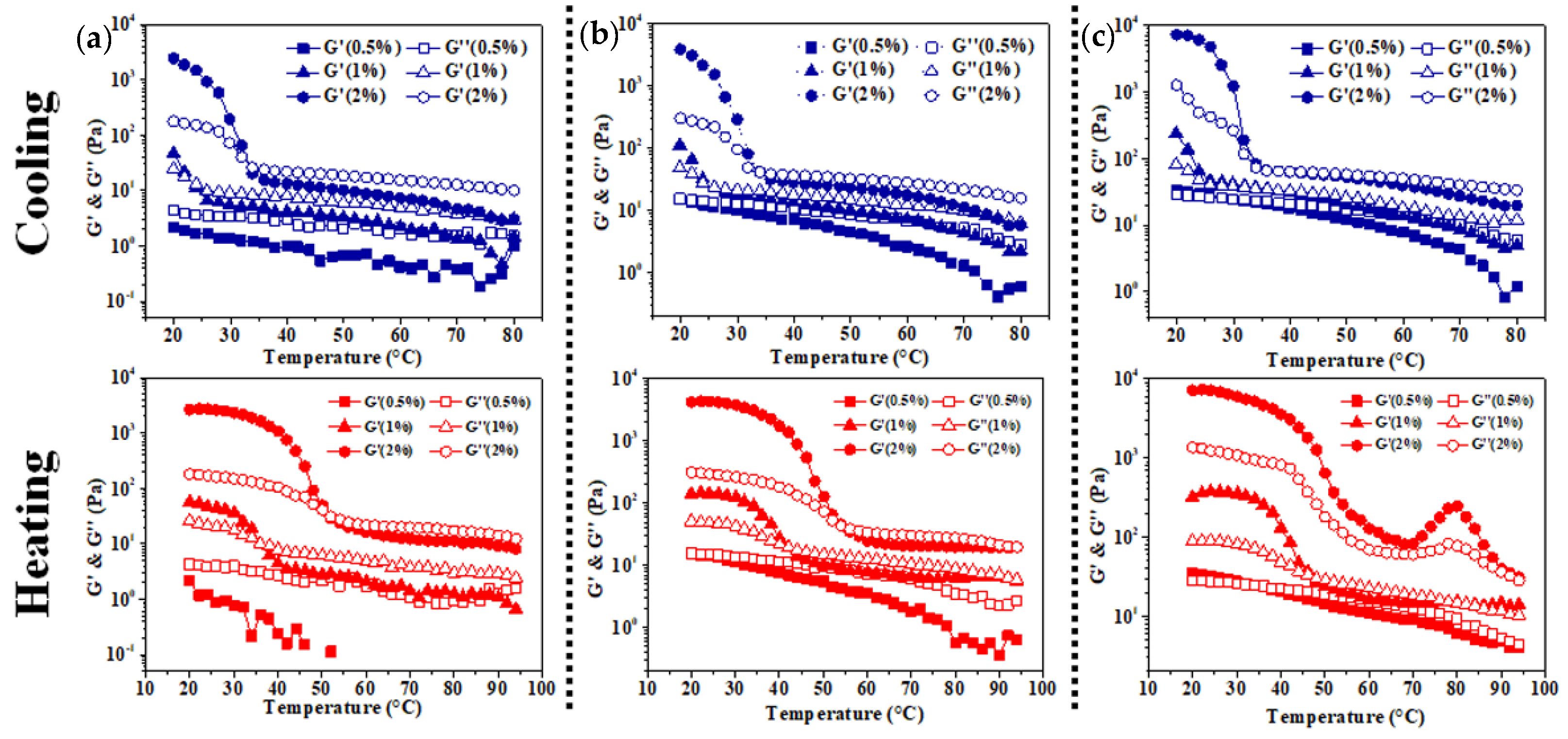
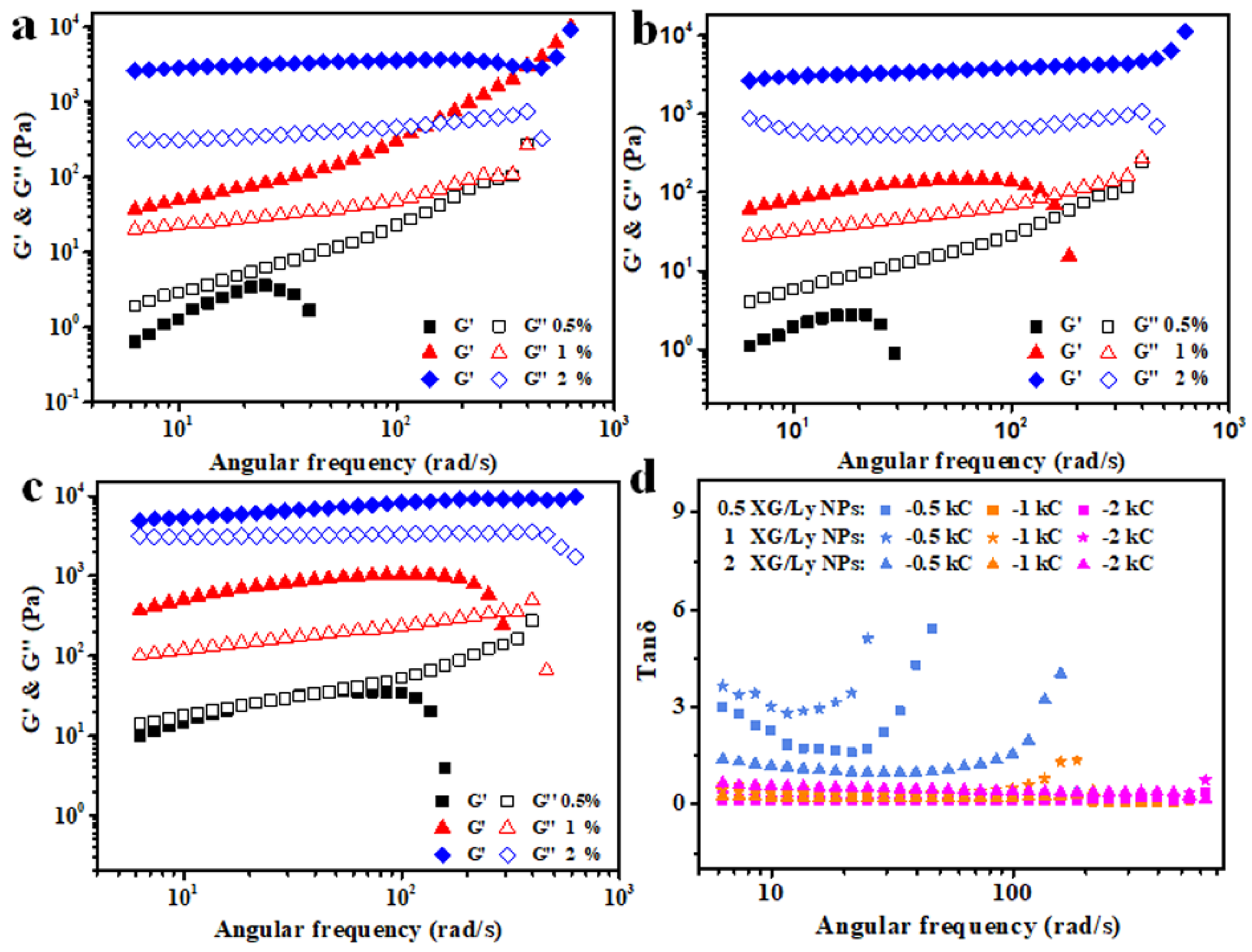
| XG/Ly NPs (%) | kC (%) | |||||
|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | ||||
| Tgel | Tmelt | Tgel | Tmelt | Tgel | Tmelt | |
| 0.5 | — | — | 23.41 | 35.91 | 33.51 | 51.68 |
| 1 | — | 20.83 | 25.65 | 42.20 | 34.07 | 54.94 |
| 2 | 29.26 | 33.22 | 36.81 | 47.13 | 37.83 | — |
| XG/Ly NPs (%) | kC (%) | |||||
|---|---|---|---|---|---|---|
| k | n | |||||
| 0.5 | 1 | 2 | 0.5 | 1 | 2 | |
| 0.5 | 0.43 | 1.98 | 688.62 | 0.87 | 0.81 | 0.41 |
| 1 | 4.18 | 7.22 | 633.56 | 0.19 | 0.57 | 0.44 |
| 2 | 4.46 | 36.94 | 1680.92 | 0.57 | 0.62 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, T.; Sun, H.; Niu, Z.; Xu, W.; Li, Z.; He, Y.; Luo, D.; Xi, W.; Wei, J.; Zhang, C. Carrageenan-Based Pickering Emulsion Gels Stabilized by Xanthan Gum/Lysozyme Nanoparticle: Microstructure, Rheological, and Texture Perspective. Foods 2022, 11, 3757. https://doi.org/10.3390/foods11233757
Xiong T, Sun H, Niu Z, Xu W, Li Z, He Y, Luo D, Xi W, Wei J, Zhang C. Carrageenan-Based Pickering Emulsion Gels Stabilized by Xanthan Gum/Lysozyme Nanoparticle: Microstructure, Rheological, and Texture Perspective. Foods. 2022; 11(23):3757. https://doi.org/10.3390/foods11233757
Chicago/Turabian StyleXiong, Tianzhen, Haomin Sun, Ziyi Niu, Wei Xu, Zhifan Li, Yawen He, Denglin Luo, Wenjie Xi, Jingjing Wei, and Chunlan Zhang. 2022. "Carrageenan-Based Pickering Emulsion Gels Stabilized by Xanthan Gum/Lysozyme Nanoparticle: Microstructure, Rheological, and Texture Perspective" Foods 11, no. 23: 3757. https://doi.org/10.3390/foods11233757
APA StyleXiong, T., Sun, H., Niu, Z., Xu, W., Li, Z., He, Y., Luo, D., Xi, W., Wei, J., & Zhang, C. (2022). Carrageenan-Based Pickering Emulsion Gels Stabilized by Xanthan Gum/Lysozyme Nanoparticle: Microstructure, Rheological, and Texture Perspective. Foods, 11(23), 3757. https://doi.org/10.3390/foods11233757






