Effect of Pullulanase Debranching Time Combined with Autoclaving on the Structural, Physicochemical Properties, and In Vitro Digestibility of Purple Sweet Potato Starch
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemical Reagents
2.2. Extraction of Starch from Jinshu No.17
2.3. Debranching Treated and Debranching Combined Autoclave Treated Starch
2.4. Morphology Observation and Granule Size Analysis
2.5. Gel Permeation Chromatography (GPC)
2.6. X-ray Diffraction Pattern
2.7. Solid-State 13C CP/MAS NMR Spectroscopy
2.8. Determination of Apparent Amylose Content
2.9. Differential Scanning Calorimetry (DSC)
2.10. Fourier Transform Infrared (FT-IR) Spectroscopy
2.11. Swelling Power and Percentage of Soluble Solids
2.12. Determination of In Vitro Digestibility
2.13. Statistical Analysis
3. Results and Discussion
3.1. Morphology and Size Distribution
3.2. Molecular Weight Distribution
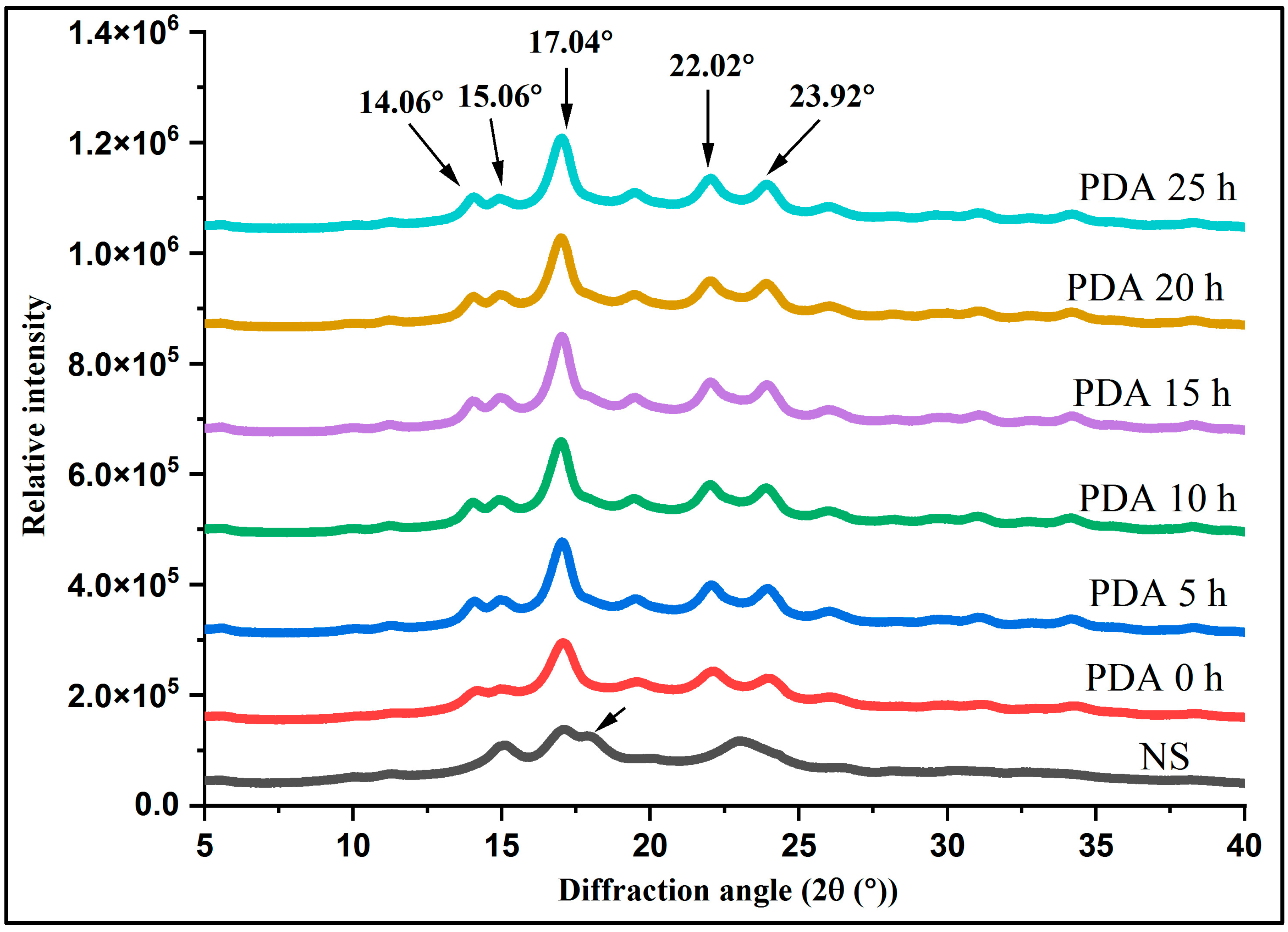
3.3. Crystalline Characteristics
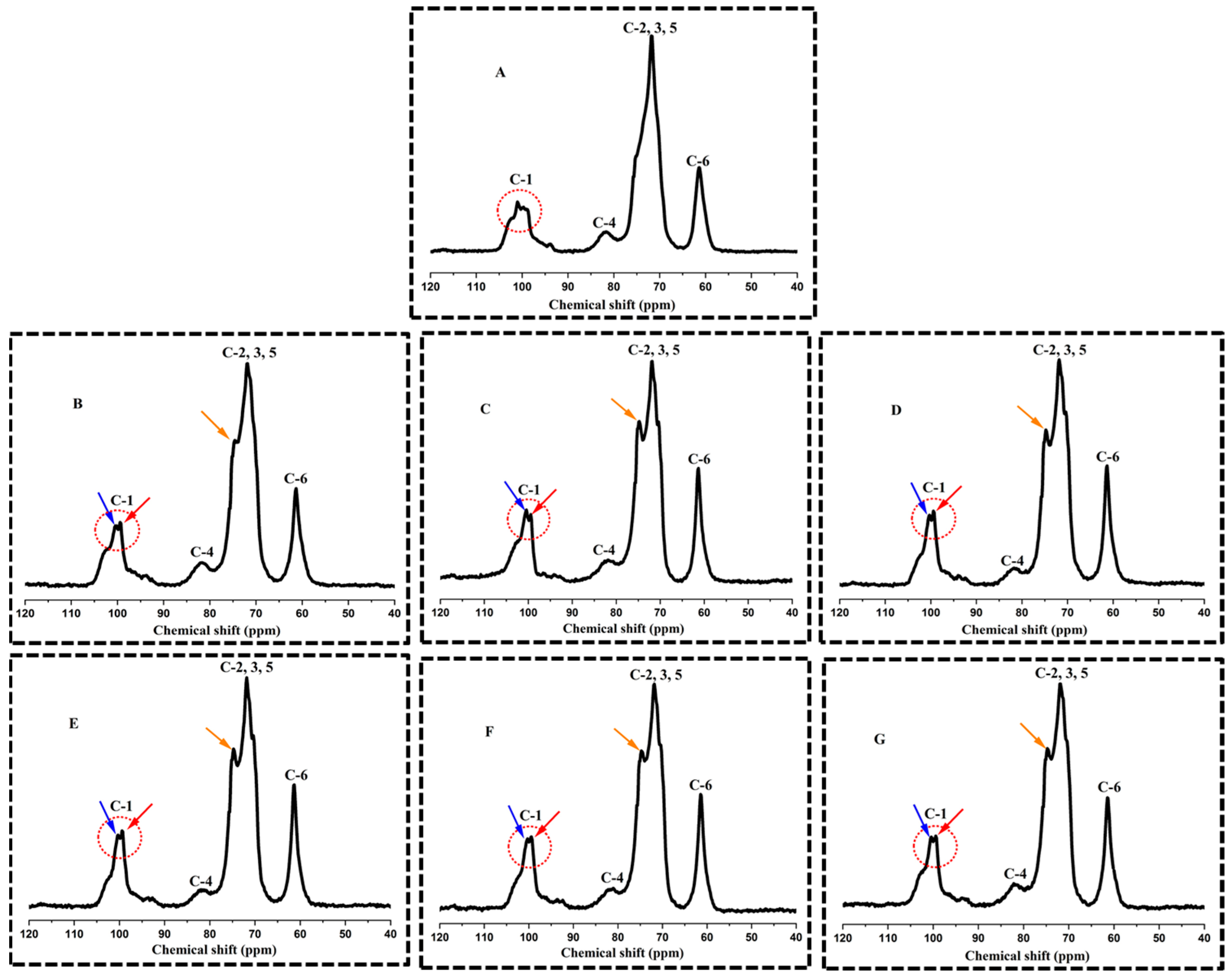
3.4. Ordered Structural Characteristics Determined by 13C CP/MAS NMR
3.5. Apparent Amylose Content
3.6. Thermal Parameters Analysis
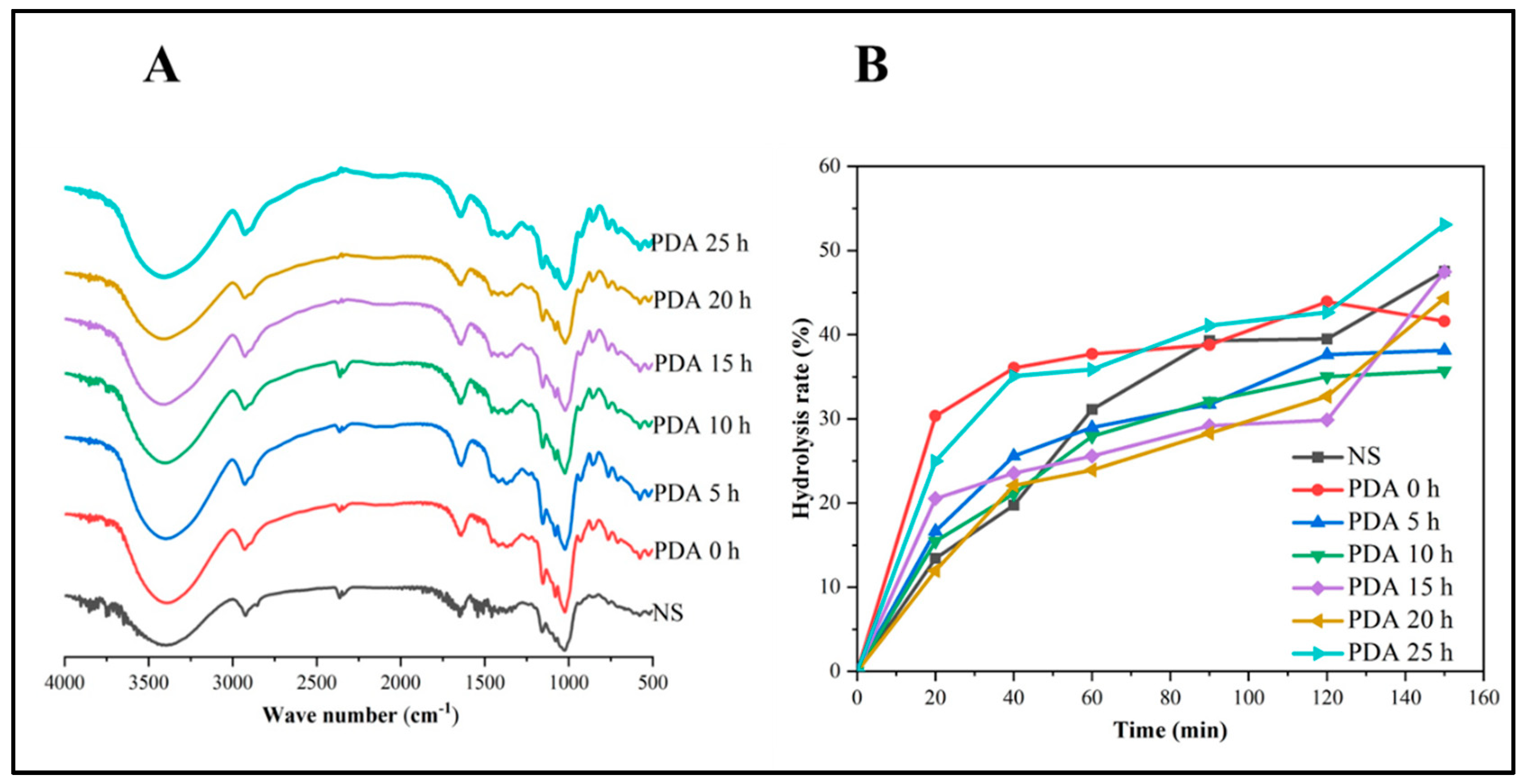
3.7. Fourier Transform Infrared (FT-IR) Analysis
3.8. Swelling Power and Percentage of Soluble Solids
3.9. In Vitro Digestion Property
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yong, H.; Wang, X.; Sun, J.; Fang, Y.; Liu, J. Changhai Jin of the Structural Characterization and Physicochemical Properties of Starches from Seven Purple Sweet Potato Varieties Cultivated in China. Int. J. Biol. Macromol. 2018, 120, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Escobar-puentes, A.A.; Rodr, L.; Fuentes, E.; Villegas-ochoa, M.A.; Gonz, G.A.; Olivas-aguirre, F.J.; Wall-medrano, A. Sweet Potato (Ipomoea batatas L.) Phenotypes: From Agroindustry to Health Effects. Foods 2022, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Sun, J.; Zhou, B.; Jin, C.; Liu, J.; Kan, J.; Qian, C.; Zhang, N. Effects of Polysaccharides from Purple Sweet Potatoes on Immune Response and Gut Microbiota Composition in Normal and Cyclophosphamide Treated Mice. Food Funct. 2018, 9, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Qu, H.; Jia, J.; Kuang, C.; Wen, Y.; Yan, H.; Gui, Z. Characterization, Antioxidant and Antitumor Activities of Polysaccharides from Purple Sweet Potato. Carbohydr. Polym. 2015, 132, 31–40. [Google Scholar] [CrossRef]
- Phomkaivon, N.; Surojanametakul, V.; Satmalee, P.; Poolperm, N.; Dangpium, N. Thai Purple Sweet Potato Flours: Characteristic and Application on Puffed Starch-Based Snacks. J. Agric. Sci. 2018, 10, 171. [Google Scholar] [CrossRef]
- Kim, J.; Ren, C.; Shin, M. Physicochemical Properties of Starch Isolated from Eight Different Varieties of Korean Sweet Potatoes. Starch/Staerke 2013, 65, 923–930. [Google Scholar] [CrossRef]
- dos Santos, T.P.R.; Fernandes, D.d.S.; Borges, C.V.; Leonel, M.; Lima, G.P.P. Orange-Fleshed Sweet Potato Chips: Processing Effect on Carotenoid Content and Resistant Starch and Sensory Acceptance. Food/Feed Sci. Technol. 2021, 64, e21200512. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, L.; Bian, X.; Guo, K.; Zhou, L.; Wei, C. Characterization and Comparative Study of Starches from Seven Purple Sweet Potatoes. Food Hydrocoll. 2018, 80, 168–176. [Google Scholar] [CrossRef]
- Trung, P.T.B.; Ngoc, L.B.B.; Hoa, P.N.; Tien, N.N.T.; Hung, P. Van Impact of Heat-Moisture and Annealing Treatments on Physicochemical Properties and Digestibility of Starches from Different Colored Sweet Potato Varieties. Int. J. Biol. Macromol. 2017, 105, 1071–1078. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Liu, Y.; Liu, X. Starch modification and application. In Starch Structure, Functionality and Application in Foods; Wang, S., Ed.; Springer: Singapore, 2020; pp. 131–149. ISBN 9789811506215. [Google Scholar]
- Verma, D.K.; Srivastav, P.P. Isolation, Modification, and Characterization of Rice Starch with Emphasis on Functional Properties and Industrial Application: A Review. Crit. Rev. Food Sci. Nutr. 2021, 5, 1–28. [Google Scholar] [CrossRef]
- Meenu, M.; Xu, B. A Critical Review on Anti-Diabetic and Anti-Obesity Effects of Dietary Resistant Starch. Crit. Rev. Food Sci. Nutr. 2019, 59, 3019–3031. [Google Scholar] [CrossRef] [PubMed]
- Guti, T.J.; Tovar, J. Trends in Food Science & Technology Update of the Concept of Type 5 Resistant Starch (RS5): Self-Assembled Starch V-Type Complexes. Trends Food Sci. Technol. 2021, 109, 711–724. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Fang, Y. Structure, Properties and Applications of Kudzu Starch. Food Hydrocoll. 2021, 119, 106817. [Google Scholar] [CrossRef]
- Ashogbon, A.O.; Akintayo, E.T. Recent Trend in the Physical and Chemical Modification of Starches from Different Botanical Sources: A Review. Starch/Staerke 2014, 66, 41–57. [Google Scholar] [CrossRef]
- Liu, G.; Gu, Z.; Hong, Y.; Cheng, L.; Li, C. Structure, Functionality and Applications of Debranched Starch: A Review. Trends Food Sci. Technol. 2017, 63, 70–79. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Q.; Li, B.; Lin, L.; Tundis, R.; Loizzo, M.R.; Zheng, B.; Xiao, J. Characterization and Prebiotic Effect of the Resistant Starch from Purple Sweet Potato. Molecules 2016, 21, 932. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Zhou, D.N.; Jin, Z.Y.; Xu, X.M.; Chen, H.Q. Effect of Debranching and Heat-Moisture Treatments on Structural Characteristics and Digestibility of Sweet Potato Starch. Food Chem. 2015, 187, 218–224. [Google Scholar] [CrossRef]
- Surendra Babu, A.; Parimalavalli, R. Effect of Pullulanase Debranching and Storage Temperatures on Structural Characteristics and Digestibility of Sweet Potato Starch. J. Saudi Soc. Agric. Sci. 2018, 17, 208–216. [Google Scholar] [CrossRef]
- Li, M.; Zhang, B.; Xie, Y.; Chen, H. Effects of Debranching and Repeated Heat-Moisture Treatments on Structure, Physicochemical Properties and in Vitro Digestibility of Wheat Starch. Food Chem. 2019, 294, 440–447. [Google Scholar] [CrossRef]
- Boonna, S.; Tongta, S. Structural Transformation of Crystallized Debranched Cassava Starch during Dual Hydrothermal Treatment in Relation to Enzyme Digestibility. Carbohydr. Polym. 2018, 192, 1–7. [Google Scholar] [CrossRef]
- Shi, M.; Chen, Y.; Yu, S.; Gao, Q. Preparation and Properties of RS III from Waxy Maize Starch with Pullulanase. Food Hydrocoll. 2013, 33, 19–25. [Google Scholar] [CrossRef]
- Shi, J.; Sweedman, M.C.; Shi, Y. Structural Changes and Digestibility of Waxy Maize Starch Debranched by Di Ff Erent Levels of Pullulanase. Carbohydr. Polym. 2018, 194, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Tian, Y.; Lu, H.; Sun, C.; Jin, Z. Effects of Fractionation and Heat-Moisture Treatment on Structural Changes and Digestibility of Debranched Waxy Maize Starch. Food Hydrocoll. 2020, 101, 105488. [Google Scholar] [CrossRef]
- Li, T.; An, F.; Teng, H.; Huang, Q.; Zeng, F. Comparison of Structural Features and in Vitro Digestibility of Purple Yam (Dioscorea alata L.) Resistant Starches by Autoclaving and Multi-Enzyme Hydrolysis. Food Sci. Biotechnol. 2018, 27, 27–36. [Google Scholar] [CrossRef]
- Gou, M.; Wu, H.; Saleh, A.S.M.; Jing, L.; Liu, Y.; Zhao, K.; Su, C.; Zhang, B.; Jiang, H.; Li, W. Effects of Repeated and Continuous Dry Heat Treatments on Properties of Sweet Potato Starch. Int. J. Biol. Macromol. 2019, 129, 869–877. [Google Scholar] [CrossRef]
- Reddy, C.K.; Son, S.Y.; Lee, C.H. Effects of Pullulanase Debranching and Octenylsuccinic Anhydride Modification on the Structural Properties of Maize Starch-Green Tea Extract Complexes. Food Hydrocoll. 2021, 115, 106630. [Google Scholar] [CrossRef]
- Li, Y.; Hu, A.; Wang, X.; Zheng, J. Physicochemical and in Vitro Digestion of Millet Starch: Effect of Moisture Content in Microwave. Int. J. Biol. Macromol. 2019, 134, 308–315. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, Z.; Zhang, B.; Chen, J.; Liu, R.; Song, D.; Li, W.; Lin, N.; Zou, X.; Wang, J.; et al. Purification, Characterization, and in Vitro Antitumor Activity of a Novel Glucan from the Purple Sweet Potato Ipomoea batatas (L.) Lam. Carbohydr. Polym. 2021, 257, 117605. [Google Scholar] [CrossRef]
- Zheng, Y.; Ou, Y.; Zhang, Y.; Zheng, B.; Zeng, S.; Zeng, H. Effects of Pullulanase Pretreatment on the Structural Properties and Digestibility of Lotus Seed Starch-Glycerin Monostearin Complexes. Carbohydr. Polym. 2020, 240, 116324. [Google Scholar] [CrossRef]
- Wang, R.; Li, M.; Liu, J.; Wang, F.; Wang, J.; Zhou, Z. Dual Modification Manipulates Rice Starch Characteristics Following Debranching and Propionate Esterification. Food Hydrocoll. 2021, 119, 106833. [Google Scholar] [CrossRef]
- Deka, D.; Sit, N. Dual Modification of Taro Starch by Microwave and Other Heat Moisture Treatments. Int. J. Biol. Macromol. 2016, 92, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Demirkesen-bicak, H.; Tacer-caba, Z.; Nilufer-erdil, D. Pullulanase Treatments to Increase Resistant Starch Content of Black Chickpea (Cicer Arietinum L.) Starch and the Effects on Starch Properties. Int. J. Biol. Macromol. 2018, 111, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, H.; Wang, Y.; Zeng, S.; Zheng, B. Structural Characteristics and Crystalline Properties of Lotus Seed Resistant Starch and Its Prebiotic Effects. Food Chem. 2014, 155, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.K.; Suriya, M.; Haripriya, S. Physico-Chemical and Functional Properties of Resistant Starch Prepared from Red Kidney Beans (Phaseolus vulgaris L.) Starch by Enzymatic Method. Carbohydr. Polym. 2013, 95, 220–226. [Google Scholar] [CrossRef]
- Lee, B.H.; Lee, Y.T. Physicochemical and Structural Properties of Different Colored Sweet Potato Starches. Starch/Staerke 2017, 69, 1–9. [Google Scholar] [CrossRef]
- Guo, K.; Liu, T.; Xu, A.; Zhang, L.; Bian, X.; Wei, C. Structural and Functional Properties of Starches from Root Tubers of White, Yellow, and Purple Sweet Potatoes. Food Hydrocoll. 2019, 89, 829–836. [Google Scholar] [CrossRef]
- Javaid, M.A.; Zia, K.M.; Iqbal, A.; Ahmad, S.; Akram, N.; Liu, X.; Nawaz, H.; Khosa, M.K.; Awais, M. Utilization of Waxy Corn Starch as an Efficient Chain Extender for the Preparation of Polyurethane Elastomers. Int. J. Biol. Macromol. 2020, 148, 415–423. [Google Scholar] [CrossRef]
- Liu, G.; Gu, Z.; Hong, Y.; Wei, H.; Zhang, C.; Huang, S.; Chen, Y.; Lu, Y.; Li, Y. Effects of Molecular Interactions in Debranched High Amylose Starch on Digestibility and Hydrogel Properties. Food Hydrocoll. 2020, 101, 105498. [Google Scholar] [CrossRef]
- Ning, Y.; Cui, B.; Yuan, C.; Zou, Y.; Liu, W.; Pan, Y. Food Hydrocolloids E Ff Ects of Konjac Glucomannan on the Rheological, Microstructure and Digestibility Properties of Debranched Corn Starch. Food Hydrocoll. 2020, 100, 105342. [Google Scholar] [CrossRef]
- Ma, Z.; Yin, X.; Chang, D.; Hu, X.; Boye, J.I. Long- and Short-Range Structural Characteristics of Pea Starch Modified by Autoclaving, α -Amylolysis, and Pullulanase Debranching. Int. J. Biol. Macromol. 2018, 120, 650–656. [Google Scholar] [CrossRef]
- Zeng, S.; Wu, X.; Lin, S.; Zeng, H.; Lu, X.; Zhang, Y.; Zheng, B. Structural Characteristics and Physicochemical Properties of Lotus Seed Resistant Starch Prepared by Different Methods. Food Chem. 2015, 186, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Chen, X.; Wang, J.; Wang, X.; Zheng, J.; Wang, L. Effects on the Structure and Properties of Native Corn Starch Modified by Enzymatic Debranching (ED), Microwave Assisted Esterification with Citric Acid (MCAE) and by the Dual ED/MCAE Treatment. Int. J. Biol. Macromol. 2021, 171, 123–129. [Google Scholar] [CrossRef] [PubMed]

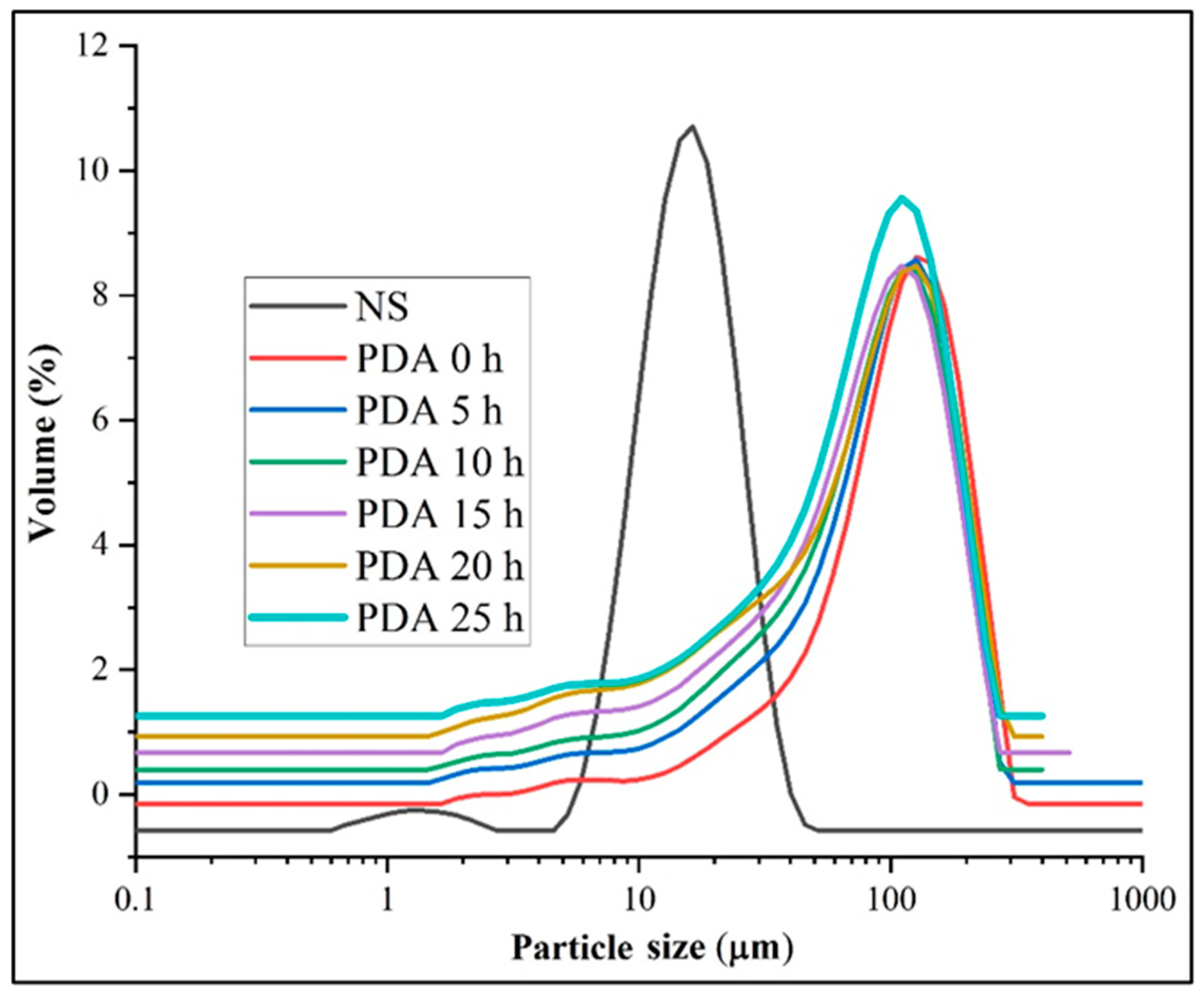
| Samples | Particle Size (μm) | ||||
|---|---|---|---|---|---|
| D [3,2] | D [4,3] | Dx (10) | Dx (50) | Dx (90) | |
| NS | 12.04 ± 0.05 g | 17.51 ± 0.01 f | 9.04 ± 0.05 f | 16.44 ± 0.05 f | 28.14 ± 0.05 e |
| PDA 0 h | 51.40 ± 0.00 a | 119.07 ± 0.11 e | 30.01 ± 0.00 a | 114.01 ± 0.01 a | 214.07 ± 0.11 a |
| PDA 5 h | 40.50 ± 0.00 b | 105.03 ± 0.05 a | 22.04 ± 0.05 b | 99.87 ± 0.11 b | 193.33 ± 0.57 bc |
| PDA 10 h | 38.44 ± 0.05 d | 98.73 ± 0.22 b | 20.32 ± 0.03 c | 93.11 ± 0.00 c | 185.33 ± 0.57 c |
| PDA 15 h | 35.64 ± 0.05 e | 93.94 ± 0.05 d | 17.54 ± 0.05 d | 88.17 ± 0.28 e | 179.34 ± 0.57 d |
| PDA 20 h | 32.93 ± 0.05 f | 98.37 ± 0.55 b | 15.10 ± 0.00 e | 91.73 ± 0.23 d | 191.37 ± 0.55 b |
| PDA 25 h | 40.00 ± 0.01 c | 97.34 ± 0.57 c | 21.73 ± 0.57 b | 91.87 ± 0.11 d | 179.34 ± 0.57 d |
| Samples | Mn (g/mol) | Mw (g/mol) | PD |
|---|---|---|---|
| NS | 5.74 × 106 | 8.54 × 106 | 1.49 |
| PDA 0 h | 1.98 × 106 | 4.37 × 106 | 2.20 |
| PDA 5 h | 1.68 × 106 | 3.46 × 106 | 2.06 |
| PDA 10 h | 1.32 × 106 | 2.89 × 106 | 2.18 |
| PDA 15 h | 1.49 × 106 | 3.81 × 106 | 2.55 |
| PDA 20 h | 1.39 × 106 | 3.60 × 106 | 2.58 |
| PDA 25 h | 2.15 × 106 | 5.84 × 106 | 2.71 |
| Samples | Relative Crystallinity (%) | Crystallinity Type | Diffraction Peaks (O) | Amylose Content (%) |
|---|---|---|---|---|
| NS | 26.46 ± 1.14 d | C | 15.12, 17.08, 17.88, 22.96 | 21.53 ± 0.37 a |
| PDA 0 h | 29.00 ± 1.07 c | B | 14.26, 15.06, 17.10, 22.18, 24.02 | 16.55 ± 0.07 b |
| PDA 5 h | 30.59 ± 1.78 bc | B | 14.08, 14.94, 17.02, 22.08, 23.94 | 11.37 ± 0.11 c |
| PDA 10 h | 31.08 ± 1.05 bc | B | 14.02, 14.92, 17.02, 23.94 | 9.33 ± 0.17 d |
| PDA 15 h | 32.34 ± 0.04 ab | B | 14.04, 15.02, 17.04, 22.02, 23.92 | 8.34 ± 0.15 e |
| PDA 20 h | 32.45 ± 0.13 bc | B | 14.08, 14.96, 17.04, 22.02, 23.92 | 8.90 ± 0.12 d |
| PDA 25 h | 35.23 ± 1.03 a | B | 14.06, 15.06, 17.04, 22.02, 23.96 | 8.99 ± 0.12 d |
| Samples | Melting Temperature (°C) | ||||
|---|---|---|---|---|---|
| To | Tp | Tc | Tc–To | ΔH (J/g) | |
| NS | 59.00 ± 0.10 f | 75.80 ± 0.20 d | 84.73 ± 0.23 g | 25.60 ± 0.40 f | 11.47 ± 0.06 d |
| PDA 0 h | 60.70 ± 0.10 e | 75.13 ± 0.04 e | 106.82 ± 0.44 e | 46.12 ± 0.25 cd | 9.68 ± 0.02 f |
| PDA 5 h | 68.63 ± 0.18 d | 78.86 ± 0.01 c | 114.32 ± 0.42 c | 45.69 ± 0.07 d | 12.26 ± 0.06 c |
| PDA 10 h | 73.05 ± 0.21 c | 78.74 ± 0.10 c | 121.95 ± 0.13 b | 48.90 ± 0.21 b | 12.45 ± 0.28 bc |
| PDA 15 h | 75.40 ± 0.13 b | 95.50 ± 0.05 a | 127.20 ± 0.27 a | 51.80 ± 0.05 a | 13.23 ± 0.09 a |
| PDA 20 h | 78.56 ± 0.06 a | 88.60 ± 0.06 b | 113.38 ± 0.06 d | 34.82 ± 0.09 e | 12.87 ± 0.08 ab |
| PDA 25 h | 48.30 ± 0.54 g | 72.30 ± 0.28 d | 95.33 ± 0.14 f | 47.03 ± 0.96 c | 10.92 ± 0.26 e |
| Samples | 65 °C | 75 °C | 85 °C | 95 °C |
|---|---|---|---|---|
| Swelling Power (g/g) | ||||
| NS | 28.52 ± 3.14 a | 29.09 ± 1.75 a | 29.85 ± 0.62 a | 30.32 ± 2.02 a |
| PDA 0 h | 14.92 ± 0.54 b | 15.63 ± 0.10 b | 15.91 ± 0.33 b | 16.64 ± 1.18 b |
| PDA 5 h | 8.17 ± 1.43 c | 8.45 ± 0.59 c | 8.95 ± 0.73 c | 9.27 ± 0.77 c |
| PDA 10 h | 6.66 ± 0.76 c | 7.04 ± 0.82 c | 7.23 ± 0.49 de | 7.66 ± 0.30 c |
| PDA 15 h | 5.63 ± 0.18 c | 6.27 ± 0.70 c | 6.46 ± 0.34 e | 7.00 ± 0.60 c |
| PDA 20 h | 6.54 ± 0.68 c | 6.80 ± 0.38 c | 6.94 ± 0.63 e | 6.90 ± 0.58 c |
| PDA 25 h | 7.66 ± 0.95 c | 8.29 ± 0.15 c | 8.85 ± 0.76 cd | 9.00 ± 0.84 c |
| Soluble Solids (%) | ||||
| NS | 0.32 ± 0.02 b | 0.33 ± 0.03 d | 0.37 ± 0.06 d | 0.41 ± 0.00 d |
| PDA 0 h | 0.48 ± 0.05 bc | 0.48 ± 0.04 c | 0.48 ± 0.01 c | 0.47 ± 0.04 cd |
| PDA 5 h | 0.40 ± 0.04 cd | 0.46 ± 0.00 c | 0.48 ± 0.02 c | 0.51 ± 0.02 c |
| PDA 10 h | 0.45 ± 0.05 bc | 0.45 ± 0.00 c | 0.47 ± 0.00 c | 0.51 ± 0.04 c |
| PDA 15 h | 0.51 ± 0.03 b | 0.52 ± 0.01 c | 0.53 ± 0.01 c | 0.54 ± 0.03 c |
| PDA 20 h | 0.62 ± 0.01 a | 0.63 ± 0.01 b | 0.62 ± 0.00 b | 0.63 ± 0.02 b |
| PDA 25 h | 0.66 ± 0.02 a | 0.73 ± 0.02 a | 0.78 ± 0.04 a | 0.85 ± 0.01 a |
| Samples | Starch Fractions (%) | ||
|---|---|---|---|
| RDS | SDS | RS | |
| NS | 13.42 ± 0.16 e | 26.06 ± 0.01 a | 60.51 ± 0.00 e |
| PDA 0 h | 30.34 ± 0.05 a | 13.58 ± 0.05 f | 56.07 ± 0.00 g |
| PDA 5 h | 16.63 ± 0.13 c | 21.01 ± 0.15 b | 62.36 ± 0.00 d |
| PDA 10 h | 15.46 ± 0.16 d | 19.55 ± 0.61 c | 64.98 ± 0.00 c |
| PDA 15 h | 13.52 ± 0.48 e | 16.34 ± 0.63 e | 70.14 ± 0.00 a |
| PDA 20 h | 11.96 ± 0.34 f | 20.71 ± 0.13 bc | 67.32 ± 0.00 b |
| PDA 25 h | 24.96 ± 0.06 b | 17.70 ± 0.36 d | 57.33 ± 0.05 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodjrenou, D.M.; Li, X.; Chen, W.; Zhang, Y.; Zheng, B.; Zeng, H. Effect of Pullulanase Debranching Time Combined with Autoclaving on the Structural, Physicochemical Properties, and In Vitro Digestibility of Purple Sweet Potato Starch. Foods 2022, 11, 3779. https://doi.org/10.3390/foods11233779
Bodjrenou DM, Li X, Chen W, Zhang Y, Zheng B, Zeng H. Effect of Pullulanase Debranching Time Combined with Autoclaving on the Structural, Physicochemical Properties, and In Vitro Digestibility of Purple Sweet Potato Starch. Foods. 2022; 11(23):3779. https://doi.org/10.3390/foods11233779
Chicago/Turabian StyleBodjrenou, David Mahoudjro, Xin Li, Wei Chen, Yi Zhang, Baodong Zheng, and Hongliang Zeng. 2022. "Effect of Pullulanase Debranching Time Combined with Autoclaving on the Structural, Physicochemical Properties, and In Vitro Digestibility of Purple Sweet Potato Starch" Foods 11, no. 23: 3779. https://doi.org/10.3390/foods11233779
APA StyleBodjrenou, D. M., Li, X., Chen, W., Zhang, Y., Zheng, B., & Zeng, H. (2022). Effect of Pullulanase Debranching Time Combined with Autoclaving on the Structural, Physicochemical Properties, and In Vitro Digestibility of Purple Sweet Potato Starch. Foods, 11(23), 3779. https://doi.org/10.3390/foods11233779








