Development of a Portable Near-Infrared Spectroscopy Tool for Detecting Freshness of Commercial Packaged Pork
Abstract
:1. Introduction
2. Materials and Methods
2.1. Meat Samples and Treatments
2.2. Microbiological Analysis
2.3. Meat Quality Traits
2.4. NIR Spectroscopy
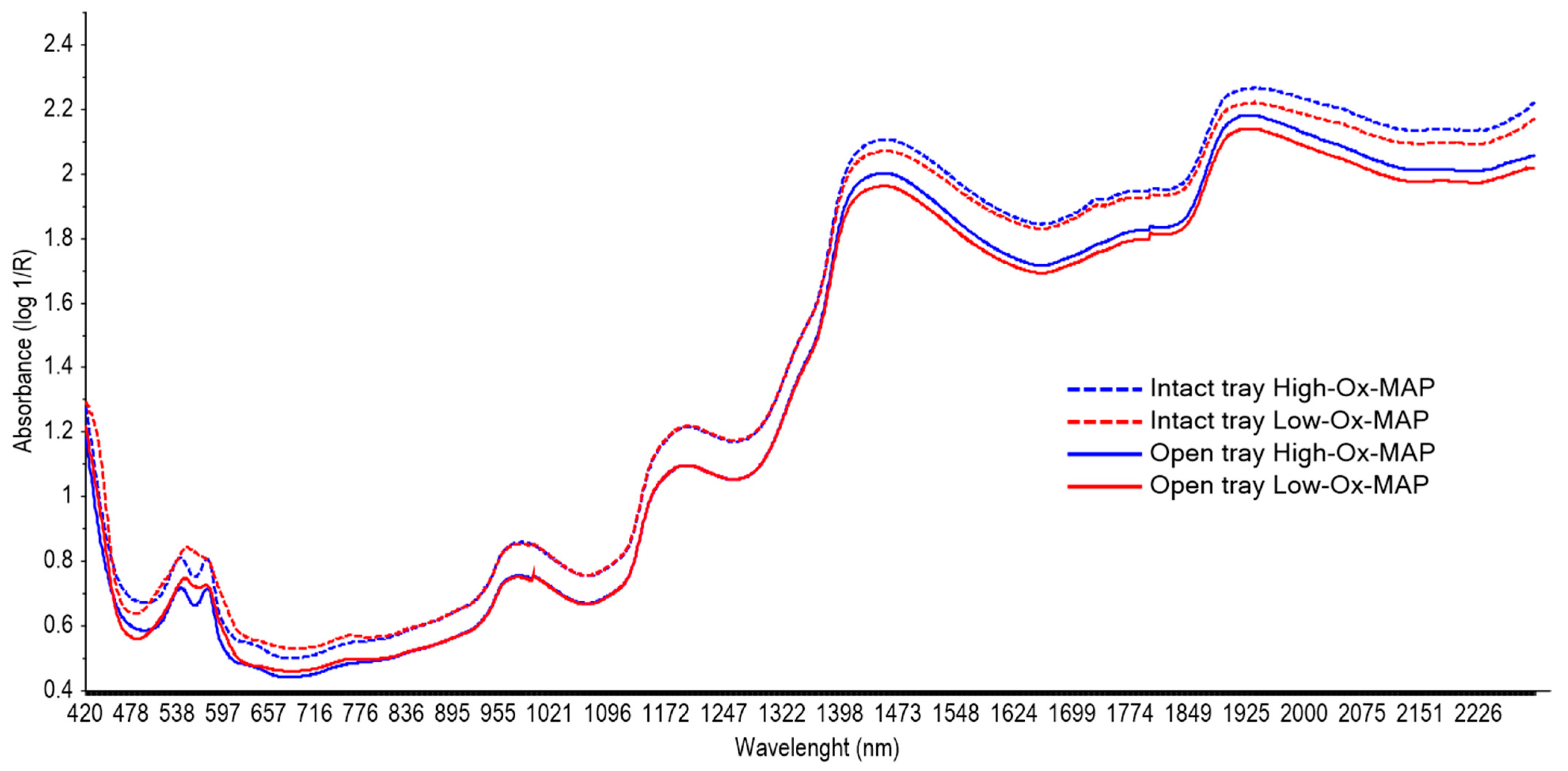
2.4.1. Spectral Collection and Pre-Treatment
2.4.2. NIR Quantitative Models for Quality Traits
2.4.3. NIR Classification Models for Fresh or Spoiled Pork
- Total viable counts: fresh (acceptable for consumption) if <6.69 log cfu/g; spoiled (unacceptable for consumption) if >6.69 log cfu/g.
- Enterobacteriaceae counts: fresh if <4 log cfu/g; spoiled if >4 log cfu/g.
- Lactic acid bacteria counts: fresh if <6.69 log cfu/g; spoiled if >6.69 log cfu/g.
2.5. Statistical Analysis
3. Results and Discussion
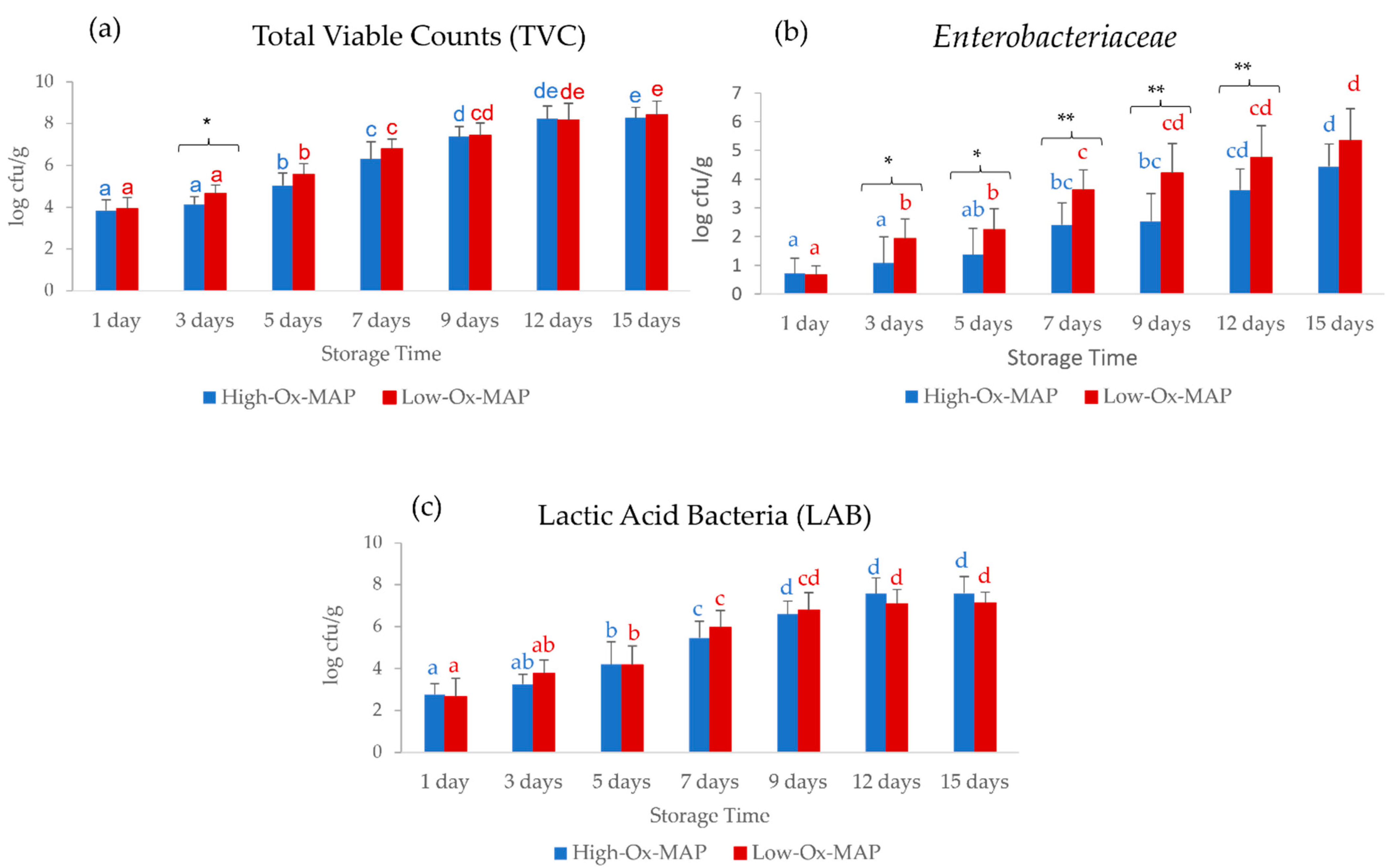
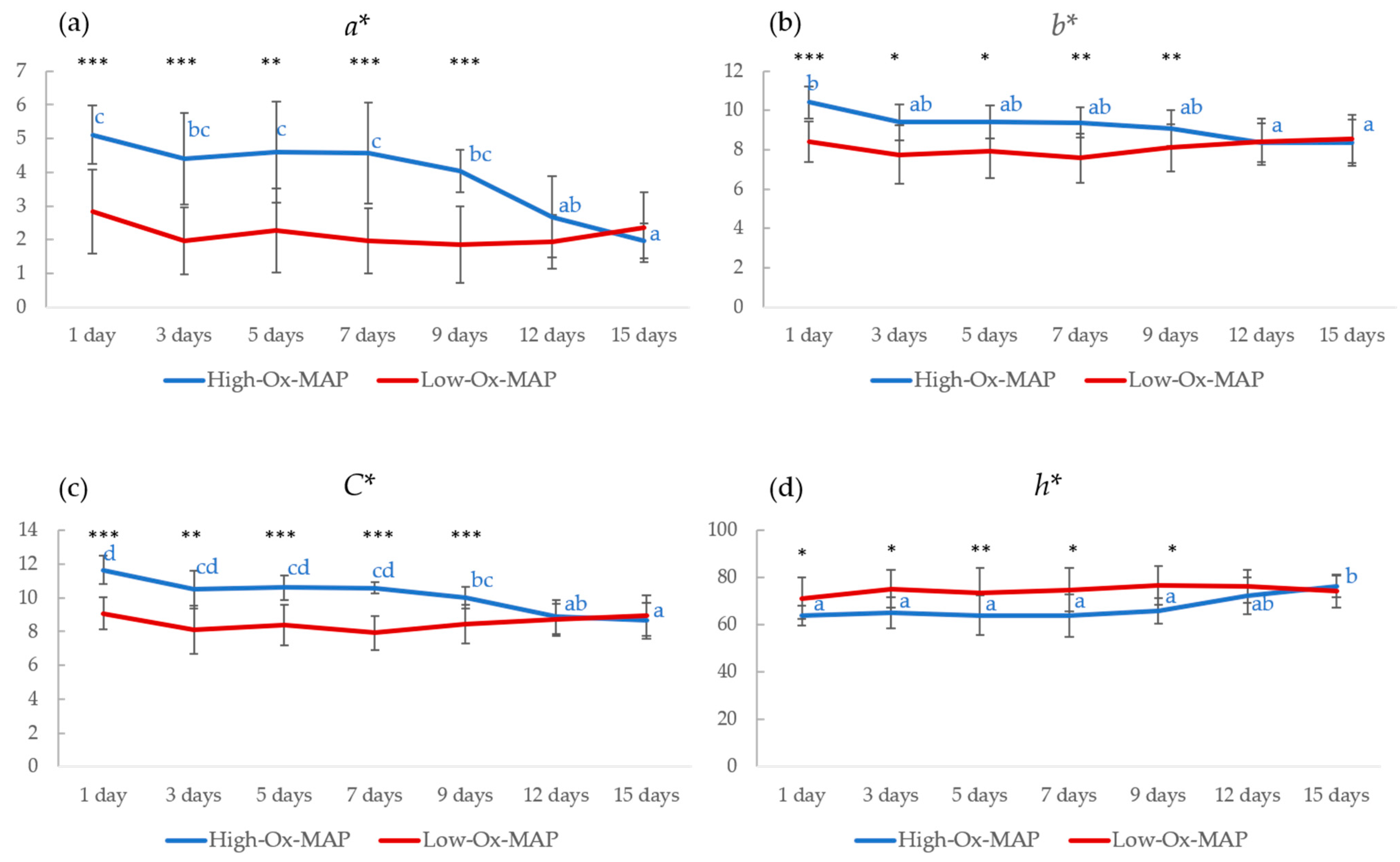
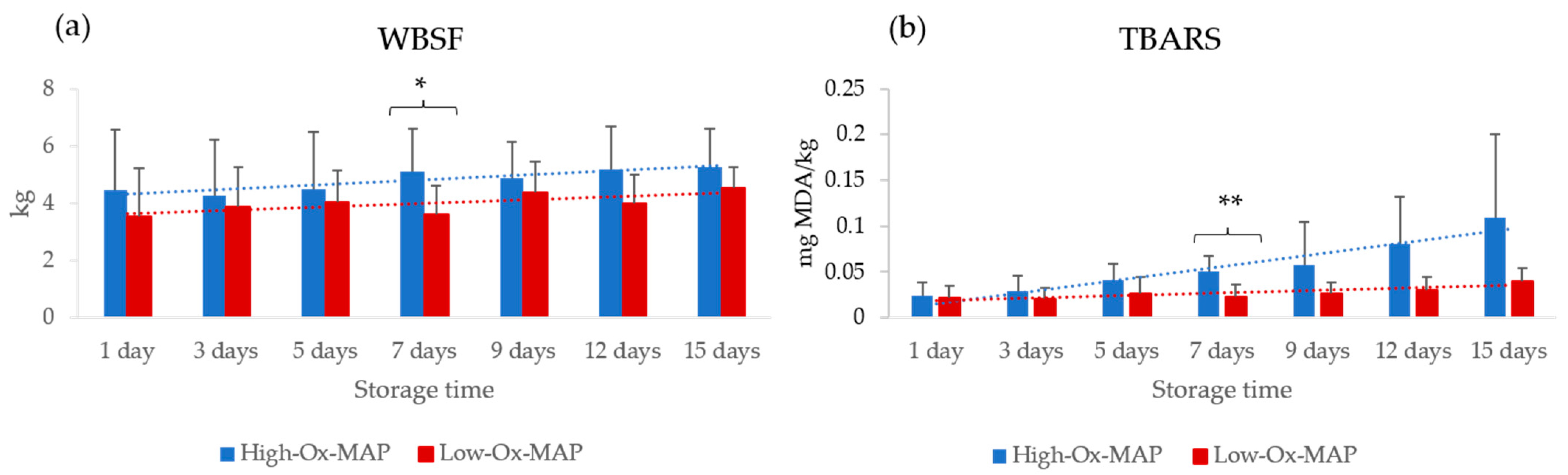
3.1. Effect of Storage Conditions on Meat Quality Traits and Microbial Loads
3.2. NIR Quantitative Models for Quality Traits
3.3. Classification and Validation of PLS-DA Models for Discriminating Fresh and Spoiled Pork Loin Packed in MAP Trays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elmasry, G.; Barbin, D.F.; Sun, D.W.; Allen, P. Meat quality evaluation by hyperspectral imaging technique: An overview. Crit. Rev. Food Sci. Nutr. 2012, 52, 689–711. [Google Scholar] [CrossRef] [PubMed]
- Prado, N.; Fernández-Ibáñez, V.; González, P.; Soldado, A. On-Site NIR spectroscopy to control the shelf life of pork meat. Food Anal. Methods 2011, 4, 582–589. [Google Scholar] [CrossRef]
- Yam, K.L.; Takhistov, P.T.; Miltz, J. Inteligent packaging: Concepts and applications. J. Food Sci. 2005, 70, R1–R10. [Google Scholar] [CrossRef]
- McMillin, K.W. Where is MAP Going? A review and future potential of modified atmosphere packaging for meat. Meat Sci. 2008, 80, 43–65. [Google Scholar] [CrossRef]
- McMillin, K.W. Advancements in meat packaging. Meat Sci. 2017, 132, 153–162. [Google Scholar] [CrossRef]
- Tejerina, D.; Contador, R.; Ortiz, A. Near infrared spectroscopy (NIRS) as tool for classification into official commercial categories and shelf-life storage times of pre-sliced modified atmosphere packaged Iberian dry-cured loin. Food Chem. 2021, 356, 129733. [Google Scholar] [CrossRef]
- Zakrys-Waliwander, P.I.; O’Sullivan, M.G.; Walsh, H.; Allen, P.; Kerry, J.P. Sensory comparison of commercial low and high oxygen modified atmosphere packed sirloin beef steaks. Meat Sci. 2011, 88, 198–202. [Google Scholar] [CrossRef]
- Ellis, D.I.; Broadhurst, D.; Kell, D.B.; Rowland, J.J.; Goodacre, R. Rapid and quantitative detection of the microbial spoilage of meat by fourier transform infrared spectroscopy and machine learning. Appl. Environ. Microbiol. 2002, 68, 2822–2828. [Google Scholar] [CrossRef] [Green Version]
- Olafsdottir, G.; Nesvadba, P.; Di Natale, C.; Careche, M.; Oehlenschläger, J.; Tryggvadóttir, S.V.; Schubring, R.; Kroeger, M.; Heia, K.; Esaiassen, M.; et al. Multisensor for fish quality determination. Trends Food Sci. Technol. 2004, 15, 86–93. [Google Scholar] [CrossRef]
- Weeranantanaphan, J.; Downey, G.; Allen, P.; Sun, D.W. A review of near infrared spectroscopy in muscle food analysis: 2005-2010. J. Near Infrared Spectrosc. 2011, 19, 61–104. [Google Scholar] [CrossRef]
- Prieto, N.; Roehe, R.; Lavín, P.; Batten, G.; Andrés, S. Application of near infrared reflectance spectroscopy to predict meat and meat products quality: A review. Meat Sci. 2009, 83, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Prieto, N.; Pawluczyk, O.; Dugan, M.E.R.; Aalhus, J.L. A Review of the principles and applications of Near-Infrared Spectroscopy to characterize meat, fat, and meat products. Appl. Spectrosc. 2017, 71, 1403–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.; Al-Holy, M.; Mousavi-Hesary, M.; Al-Qadiri, H.; Cavinato, A.G.; Rasco, B.A. Rapid and quantitative detection of the microbial spoilage in chicken meat by diffuse reflectance spectroscopy (600–1100 nm). Lett. Appl. Microbiol. 2004, 39, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Grau, R.; Sánchez, A.J.; Girón, J.; Iborra, E.; Fuentes, A.; Barat, J.M. Nondestructive assessment of freshness in packaged sliced chicken breasts using SW-NIR spectroscopy. Food Res. Int. 2011, 44, 331–337. [Google Scholar] [CrossRef]
- Andersen, P.V.; Wold, J.P.; Gjerlaug-Enger, E.; Veiseth-Kent, E. Predicting post-mortem meat quality in porcine longissimus lumborum using Raman, near infrared and fluorescence spectroscopy. Meat Sci. 2018, 145, 94–100. [Google Scholar] [CrossRef]
- Horváth, K.; Seregély, Z.; Andrássy, É.; Dalmadi, I.; Farkas, J. A preliminary study using near infrared spectroscopy to evaluate freshness and detect spoilage in sliced pork meat. Acta Aliment. 2008, 37, 93–102. [Google Scholar] [CrossRef]
- Lanza, I.; Conficoni, D.; Balzan, S.; Cullere, M.; Fasolato, L.; Serva, L.; Contiero, B.; Trocino, A.; Marchesini, G.; Xiccato, G.; et al. Assessment of chicken breast shelf life based on bench-top and portable near-infrared spectroscopy tools coupled with chemometrics. Food Qual. Saf. 2021, 5, 1–11. [Google Scholar] [CrossRef]
- American Medical Student Association. AMSA Meat Color Measurement Guidelines; AMSA: Washington, WA, USA, 2012; ISBN 8005172672.
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Downey, G.; Hildrum, K.I. Analysis of Meats. In Near-Infrared Spectroscopy in Agriculture; John Wiley & Sons, Ltd.: New York, NY, USA, 2004; pp. 599–632. ISBN 9780891182368. [Google Scholar]
- Faber, N.K.N. A closer look at the bias-variance trade-off in multivariate calibration. J. Chemom. 1999, 13, 185–192. [Google Scholar] [CrossRef]
- Barbin, D.F.; Elmasry, G.; Sun, D.W.; Allen, P.; Morsy, N. Non-destructive assessment of microbial contamination in porcine meat using NIR hyperspectral imaging. Innov. Food Sci. Emerg. Technol. 2013, 17, 180–191. [Google Scholar] [CrossRef]
- Matthews, B.W. Comparison of the predicted and observed secondary structure of T4 phage lysozyme. BBA Protein Struct. 1975, 405, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Cáceres-Nevado, J.M.; Garrido-Varo, A.; De Pedro-Sanz, E.; Tejerina-Barrado, D.; Pérez-Marín, D.C. Non-destructive Near Infrared Spectroscopy for the labelling of frozen Iberian pork loins. Meat Sci. 2021, 175, 108440. [Google Scholar] [CrossRef] [PubMed]
- Stella, S.; Bernardi, C.; Tirloni, E. Influence of skin packaging on raw beef quality: A review. J. Food Qual. 2018, 464578. [Google Scholar] [CrossRef] [Green Version]
- Jaberi, R.; Kaban, G.; Kaya, M. Effects of vacuum and high-oxygen modified atmosphere packaging on physico-chemical and microbiological properties of minced water buffalo meat. Asian Australas. J. Anim. Sci. 2019, 32, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Zakrys, P.I.; Hogan, S.A.; O’Sullivan, M.G.; Allen, P.; Kerry, J.P. Effects of oxygen concentration on the sensory evaluation and quality indicators of beef muscle packed under modified atmosphere. Meat Sci. 2008, 79, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Adhiputra, K.; Padayachee, A.; Channon, H.; Ha, M.; Warner, R.D. High oxygen modified atmosphere packaging negatively influences consumer acceptability traits of pork. Foods 2019, 8, 567. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Huff-Lonergan, E.; Sebranek, J.G.; Lonergan, S.M. High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Sci. 2010, 85, 759–767. [Google Scholar] [CrossRef]
- Lund, M.N.; Lametsch, R.; Hviid, M.S.; Jensen, O.N.; Skibsted, L.H. High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Sci. 2007, 77, 295–303. [Google Scholar] [CrossRef]
- Andrés, S.; Silva, A.; Soares-Pereira, A.L.; Martins, C.; Bruno-Soares, A.M.; Murray, I. The use of visible and near infrared reflectance spectroscopy to predict beef M. longissimus thoracis et lumborum quality attributes. Meat Sci. 2008, 78, 217–224. [Google Scholar] [CrossRef] [Green Version]
- De Marchi, M. On-line prediction of beef quality traits using near infrared spectroscopy. Meat Sci. 2013, 94, 455–460. [Google Scholar] [CrossRef]
- De Marchi, M.; Penasa, M.; Battagin, M.; Zanetti, E.; Pulici, C.; Cassandro, M. Feasibility of the direct application of near-infrared reflectance spectroscopy on intact chicken breasts to predict meat color and physical traits. Poult. Sci. 2011, 90, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Barlocco, N.; Vadell, A.; Ballesteros, F.; Gallieta, G. The use of visible and near-infrared reflectance spectroscopy to predict colour on both intact and homogenised pork muscle. LWT Food Sci. Technol. 2003, 36, 195–202. [Google Scholar] [CrossRef]
- Barlocco, N.; Vadell, A.; Ballesteros, F.; Galietta, G.; Cozzolino, D. Predicting intramuscular fat, moisture and Warner-Bratzler shear force in pork muscle using near infrared reflectance spectroscopy. Anim. Sci. 2006, 82, 111–116. [Google Scholar] [CrossRef]
- Prieto, N.; Juárez, M.; Larsen, I.L.; López-Campos, O.; Zijlstra, R.T.; Aalhus, J.L. Rapid discrimination of enhanced quality pork by visible and near infrared spectroscopy. Meat Sci. 2015, 110, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Murray, I. Identification of animal meat muscles by visible and near infrared reflectance spectroscopy. LWT Food Sci. Technol. 2004, 37, 447–452. [Google Scholar] [CrossRef]
- Xing, J.; Ngadi, M.; Gunenc, A.; Prasher, S.; Gariepy, C. Use of visible spectroscopy for quality classification of intact pork meat. J. Food Eng. 2007, 82, 135–141. [Google Scholar] [CrossRef]
- Balage, J.M.; e Silva, S.D.L.; Gomide, C.A.; de Nadai Bonin, M.; Figueira, A.C. Predicting pork quality using Vis/NIR spectroscopy. Meat Sci. 2015, 108, 37–43. [Google Scholar] [CrossRef]
- Furtado, E.J.G.; Bridi, A.M.; Barbin, D.F.; Barata, C.C.P.; Peres, L.M.; Barbon, A.P.A.D.C.; Andreo, N.; Giangareli, B.D.L.; Terto, D.K.; Batista, J.P. Prediction of pH and color in pork meat using VIS-NIR near-infrared spectroscopy (NIRS). Food Sci. Technol. 2019, 39, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Aalhus, J.L.; Best, D.R.; Murray, A.C.; Jones, S.D.M. A comparison of the quality characteristics of pale, soft and exudative beef and pork. J. Muscle Foods 1998, 9, 267–280. [Google Scholar] [CrossRef]
- Franco, D.; Mato, A.; Salgado, F.J.; López-Pedrouso, M.; Carrera, M.; Bravo, S.; Parrado, M.; Gallardo, J.M.; Zapata, C. Tackling proteome changes in the longissimus thoracis bovine muscle in response to pre-slaughter stress. J. Proteom. 2015, 122, 73–85. [Google Scholar] [CrossRef]
- Kapper, C.; Klont, R.E.; Verdonk, J.M.A.J.; Urlings, H.A.P. Prediction of pork quality with near infrared spectroscopy (NIRS). 1. Feasibility and robustness of NIRS measurements at laboratory scale. Meat Sci. 2012, 91, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Savenije, B.; Geesink, G.H.; Van Der Palen, J.G.P.; Hemke, G.; Hopkins, D.; Ouali, A. Prediction of pork quality using visible/near-infrared reflectance spectroscopy. Meat Sci. 2006, 73, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.T.; Fan, Y.X.; Cheng, F. On-line prediction of fresh pork quality using visible/near-infrared reflectance spectroscopy. Meat Sci. 2010, 86, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Geesink, G.H.; Schreutelkamp, F.H.; Frankhuizen, R.; Vedder, H.W.; Faber, N.M.; Kranen, R.W.; Gerritzen, M.A. Prediction of pork quality attributes from near infrared reflectance spectra. Meat Sci. 2003, 65, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Sun, D.W.; Pu, H.; Xie, A.; Han, Z.; Luo, M. Non-destructive prediction of thiobarbituric acid reactive substances (TBARS) value for freshness evaluation of chicken meat using hyperspectral imaging. Food Chem. 2015, 179, 175–181. [Google Scholar] [CrossRef]
- Atanassova, S.; Stoyanchev, T.; Ribarski, S. Evaluation of pork meat quality and freshness using colorimetric and spectral methods. Agric. Sci. Technol. 2013, 5, 115–120. [Google Scholar]
- Hoving-Bolink, A.H.; Vedder, H.W.; Merks, J.W.M.; De Klein, W.J.H.; Reimert, H.G.M.; Frankhuizen, R.; Van Den Broek, W.H.A.M.; Lambooij, E. Perspective of NIRS measurements early post mortem for prediction of pork quality. Meat Sci. 2005, 69, 417–423. [Google Scholar] [CrossRef]
- Boughorbel, S.; Jarray, F.; El-Anbari, M. Optimal classifier for imbalanced data using Matthews Correlation Coefficient metric. PLoS ONE 2017, 12, e0177678. [Google Scholar] [CrossRef]
- Atanassova, S.; Stoyanchev, T. Prediction of pork meat freshness by fiber-optics near-infrared instrument. In Proceedings of the FAC Workshop on Dynamics and Control in Agriculture and Food Processing, Plovdiv, Bulgaria, 13–16 June 2012; pp. 51–54. [Google Scholar]
- Workman Jr, J.; Weyer, L. Practical Guide to Interpretive Near-Infrared Spectroscopy, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
| MAP | T | MAP × T | |
|---|---|---|---|
| TVC (log cfu/g) | 0.021 * | 0.000 *** | 0.547 |
| Enterobacteriaceae (log cfu/g) | 0.000 *** | 0.000 *** | 0.154 |
| BAL (log cfu/g) | 0.949 | 0.000 *** | 0.264 |
| pH | 0.554 | 0.122 | 0.529 |
| WBSF (kg) | 0.002 ** | 0.506 | 0.983 |
| TBARS (mg MDA/kg) | 0.021 * | 0.072 | 0.320 |
| L* | 0.158 | 0.419 | 0.997 |
| a* | 0.000 *** | 0.000 *** | 0.000 *** |
| b* | 0.000 *** | 0.069 | 0.005 ** |
| C* | 0.000 *** | 0.000 *** | 0.000 *** |
| h* | 0.000 *** | 0.009 * | 0.055 |
| Calibration (n = 106) | Validation (n = 20) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max | Min | Range | Mean | SD | CV (%) | Max | Min | Range | Mean | SD | CV (%) | |
| pH | 6.84 | 5.29 | 1.55 | 5.72 | 0.23 | 4.09 | 6.3 | 5.42 | 0.87 | 5.73 | 0.21 | 3.70 |
| WBSF (kg) | 8.9 | 1.44 | 7.46 | 4.32 | 1.56 | 36.1 | 6.96 | 2.64 | 4.32 | 4.06 | 1.06 | 26.1 |
| TBARS (mg MDA/kg) | 0.27 | 0 | 0.27 | 0.04 | 0.03 | 71.43 | 0.07 | 0.009 | 0.06 | 0.03 | 0.02 | 54.29 |
| L* | 61.75 | 47.58 | 14.18 | 55.36 | 3.37 | 6.09 | 61.37 | 49.63 | 11.74 | 55.48 | 2.81 | 5.07 |
| a* | 7.36 | 0.02 | 7.34 | 2.89 | 1.53 | 53.05 | 6.75 | 0.76 | 5.98 | 2.99 | 1.70 | 56.86 |
| b* | 11.58 | 4.58 | 7.00 | 8.58 | 1.29 | 15.12 | 10.96 | 6.27 | 4.69 | 8.65 | 1.19 | 13.74 |
| C* | 12.70 | 5.27 | 7.43 | 9.20 | 1.42 | 15.47 | 12.63 | 6.64 | 5.99 | 9.3 | 1.49 | 15.99 |
| h* | 89.9 | 47.65 | 42.25 | 71.9 | 8.81 | 12.26 | 85.89 | 54.17 | 31.71 | 71.74 | 8.55 | 11.92 |
| TVC (log cfu/g) | 9.25 | 3.04 | 6.21 | 6.37 | 1.74 | 27.36 | 9.12 | 3.16 | 5.96 | 6.19 | 1.93 | 31.10 |
| Enterobacteriaceae (log cfu/g) | 7.23 | 0 | 7.23 | 2.82 | 1.66 | 58.93 | 6.38 | 0.69 | 5.68 | 3.03 | 1.86 | 61.39 |
| LAB (log cfu/g) | 9.03 | 1 | 8.03 | 5.45 | 1.91 | 35.11 | 7.42 | 2.24 | 5.18 | 4.96 | 1.73 | 34.85 |
| Intact Trays | Treat. | n | LVs | RMSECAL | R2CAL | RMSECV | R2CV | RERCV | RPDCV | RMSEP | R2VAL | SEP | RERVAL | RPDVAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | SNVD | 101 | 11 | 0.097 | 0.772 | 0.170 | 0.312 | 7.618 | 1.203 | 0.185 | 0.199 | 0.187 | 4.730 | 1.148 |
| WBSF (kg) | SNVD | 98 | 4 | 1.14 | 0.380 | 1.266 | 0.251 | 5.882 | 1.151 | 0.912 | 0.209 | 0.926 | 4.728 | 1.156 |
| TBARS (mg MDA/kg) | SNVD | 100 | 9 | 0.025 | 0.615 | 0.036 | 0.260 | 7.491 | 1.162 | 0.025 | 0.02 | 0.026 | 2.610 | 0.775 |
| L* | SG 1,4,4,1 | 97 | 4 | 1.236 | 0.858 | 1.980 | 0.645 | 7.160 | 1.671 | 1.516 | 0.673 | 1.547 | 7.745 | 1.855 |
| a* | SNVD | 101 | 6 | 0.577 | 0.861 | 0.688 | 0.807 | 10.671 | 2.271 | 0.795 | 0.770 | 0.750 | 7.529 | 2.142 |
| b* | SNVD | 102 | 8 | 0.634 | 0.722 | 0.837 | 0.525 | 7.859 | 1.445 | 0.726 | 0.606 | 0.737 | 6.460 | 1.636 |
| C* | SNV | 96 | 8 | 0.619 | 0.788 | 0.867 | 0.594 | 7.860 | 1.563 | 0.841 | 0.662 | 0.843 | 7.121 | 1.767 |
| h* | SNVD | 102 | 9 | 2.421 | 0.922 | 3.945 | 0.798 | 10.710 | 2.217 | 3.826 | 0.789 | 3.796 | 8.289 | 2.236 |
| TVC (log cfu/g) | SNV | 98 | 7 | 0.753 | 0.796 | 0.942 | 0.688 | 6.517 | 1.783 | 0.888 | 0.776 | 0.891 | 6.715 | 2.170 |
| ENT (log cfu/g) | SNVD | 98 | 6 | 0.725 | 0.787 | 0.851 | 0.712 | 7.117 | 1.856 | 0.927 | 0.738 | 0.932 | 6.132 | 2.009 |
| LAB (log cfu/g) | SNV | 106 | 8 | 0.827 | 0.810 | 1.044 | 0.704 | 7.694 | 1.830 | 0.882 | 0.725 | 0.792 | 5.874 | 1.960 |
| Open Trays | Treat. | n | LVs | RMSECAL | R2CAL | RMSECV | R2CV | RERCV | RPDCV | RMSEP | R2VAL | SEP | RERVAL | RPDVAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | SG 1,4,4,1 | 106 | 10 | 0.095 | 0.833 | 0.164 | 0.508 | 9.421 | 1.426 | 0.148 | 0.483 | 0.152 | 5.912 | 1.435 |
| WBSF (kg) | SG 1,4,4,1 | 98 | 4 | 0.976 | 0.532 | 1.076 | 0.444 | 6.917 | 1.336 | 0.888 | 0.252 | 0.882 | 4.856 | 1.187 |
| TBARS (mg MDA/kg) | SG 1,4,4,1 | 86 | 9 | 0.013 | 0.528 | 0.016 | 0.329 | 6.907 | 1.261 | 0.017 | 0.154 | 0.017 | 3.848 | 1.141 |
| L* | ABS | 97 | 5 | 1.126 | 0.878 | 1.247 | 0.854 | 11.368 | 2.604 | 0.988 | 0.869 | 1.013 | 11.884 | 2.846 |
| a* | ABS | 99 | 5 | 0.540 | 0.869 | 0.589 | 0.848 | 11.330 | 2.559 | 0.894 | 0.709 | 0.806 | 6.695 | 1.905 |
| b* | SNVD | 106 | 5 | 0.657 | 0.740 | 0.718 | 0.695 | 9.753 | 1.806 | 0.498 | 0.814 | 0.506 | 9.418 | 2.386 |
| c* | SNV | 106 | 9 | 0.597 | 0.822 | 0.814 | 0.676 | 9.127 | 1.748 | 0.750 | 0.731 | 0.714 | 7.985 | 1.981 |
| h* | SG 1,4,4,1 | 106 | 4 | 3.763 | 0.815 | 4.145 | 0.780 | 10.194 | 2.126 | 4.682 | 0.684 | 4.717 | 6.774 | 1.827 |
| TVC (log UF C/g) | SG 2,5,5,2 | 106 | 12 | 0.556 | 0.897 | 0.992 | 0.679 | 6.260 | 1.692 | 1.037 | 0.694 | 0.898 | 5.750 | 1.858 |
| ENT (log CFU/g) | SG 2,5,5,2 | 98 | 11 | 0.473 | 0.911 | 0.940 | 0.657 | 6.847 | 1.700 | 0.903 | 0.752 | 0.925 | 6.295 | 2.062 |
| LAB (log CFU/g) | SNVD | 95 | 10 | 0.587 | 0.900 | 0.963 | 0.735 | 8.341 | 1.936 | 0.800 | 0.682 | 0.541 | 6.476 | 2.161 |
| Total Viable Counts (Fresh/Spoiled) | Intact Trays | Open Trays | ||||
|---|---|---|---|---|---|---|
| Pre-treatment | SG 2,5,5,2 SNVD | SNV | ||||
| CAL | CV | VAL | CAL | CV | VAL | |
| Sensitivity (%) | 97.72 | 93.18 | 90 | 97.72 | 90.9 | 80 |
| Specificity (%) | 98.2 | 92.85 | 90 | 100 | 100 | 100 |
| Accuracy (%) | 98 | 93 | 90 | 99 | 96.11 | 90 |
| Matthews correlation | 0.959 | 0.858 | 0.8 | 0.98 | 0.922 | 0.816 |
| Enterobacteriaceae (fresh/spoiled) | Intact trays | Open trays | ||||
| Pre-treatment | SNV | SG 2,5,5,2 SNV | ||||
| CAL | CV | VAL | CAL | CV | VAL | |
| Sensitivity (%) | 91.89 | 90.5 | 100 | 98.66 | 97.33 | 92.85 |
| Specificity (%) | 89.47 | 89.47 | 100 | 95 | 70 | 83.33 |
| Accuracy (%) | 91.39 | 90.32 | 100 | 97.89 | 91.57 | 90 |
| Matthews correlation | 0.76 | 0.737 | 1 | 0.936 | 0.733 | 0.761 |
| Lactic Acid Bacteria (fresh/spoiled) | Intact trays | Open trays | ||||
| Pre-treatment | SG 1,4,4,1 SNVD | SG 1,4,4,1 SNV | ||||
| CAL | CV | VAL | CAL | CV | VAL | |
| Sensitivity (%) | 98 | 98 | 91.66 | 98 | 94.23 | 100 |
| Specificity (%) | 86.95 | 84.78 | 100 | 100 | 97.77 | 100 |
| Accuracy (%) | 92.85 | 91.83 | 95 | 98.96 | 95.87 | 100 |
| Matthews correlation | 0.86 | 0.835 | 0.902 | 0.979 | 0.918 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias, E.; Sierra, V.; Prado, N.; González, P.; Fiorentini, G.; Díaz, J.; Oliván, M. Development of a Portable Near-Infrared Spectroscopy Tool for Detecting Freshness of Commercial Packaged Pork. Foods 2022, 11, 3808. https://doi.org/10.3390/foods11233808
Arias E, Sierra V, Prado N, González P, Fiorentini G, Díaz J, Oliván M. Development of a Portable Near-Infrared Spectroscopy Tool for Detecting Freshness of Commercial Packaged Pork. Foods. 2022; 11(23):3808. https://doi.org/10.3390/foods11233808
Chicago/Turabian StyleArias, Eduardo, Verónica Sierra, Natalia Prado, Pelayo González, Giovani Fiorentini, Juan Díaz, and Mamen Oliván. 2022. "Development of a Portable Near-Infrared Spectroscopy Tool for Detecting Freshness of Commercial Packaged Pork" Foods 11, no. 23: 3808. https://doi.org/10.3390/foods11233808
APA StyleArias, E., Sierra, V., Prado, N., González, P., Fiorentini, G., Díaz, J., & Oliván, M. (2022). Development of a Portable Near-Infrared Spectroscopy Tool for Detecting Freshness of Commercial Packaged Pork. Foods, 11(23), 3808. https://doi.org/10.3390/foods11233808






