Antimicrobial Active Packaging Containing Nisin for Preservation of Products of Animal Origin: An Overview
Abstract
:1. Introduction
2. Background Theory
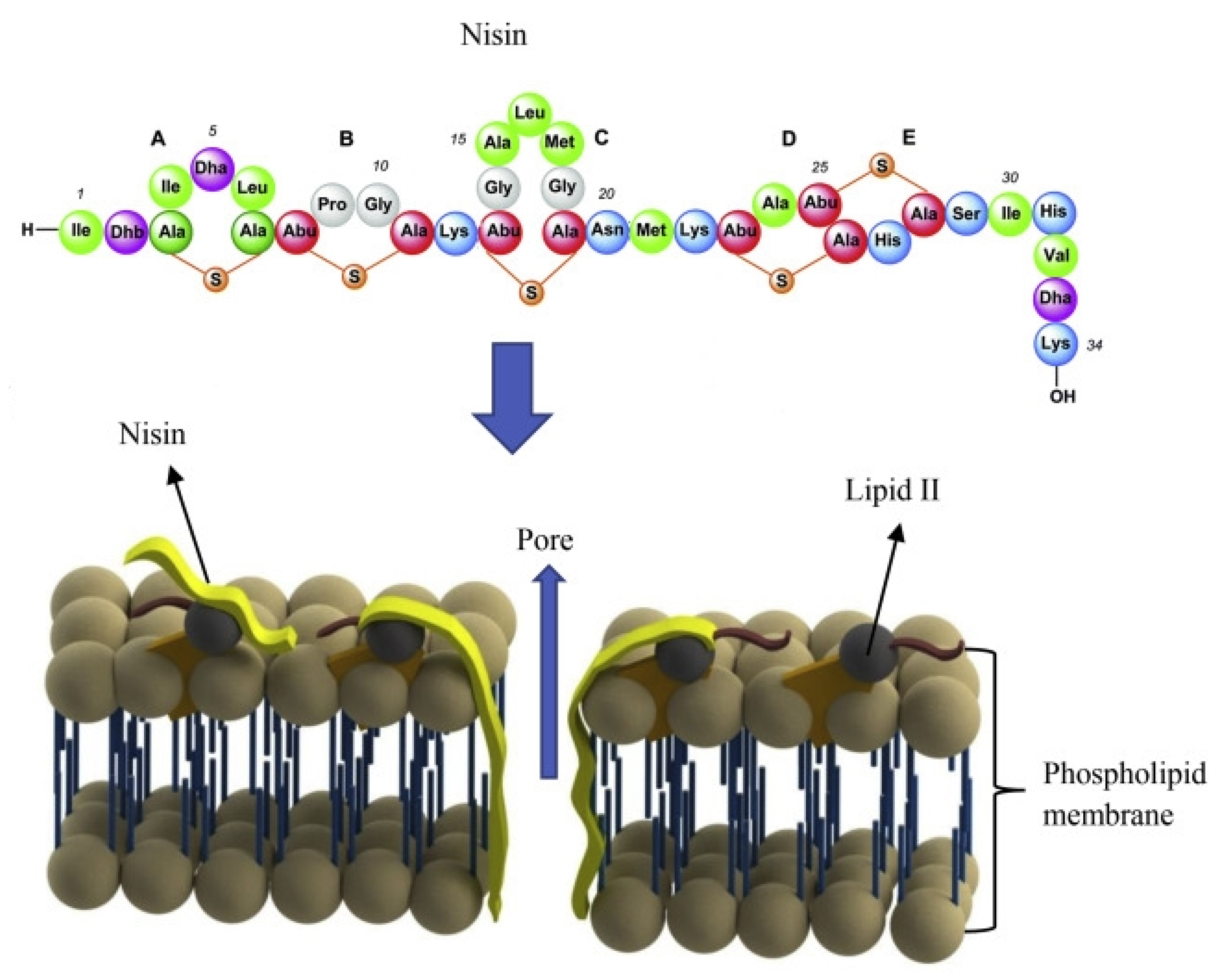
2.1. Nisin Mechanism of Action
2.2. Incorporation of Nisin in Packaging Materials
3. Application and Effects of Nisin Incorporated Packaging Materials
3.1. Antimicrobial Activity of Materials Containing Nisin
3.2. Effects of Materials Containing Nisin on Meat and Dairy Products
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Anumudu, C.; Hart, A.; Miri, T.; Onyeaka, H. Recent Advances in the Application of the Antimicrobial Peptide Nisin in the Inactivation of Spore-Forming Bacteria in Foods. Molecules 2021, 26, 5552. [Google Scholar] [CrossRef]
- Mukurumbira, A.R.; Shellie, R.A.; Keast, R.; Palombo, E.A.; Jadhav, S.R. Encapsulation of essential oils and their application in antimicrobial active packaging. Food Control 2022, 136, 108883. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Dong, M.; Zhang, H.; Li, L.; Wang, L. Food spoilage, bioactive food fresh-keeping films and functional edible coatings: Research status, existing problems and development trend. Trends Food Sci. Technol. 2022, 119, 122–132. [Google Scholar] [CrossRef]
- Kuai, L.; Liu, F.; Chiou, B.S.; Avena-Bustillos, R.J.; McHugh, T.H.; Zhong, F. Controlled release of antioxidants from active food packaging: A review. Food Hydrocoll. 2021, 120, 106992. [Google Scholar] [CrossRef]
- Kay, I.P.; Herskovitz, J.E.; Goddard, J.M. Interfacial behavior of a polylactic acid active packaging film dictates its performance in complex food matrices. Food Packag. Shelf Life 2022, 32, 100832. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Ezati, P.; Rhim, J.W. Curcumin and its uses in active and smart food packaging applications—A comprehensive review. Food Chem. 2022, 375, 131885. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Salehabadi, A.; Nafchi, A.M.; Oladzadabbasabadi, N.; Jafari, S.M. Cheese packaging by edible coatings and biodegradable nanocomposites; improvement in shelf life, physicochemical and sensory properties. Trends Food Sci. Technol. 2021, 116, 218–231. [Google Scholar] [CrossRef]
- Lim, C.W.; Lai, K.Y.; Ho, W.T.; Chan, S.H. Isotopic dilution assay development of nisin A in cream cheese, mascarpone, processed cheese and ripened cheese by LC-MS/MS method. Food Chem. 2019, 292, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Meister Meira, S.M.; Jardim, A.I.; Brandelli, A. Adsorption of nisin and pediocin on nanoclays. Food Chem. 2015, 188, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Rios, V.; Pedersen, M.; Pedrazzi, M.; Gkogka, E.; Smedsgaard, J.; Dalgaard, P. Antimicrobial effect of nisin in processed cheese—Quantification of residual nisin by LC-MS/MS and development of new growth and growth boundary model for Listeria monocytogenes. Int. J. Food Microbiol. 2021, 338, 108952. [Google Scholar] [CrossRef]
- Novickij, V.; Stanevičienė, R.; Grainys, A.; Lukša, J.; Badokas, K.; Krivorotova, T.; Sereikaitė, J.; Novickij, J.; Servienė, E. Electroporation-assisted inactivation of Escherichia coli using nisin-loaded pectin nanoparticles. Innov. Food Sci. Emerg. Technol. 2016, 38, 98–104. [Google Scholar] [CrossRef]
- Dong, A.; Malo, A.; Leong, M.; Ho, V.T.T.; Turner, M.S. Control of Listeria monocytogenes on ready-to-eat ham and fresh cut iceberg lettuce using a nisin containing Lactococcus lactis fermentate. Food Control 2021, 119, 107420. [Google Scholar] [CrossRef]
- Monfared, Y.K.; Mahmoudian, M.; Hoti, G.; Caldera, F.; Lopez Nicolas, J.M.; Zakeri-Milani, P.; Matencio, A.; Trotta, F. Cyclodextrin-Based Nanosponges as Perse Antimicrobial Agents Increase the Activity of Natural Antimicrobial Peptide Nisin. Pharmaceutics 2022, 14, 685. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhao, X.; Meng, R.; Liu, Z.; Zhang, G.; Guo, N. Synergistic antimicrobial effects of nisin and p-Anisaldehyde on Staphylococcus aureus in pasteurized milk. LWT 2017, 84, 222–230. [Google Scholar] [CrossRef]
- Bolt, H.L.; Kleijn, L.H.J.; Martin, N.I.; Cobb, S.L. Synthesis of Antibacterial Nisin–Peptoid Hybrids Using Click Methodology. Molecules 2018, 23, 1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Lu, N.; Wang, J.; Chen, Z.; Chen, C.; Regenstein, J.M.; Zhou, P. Effect of N-terminal modification on the antimicrobial activity of nisin. Food Control 2020, 114, 107227. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, Q. Lactobionic acid enhances the synergistic effect of nisin and thymol against Listeria monocytogenes Scott A in tryptic soy broth and milk. Int. J. Food Microbiol. 2017, 260, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Remedio, L.N.; Silva dos Santos, J.W.; Vieira Maciel, V.B.; Pedroso Yoshid, C.M.; Aparecida de Carvalho, R. Characterization of active chitosan films as a vehicle of potassium sorbate or nisin antimicrobial agents. Food Hydrocoll. 2019, 87, 830–838. [Google Scholar] [CrossRef]
- Figueiredo, A.C.L.; Almeida, R.C.C. Antibacterial efficacy of nisin, bacteriophage P100 and sodium lactate against Listeria monocytogenes in ready-to-eat sliced pork ham. Braz. J. Microbiol. 2017, 48, 724–729. [Google Scholar] [CrossRef]
- Khan, A.; Vu, K.D.; Riedl, B.; Lacroix, M. Optimization of the antimicrobial activity of nisin, Na-EDTA and pH against gram-negative and gram-positive bacteria. LWT 2015, 61, 124–129. [Google Scholar] [CrossRef]
- Zimet, P.; Mombru, A.W.; Faccio, R.; Brugnini, G.; Miraballes, I.; Rufo, C.; Pardo, H. Optimization and characterization of nisin-loaded alginate-chitosan nanoparticles with antimicrobial activity in lean beef. LWT 2018, 91, 107–116. [Google Scholar] [CrossRef]
- Ibarra-Sánchez, L.A.; El-Haddad, N.; Mahnoud, D.; Miller, M.J.; Karam, L. Invited review: Advances in nisin use for preservation of dairy products. J. Dairy Sci. 2020, 103, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Costello, K.M.; Smet, C.; Gutierrez-Merino, J.; Bussemaker, M.; Van Impe, J.F.; Velliou, E.G. The impact of food model system structure on the inactivation of Listeria innocua by cold atmospheric plasma and nisin combined treatments. Int. J. Food Microbiol. 2021, 337, 108948. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Iturmendi, N.; Mate, J.I.; Fernandez-Garcia, T. Combined effect of nisin addition and high pressure processing on the stability of liquid micellar casein concentrates. Int. Dairy J. 2022, 130, 105361. [Google Scholar] [CrossRef]
- Thébault, P.; Ammoun, M.; Boudjemaa, R.; Ouvrard, A.; Steenkeste, K.; Bourguignon, B.; Fontaine-Aupart, M.P. Surface functionalization strategy to enhance the antibacterial effect of nisin Z peptide. Surf. Interfaces 2022, 30, 101822. [Google Scholar] [CrossRef]
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32008R1333 (accessed on 9 November 2022).
- EFSA Panel on Food Additives and Nutrient Sources added to Food, ANS. Safety of nisin (E 234) as a food additive in the light of new toxicological data and the proposed extension of use. EFSA J. 2017, 15, e05063.
- Juncioni de Arauz, L.; Jozala, A.F.; Mazzola, P.G.; Penna, T.C.V. Nisin biotechnological production and application: A review. Trends Food Sci. Technol. 2009, 20, 146–154. [Google Scholar] [CrossRef]
- Amara, C.B.; Kim, L.; Oulahal, N.; Degraeve, P.; Gharsallaoui, A. Using complexation for the microencapsulation of nisin in biopolymer matrices by spray-drying. Food Chem. 2017, 236, 32–40. [Google Scholar] [CrossRef]
- Eghbal, N.; Viton, C.; Gharsallaoui, A. Nano and microencapsulation of bacteriocins for food applications: A review. Food Biosci. 2022; 102173, in press. [Google Scholar] [CrossRef]
- Santos, J.C.P.; Sousa, R.C.S.; Otoni, C.G.; Moraes, A.R.F.; Souza, V.G.L.; Medeiros, E.A.A.; Espitia, P.J.P.; Pires, A.C.S.; Coimbra, J.S.R.; Soares, N.F.F. Nisin and other antimicrobial peptides: Production, mechanisms of action, and application in active food packaging. Innov. Food Sci. Emerg. Technol. 2018, 48, 179–194. [Google Scholar] [CrossRef]
- Khan, I.; Oh, D.H. Integration of nisin into nanoparticles for application in foods. Innov. Food Sci. Emerg. Technol. 2016, 34, 376–384. [Google Scholar] [CrossRef]
- Sozbilen, G.S.; Yemenicioglu, A. Antilisterial effects of lysozyme-nisin combination at temperature and pH ranges optimal for lysozyme activity: Test of key findings to inactivate Listeria in raw milk. LWT 2021, 137, 110447. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Hussein, D.E.; Algammal, A.M.; George, T.T.; Jeandet, P.; Al-Snafi, A.E.; Tiwari, A.; Pagnossa, J.P.; Lima, C.M.; Thorat, N.D.; et al. Application of natural antimicrobials in food preservation: Recent views. Food Control 2021, 126, 108066. [Google Scholar] [CrossRef]
- Bai, F.; Guo, D.; Wang, Y.; Zhang, S.; Li, J.; Zhi, K.; Shi, C.; Xia, X. The combined bactericidal effect of nisin and thymoquinone against Listeria monocytogenes in Tryptone Soy Broth and sterilized milk. Food Control 2022, 135, 108771. [Google Scholar] [CrossRef]
- Bahrami, A.; Delshadi, R.; Jafari, S.M.; Williams, L. Nanoencapsulated nisin: An engineered natural antimicrobial system for the food industry. Trends Food Sci. Technol. 2019, 94, 20–31. [Google Scholar] [CrossRef]
- Sanjurjo, K.; Flores, S.; Gerschenson, L.; Jagus, R. Study of the performance of nisin supported in edible films. Food Res. Int. 2006, 39, 749–754. [Google Scholar] [CrossRef]
- Bhowmik, S.; Agyei, D.; Ali, A. Bioactive chitosan and essential oils in sustainable active food packaging: Recent trends, mechanisms, and applications. Food Packag. Shelf Life 2022, 34, 100962. [Google Scholar]
- Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC, 2004 within the General Requirements for Food Contact Materials. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:338:0004:0017:en:PDF (accessed on 9 November 2022).
- Szabó, B.S.; Petrovics, N.; Kirchkeszner, C.; Nyiri, Z.; Bodai, Z.; Eke, Z. Stability study of primary aromatic amines in aqueous food simulants under storage conditions of food contact material migration studies. Food Packag. Shelf Life 2022, 33, 100909. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:012:0001:0089:en:PDF (accessed on 9 November 2022).
- Baicu, A. Utilizarea Nanomaterialelor în Industria Alimentară (Nanomaterials Usage in Food Industry). Ph.D. Thesis, Faculty of Biotechnology, University of Agriculture and Veterinary Medicine of Bucharest, Bucharest, Romania, 2022. [Google Scholar]
- Perera, K.Y.; Jaiswal, S.; Jaiswal, A.K. A review on nanomaterials and nanohybrids based bio-nanocomposites for food packaging. Food Chem. 2022, 376, 131912. [Google Scholar] [CrossRef] [PubMed]
- Emamhadi, M.A.; Sarafraz, M.; Akbari, M.; Thai, V.N.; Fakhri, Y.; Linh, N.T.T.; Khaneghah, A.M. Nanomaterials for food packaging applications: A systematic review. Food Chem. Toxicol. 2020, 146, 111825. [Google Scholar] [CrossRef]
- Maisanaba, S.; Prieto, A.; Pichardo, S.; Jorda-Beneyto, M.; Aucejo, S.; Jos, A. Cytotoxicity and mutagenicity assessment of organomodified clays potentially used in food packaging. Toxicol. Vitr. 2015, 29, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.; Masoodi, F.; Baba, W.; Ahmad, M.; Rahmanian, N.; Jafari, S.M. Nanoencapsulation of Agrochemicals, Fertilizers, and Pesticides for Improved Plant Production. In Advances in Phytonanotechnology; Ghorbanpour, M., Wani, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 279–298. [Google Scholar]
- Cushen, M.; Kerry, J.; Morris, M.; Cruz-Romero, M.; Cummins, E. Nanotechnologies in the food industry—Recent developments, risks and regulation. Trends Food Sci. Technol. 2012, 24, 30–46. [Google Scholar] [CrossRef]
- Han, Y.; Wang, X.; Dai, H.; Li, S. Nanosize and surface charge effects of hydroxyapatite nanoparticles on Red blood cell suspensions. ACS Appl. Mater. Interfaces 2012, 4, 4616–4622. [Google Scholar] [CrossRef] [PubMed]
- Gedarawatte, S.T.G.; Ravensdale, J.T.; Al-Salami, H.; Dykes, G.A.; Coorey, R. Antimicrobial efficacy of nisin-loaded bacterial cellulose nanocrystals against selected meat spoilage lactic acid bacteria. Carbohydr. Polym. 2021, 251, 117096. [Google Scholar] [CrossRef] [PubMed]
- Hanušová, K.; Šťastná, M.; Votavová, L.; Klaudisová, K.; Dobiáš, J.; Voldřich, M.; Marek, M. Polymer films releasing nisin and/or natamycin from polyvinyldichloride lacquer coating: Nisin and natamycin migration, efficiency in cheese packaging. J. Food Eng. 2010, 99, 491–496. [Google Scholar] [CrossRef]
- Reichenberg, G.; Ophir, A.; Nir, Y. Nisin as an Antibacterial Substance in Active Packaging: 2. Use of Ethylene Methyl Acrylate and Co-Polyamide to Enhance Its Effectiveness. Int. J. Mater. Sci. 2015, 5, 45–53. [Google Scholar] [CrossRef]
- Chang, S.H.; Chen, Y.J.; Tseng, H.J.; Hsiao, H.; Chai, H.J.; Shang, K.C.; Pan, C.L.; Tsai, G.J. Applications of Nisin and EDTA in Food Packaging for Improving Fabricated Chitosan-Polylactate Plastic Film Performance and Fish Fillet Preservation. Membranes 2021, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, H.; Wu, J.; Ma, J.; Lu, P. Bio-based antimicrobial packaging from sugarcane bagasse nanocellulose/nisin hybrid films. Int. J. Biol. Macromol. 2020, 161, 627–635. [Google Scholar] [CrossRef]
- Behzadi, F.; Darouie, S.; Alavi, S.M.; Shariati, P.; Singh, G.; Dolatshahi-Pirouz, A.; Arpanaei, A. Stability and Antimicrobial Activity of Nisin-Loaded Mesoporous Silica Nanoparticles: A Game-Changer in the War against Maleficent Microbes. J. Agric. Food Chem. 2018, 66, 4233–4243. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wu, Y.; Liu, C.; Huang, L.; Zou, Y.; Shen, Y.; Lin, Q. Designing soluble soybean polysaccharides-based nanoparticles to improve sustained antimicrobial activity of nisin. Carbohydr. Polym. 2019, 225, 15251. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Dai, Y.J.; Lin, L. Enhancing antibacterial efficacy of nisin in pork by poly--glutamic acid/poly-l-lysine nanoparticles encapsulation. J. Food Saf. 2018, 38, e12475. [Google Scholar] [CrossRef]
- Zhao, X.; Kuipers, O.P. Synthesis of silver-nisin nanoparticles with low cytotoxicity as antimicrobials against biofilm-forming pathogens. Colloids Surf. B. 2021, 206, 11965. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, J.; Zhu, X.; Lu, Y.; Xue, Y.; Lu, Z. Effects of Salmonella bacteriophage, nisin and potassium sorbate and their combination on safety and shelf life of fresh chilled pork. Food Control 2017, 73, 869–877. [Google Scholar] [CrossRef]
- Rivera-Hernández, L.; Chavarría-Hernández, N.; del Rocío López Cuellar, M.; Martínez-Juárez, V.M.; Rodríguez-Hernández, A.I. Pectin-gellan films intended for active food packaging: Release kinetics of nisin and physico-mechanical characterization. J. Food Sci. Technol. 2021, 58, 2973–2981. [Google Scholar] [CrossRef] [PubMed]
- Gharsallaoui, A.; Joly, C.; Oulahal, N.; Degraeve, P. Nisin as a Food Preservative: Part 2: Antimicrobial Polymer Materials Containing Nisin. Crit. Rev. Food Sci. Nutr. 2016, 56, 1275–1289. [Google Scholar] [CrossRef]
- Opálková Šišková, A.; Mosnáčková, K.; Musioł, M.; Opálek, A.; Bučková, M.; Rychter, P.; Eckstein Andicsová, A. Electrospun Nisin-Loaded Poly(ε-caprolactone)-Based Active Food Packaging. Materials 2022, 15, 4540. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Li, Y.; Luo, C.; Yang, C.; Shi, W.; Li, L. Covalent Immobilization of Polypeptides on Polylactic Acid Films and Their Application to Fresh Beef Preservation. J. Agric. Food Chem. 2020, 68, 10532–10541. [Google Scholar] [CrossRef] [PubMed]
- Cha, D.S.; Chen, J.; Park, H.J.; Chinnan, M.S. Inhibition of Listeria monocytogenes in tofu by use of a polyethylene film coated with a cellulosic solution containing nisin. Int. J. Food Sci. Technol. 2003, 38, 499–503. [Google Scholar] [CrossRef]
- Lin, L.S.; Wang, B.J.; Weng, Y.M. Quality preservation of commercial fish balls with antimicrobial zein coatings. J. Food Qual. 2011, 34, 81–87. [Google Scholar] [CrossRef]
- Perez, P.F.; Resa, C.P.O.; Gerschenson, L.N.; Jagus, R.J. Addition of Zein for the improvement of Physicochemical Properties of Antimicrobial Tapioca Starch Edible Film. Food Bioproc. Tech. 2021, 14, 262–271. [Google Scholar] [CrossRef]
- Dawson, P.L.; Hirt, D.E.; Rieck, J.R.; Acton, J.C.; Sotthibandhu, A. Nisin release from films is affected by both protein type and film-forming method. Food Res. Int. 2003, 36, 959–968. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, V.; Waheed, S.M.; Pradhan, D. Efficacy of Reuterin and Bacteriocins Nisin and Pediocin in the Preservation of Raw Milk from Dairy Farms. Food Technol. Biotechnol. 2020, 58, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Alirezalu, K.; Yaghoubi, M.; Poorsharif, L.; Shadi, A.; Kahve, H.I.; Pateiro, M.; Lorenzo, J.M.; Munekata, P.E.S. Antimicrobial Polyamide-Alginate Casing Incorporated with Nisin and ε-Polylysine Nanoparticles Combined with Plant Extract for Inactivation of Selected Bacteria in Nitrite-Free Frankfurter-Type Sausage. Foods 2021, 10, 1003. [Google Scholar] [CrossRef]
- Qu, A.; Zhang, Y.; Shi, H.; Wang, H.; Ding, K.; Pan, Z.H.; Zhao, G.; Hadiatullad, H. Investigation of gas-producing bacteria in sufu and its effective method to control their growth. LWT 2022, 155, 112919. [Google Scholar] [CrossRef]
- Hemmati, F.; Bahrami, A.; Esfanjani, A.F.; Hosseini, H.; McClements, D.J.; Williams, L. Electrospun antimicrobial materials: Advanced packaging materials for food applications. Trends Food Sci. Technol. 2021, 111, 520–533. [Google Scholar] [CrossRef]
- Abarca, R.L.; Medina, J.; Alvarado, N.; Ortiz, P.A.; Carrillo Lopez, B. Biodegradable gelatin-based films with nisin and EDTA that inhibit Escherichia coli. PLoS ONE 2022, 17, e0264851. [Google Scholar] [CrossRef] [PubMed]
- Sangcharoen, N.; Klaypradit, W.; Wilaipun, P. Antimicrobial activity optimization of nisin, ascorbic acid and ethylenediamine tetraacetic acid disodium salt (EDTA) against Salmonella Enteritidis ATCC 13076 using response surface methodology. Agric. Nat. Resour. 2017, 51, 355–364. [Google Scholar] [CrossRef]
- Fang, T.J.; Tsai, H.C. Growth patterns of Escherichia coli O157: H7 in ground beef treated with nisin, chelators, organic acids and their combinations immobilized in calcium alginate gels. Food Microbiol. 2003, 20, 243–253. [Google Scholar] [CrossRef]
- Narsaiah, K.; Jha, S.N.; Wilson, R.A.; Mandge, H.M.; Manikantan, M.R. Optimizing microencapsulation of nisin with sodium alginate and guar gum. J. Food Sci. Technol. 2014, 51, 4054–4059. [Google Scholar] [CrossRef] [PubMed]
- Zimet, P.; Valadez, R.; Raffaelli, S.; Estevez, M.B.; Pardo, H.; Albores, S. Biogenic Silver Nanoparticles Conjugated with Nisin: Improving the Antimicrobial and Antibiofilm Properties of Nanomaterials. Chemistry 2021, 3, 1271–1285. [Google Scholar] [CrossRef]
- Dawson, P.L.; Harmon, L.; Sotthibandhu, A.; Han, I.Y. Antimicrobial activity of nisin-adsorbed silica and corn starch powders. Food Microbiol. 2005, 22, 93–99. [Google Scholar] [CrossRef]
- Morsy, M.; Elsabagh, R.; Trinetta, V. Evaluation of novel synergistic antimicrobial activity of nisin, lysozyme, EDTA nanoparticles, and/or ZnO nanoparticles to control foodborne pathogens on minced beef. Food Control 2018, 92, 249–254. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, Y.; Zhang, X.; Zhu, L.; Fang, Z.; Shi, Q. Thermodynamic Properties and State Diagram of Gum Ghatti-Based Edible Films: Effects of Glycerol and Nisin. Polymers 2020, 12, 449. [Google Scholar] [CrossRef] [Green Version]
- Marvdashti, L.M.; Yavarmanesh, M.; Koocheki, A. Controlled release of nisin from polyvinyl alcohol—Alyssum homolocarpum seed gum composite films: Nisin kinetics. Food Biosci. 2019, 28, 133–139. [Google Scholar] [CrossRef]
- Han, D.; Sherman, S.; Filocamo, S.; Steckl, A.J. Long-term antimicrobial effect of nisin released from electrospun triaxial fiber membranes. Acta Biomater. 2017, 53, 242–249. [Google Scholar] [CrossRef]
- Lu, P.; Guo, M.; Xu, Z.; Wu, M. Application of Nanofibrillated Cellulose on BOPP/LDPE Film as Oxygen Barrier and Antimicrobial Coating Based on Cold Plasma Treatment. Coatings 2018, 8, 207. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Wu, J.; Li, C.; Lin, L. Improving anti-listeria activity of cheese packaging via nanofiber containing nisin-loaded nanoparticles. LWT 2017, 81, 233–242. [Google Scholar] [CrossRef]
- Jiang, L.; Su, C.; Wen, Y.; Zhu, Z.; Liu, J.; He, S.; He, S.; Liu, X.; Shao, W. Antibacterial activity and long-term stable antibacterial performance of nisin grafted magnetic GO nanohybrids. Mater. Sci. Eng. C 2020, 111, 110809. [Google Scholar] [CrossRef]
- Cui, H.Y.; Wu, J.; Li, C.Z.; Lin, L. Anti-listeria effects of chitosan-coated nisin-silica liposome on Cheddar cheese. J. Dairy Sci. 2016, 99, 8598–8606. [Google Scholar] [CrossRef] [Green Version]
- Meister Meira, S.M.; Zehetmeyer, G.; Scheibel, J.M.; Orlandini Werner, J.; Brandelli, A. Starch-halloysite nanocomposites containing nisin: Characterization and inhibition of Listeria monocytogenes in soft cheese. LWT 2016, 68, 226–234. [Google Scholar] [CrossRef]
- Divsalar, E.; Tajik, H.; Moradi, M.; Farough, M.; Lotfi, M.; Kuswandi, B. Characterization of cellulosic paper coated with chitosan-zinc oxide nanocomposite containing nisin and its application in packaging of UF cheese. Int. J. Biol. Macromol. 2018, 109, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Teixeira Barbosa, A.A.; Silva de Araújo, H.G.; Nogueira Matos, P.; Guitierrez Carnelossi, M.A.; Almeida de Castro, A. Effects of nisin-incorporated films on the microbiological and, physicochemical quality of minimally processed mangoes. Int. J. Food Microbiol. 2013, 164, 135–140. [Google Scholar] [CrossRef]
- Song, Z.; Li, F.; Guan, H.; Xu, Y.; Fu, Q.; Li, D. Combination of nisin and ε-polylysine with chitosan coating inhibits the white blush of fresh-cut carrots. Food Control 2017, 74, 34–44. [Google Scholar] [CrossRef]
- Wang, H.; Guo, L.; Liu, L.; Han, B.; Niu, X. Composite chitosan films prepared using nisin and Perilla frutescense essential oil and their use to extend strawberry shelf life. Food Biosci. 2021, 41, 101037. [Google Scholar] [CrossRef]
- Sami, R.; Khojah, E.; Elhakem, A.; Benajiba, N.; Helal, M.; Alhuthal, N.; Alzahrani, S.A.; Alharbi, M.; Chavali, M. Performance Study of Nano/SiO2 Films and the Antimicrobial Application on Cantaloupe Fruit Shelf-Life. Appl. Sci. 2021, 11, 3879. [Google Scholar] [CrossRef]
- Eldib, R.; Khojah, E.; Elhakem, A.; Benajiba, N.; Helal, M. Chitosan, Nisin, Silicon Dioxide Nanoparticles Coating Films Effects on Blueberry (Vaccinium myrtillus) Quality. Coatings 2020, 10, 962. [Google Scholar] [CrossRef]
- Sami, R.; Soltane, S.; Helal, M. Microscopic Image Segmentation and Morphological Characterization of Novel Chitosan/Silica Nanoparticle/Nisin Films Using Antimicrobial Technique for Blueberry Preservation. Membranes 2021, 11, 303. [Google Scholar] [CrossRef]
- Holcapkova, P.; Hurajova, A.; Bazant, P.; Pummerova, M.; Sedlarik, V. Thermal stability of bacteriocin nisin in polylactide-based films. Polym. Degrad. Stab. 2018, 158, 31–39. [Google Scholar] [CrossRef]
- Gao, S.; Zhai, X.; Cheng, Y.; Zhang, R.; Wang, W.; Hou, H. Starch/PBAT blown antimicrobial films based on the synergistic effects of two commercial antimicrobial peptides. Int. J. Biol. Macromol. 2022, 204, 457–465. [Google Scholar] [CrossRef]
- Zehetmeyer, G.; Meira, S.M.M.; Scheibel, J.M.; Bof de Oliveira, R.V.; Brandelli, A.; Soares, R.M.D. Influence of melt processing on biodegradable nisin-PBAT films intended for active food packaging applications. J. Appl. Polym. Sci. 2015, 133, 43212. [Google Scholar] [CrossRef]
- Wu, H.; Teng, C.; Liu, B.; Tian, H.; Wang, J.G. Characterization and long term antimicrobial activity of the nisin anchored cellulose films. Int. J. Biol. Macromol. 2018, 113, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Shao, X.; Zhang, M.; Wang, Z.; Dong, J.; Yu, D. Mechanical, barrier and antimicrobial properties of corn distarch phosphate/nanocrystalline cellulose films incorporated with Nisin and ε-polylysine. Int. J. Biol. Macromol. 2019, 136, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, R.; Zhang, H.; Jiang, S.; Liu, H.; Sun, M. Kinetics and functional effectiveness of nisin loaded antimicrobial packaging film based on chitosan/poly(vinyl alcohol). Carbohydr. Polym. 2015, 127, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Zimet, P.; Mombrú, A.W.; Mombrú, D.; Castro, A.; Villanueva, J.P.; Pardo, H.; Rufo, C. Physico-chemical and antilisterial properties of nisin-incorporated chitosan/carboxymethyl chitosan films. Carbohydr. Polym. 2019, 219, 334–343. [Google Scholar] [CrossRef]
- Gao, G.; Fan, H.; Zhang, Y.; Cao, Y.; Li, T.; Qiao, W.; Wu, M.; Ma, T.; Li, G. Production of nisin-containing bacterial cellulose nanomaterials with antimicrobial properties through co-culturing Enterobacter sp. FY-07 and Lactococcus lactis N8. Carbohydr. Polym. 2021, 251, 117131. [Google Scholar] [CrossRef] [PubMed]
- Wentz Brum, L.F.; dos Santos, C.; Santos, J.H.Z.; Brandelli, A. Structured silica materials as innovative delivery systems for the bacteriocin nisin. Food Chem. 2022, 366, 130599. [Google Scholar] [CrossRef]
- Oliveira, T.V.; Freitas, P.A.V.; Pola, C.C.; Terra, L.R.; Silva, J.O.R.; Badaro, A.T.; Junior, N.S.; Oliveira, M.M.; Silva, R.R.A.; Soares, N.F.F. The Influence of Intermolecular Interactions between Maleic Anhydride, Cellulose Nanocrystal, and Nisin-Z on the Structural, Thermal, and Antimicrobial Properties of Starch-PVA Plasticized Matrix. Polysaccharides 2021, 2, 661–676. [Google Scholar] [CrossRef]
- Kour, M.; Gupta, N.; Sood, M.; Bandral, J.D.; Hameed, F.; Kour, P. Hurdle technology: A review. Int. J. Chem. Stud. 2019, 7, 2579–2585. [Google Scholar]
- Hassan, A.H.A.; Cutter, C.N. Development and evaluation of pullulan-based composite antimicrobial films (CAF) incorporated with nisin, thymol and lauric arginate to reduce foodborne pathogens associated with muscle foods. Int. J. Food Microbiol. 2020, 320, 108519. [Google Scholar] [CrossRef] [PubMed]
- Naas, H.; Martinez-Dawson, R.; Han, I.; Dawson, P. Effect of combining nisin with modified atmosphere packaging on inhibition of Listeria monocytogenes in ready-to-eat turkey bologna. Poult. Sci. 2013, 92, 1930–1935. [Google Scholar] [CrossRef]
- Bingol, E.B.; Akkaya, E.; Hampikyan, H.; Cetin, O.; Colak, H. Effect of nisin-EDTA combinations and modified atmosphere packaging on the survival of Salmonella enteritidis in Turkish type meatballs. Cyta J. Food 2018, 16, 1030–1036. [Google Scholar] [CrossRef] [Green Version]
- Economou, T.; Pournis, N.; Ntzimani, A.; Savvaidis, I.N. Nisin–EDTA treatments and modified atmosphere packaging to increase fresh chicken meat shelf-life. Food Chem. 2009, 114, 1470–1476. [Google Scholar] [CrossRef]
- Martillanes, S.; Rocha-Pimienta, J.; Llera-Oyola, J.; Gil, M.V.; Ayuso-Yuste, M.C.; García-Parra, J.; Delgado-Adamez, J. Control of Listeria monocytogenes in sliced dry-cured Iberian ham by high pressure processing in combination with an eco-friendly packaging based on chitosan, nisin and phytochemicals from rice bran. Food Control 2021, 124, 107933. [Google Scholar] [CrossRef]
- Costello, K.M.; Velliou, E.; Gutierrez-Merino, J.; Smet, C.; El Kadri, H.; Van Impe, J.F.; Bissemaker, M. The effect of ultrasound treatment in combination with nisin on the inactivation of Listeria innocua and Escherichia coli. Ultrason. Sonochem. 2021, 79, 105776. [Google Scholar] [CrossRef]
- Han, F.; Lu, S.; Chi, H. Effects of Nisin Treatment on the Shelf Life of Ready-to-Eat Roasted Shrimp (Penaeus vannamei). Adv. Biochem. 2021, 9, 18–24. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, W.; Wang, J. Recent advances in the study of modified cellulose in meat products: Modification method of cellulose, meat quality improvement and safety concern. Trends Food Sci. Technol. 2022, 122, 140–156. [Google Scholar] [CrossRef]
- Cercel, F.; Stroiu, M.; Ianitchi, D.; Alexe, P. Research on obtaining, characterization and use of edible films in food industry. AgroLife Sci. J. 2017, 6, 56–64. [Google Scholar]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Starch-polyester bilayer films with phenolic acids for pork meat preservation. Food Chem. 2022, 385, 132650. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Amara, C.B.; Oulahal, N.; Gharsallaoui, A.; Joly, C.; Tongdeesoontorn, W.; Rawdkuen, S.; Degraeve, P. Gelatin films with nisin and catechin for minced pork preservation. Food Packag. Shelf Life. 2018, 18, 173–183. [Google Scholar] [CrossRef]
- Cao, Y.; Warner, R.D.; Fang, Z. Effect of chitosan/nisin/gallic acid coating on preservation of pork loin in high oxygen modified atmosphere packaging. Food Control 2019, 101, 9–16. [Google Scholar] [CrossRef]
- Leelaphiwat, P.; Pechprankan, C.; Siripho, P.; Bumbudsanpharoke, N.; Harnkarnsujarit, N. Effects of nisin and EDTA on morphology and properties of thermoplastic starch and PBAT biodegradable films for meat packaging. Food Chem. 2022, 369, 130956. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, M.; Bhandari, B.; Xu, J.; Yang, C. Effects of nanoemulsion-based active coatings with composite mixture of star anise essential oil, polylysine, and nisin on the quality and shelf life of ready-to-eat Yao meat products. Food Control 2020, 107, 106771. [Google Scholar] [CrossRef]
- Correa, J.P.; Molina, V.; Sanchez, M.; Kainz, C.; Eisenberg, P.; Massani, M.B. Improving ham shelf life with a polyhydroxybutyrate/polycaprolactone biodegradable film activated with nisin. Food Packag. Shelf Life 2017, 11, 31–39. [Google Scholar] [CrossRef]
- Batpho, K.; Boonsupthip, W.; Rachtanapun, C. Antimicrobial activity of collagen casing impregnated with nisin against foodborne microorganisms associated with ready-to-eat sausage. Food Control 2017, 73, 1342–1352. [Google Scholar] [CrossRef]
- Pattanayaiying, R.; H-Kittikun, A.; Cutter, C.N. Incorporation of nisin Z and lauric arginate into pullulan films to inhibit foodborne pathogens associated with fresh and ready-to-eat muscle foods. Int. J. Food Microbiol. 2015, 207, 77–82. [Google Scholar] [CrossRef]
- Morgan, A.; Darby, D.; Bruce, T.; Romero, A.; Cooksey, K. Development of an antimicrobial coating containing nisin and pectin for deli meat turkey bologna. LWT 2022, 159, 113210. [Google Scholar] [CrossRef]
- Gedarawatte, S.T.G.; Ravensdale, J.T.; Johns, M.L.; Li, M.; Al-Salami, H.; Dykes, G.A.; Coorey, R. Evaluation of the water-holding and anti-spoilage effect of a bacterial cellulose nanocrystal coating for the storage of vacuum-packaged beef. Food Packag. Shelf Life 2022, 31, 100818. [Google Scholar] [CrossRef]
- Lin, L.; Luo, C.; Li, C.; Chen, X.; Cui, H. A Novel Biocompatible Ternary Nanoparticle with High Antibacterial Activity: Synthesis, Characterization, and Its Application in Beef Preservation. Foods 2022, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Guohua, H.; Wei, L.; Hailin, F.; Jian, L.; Yuanyuan, G. Effects of chitosan combined with nisin treatment on storage quality of large yellow croaker (Pseudosciaena crocea). Food Chem. 2016, 203, 276–282. [Google Scholar]
- Kakatkar, A.S.; Gautam, R.K.; Shashidhar, R. Combination of glazing, nisin treatment and radiation processing for shelf-life extension of seer fish (Scomberomorous guttatus) steaks. Radiat. Phys. Chem. 2017, 130, 303–305. [Google Scholar] [CrossRef]
- Meral, R.; Alav, A.; Karakas, C.Y.; Dertli, E.; Yilmaz, M.T.; Ceylan, Z. Effect of electrospun nisin and curcumin loaded nanomats on the microbial quality, hardness and sensory characteristics of rainbow trout fillet. LWT 2019, 113, 108292. [Google Scholar] [CrossRef]
- Oner, B.; Meral, R.; Ceylan, Z. Determination of some quality indices of rainbow trout fillets treated with nisin-loaded polyvinylalcohol-based nanofiber and packed with polyethylene package. LWT 2021, 149, 111854. [Google Scholar] [CrossRef]
- Pattanayaiying, R.; Sane, A.; Photjanataree, P.; Cutter, C.N. Thermoplastic starch/polybutylene adipate terephthalate film coated with gelatin containing nisin Z and lauric arginate for control of foodborne pathogens associated with chilled and frozen seafood. Int. J. Food Microbiol. 2019, 290, 59–67. [Google Scholar] [CrossRef]
- Cen, S.; Fang, Q.; Tong, L.; Yang, W.; Zhang, J.; Lou, Q.; Huang, T. Effects of chitosan-sodium alginate-nisin preservatives on the quality and spoilage microbiota of Penaeus vannamei shrimp during cold storage. Int. J. Food Microbiol. 2021, 349, 109227. [Google Scholar] [CrossRef]
- Cruz Lima, R.; Carvalho, A.P.A.; Vieira, C.P.; Moreira, R.V.; Conte-Junior, C.A. Green and Healthier Alternatives to Chemical Additives as Cheese Preservative: Natural Antimicrobials in Active Nanopackaging/Coatings. Polymers 2021, 13, 2675. [Google Scholar] [CrossRef] [PubMed]
- Nitu, S.; Geicu-Cristea, M.; Matei, F. Milk-clotting enzymes obtained from plants in cheesemaking—A review. Scientific Bulletin. Series F. Biotechnologies 2021, 25, 66–75. [Google Scholar]
- Nasralla, N.N.; Gomah, N.H.; Aly, M.M.; Abdel-Aleem, J.A.; Hammam, A.R.A.; Osman, D.M.; El-Derwy, Y.M.A. Compositional characteristics of dairy products and their potential nondairy applications after shelf-life. Curr. Res. Nutr. Food Sci. 2022, 5, 150–156. [Google Scholar] [CrossRef]
- Seydim, A.C.; Sarikus-Tutal, G.; Sogut, E. Effect of whey protein edible films containing plant essential oils on microbial inactivation of sliced Kasar cheese. Food Packag. Shelf Life 2020, 26, 100567. [Google Scholar] [CrossRef]
- Berti, S.; Ollé Resa, C.P.; Basanta, F.; Gerschenson, L.N.; Jagus, R.J. Edible coatings on Gouda cheese as a barrier against external contamination during ripening. Food Biosci. 2019, 31, 100447. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Silva, R.R.A.; Oliveira, T.V.; Soares, R.R.A.; Soares, N.F.F. Biodegradable film development by nisin Z addition into hydroxypropylmethylcellulose matrix for mozzarella cheese preservation. Int. J. Food Stud. 2020, 9, 360–372. [Google Scholar] [CrossRef]
- Soto, K.M.; Hernández-Iturriaga, M.; Loarca-Piña, G.; Luna-Bárcenas, G.; Mendoza, S. Antimicrobial effect of nisin electrospun amaranth: Pullulan nanofibers in apple juice and fresh cheese. Int. J. Food Microbiol. 2019, 295, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Resa, C.P.O.; Gerschenson, L.N.; Jagus, R.J. Starch edible film supporting natamycin and nisin for improving microbiological stability of refrigerated argentinian Port Salut cheese. Food Control 2016, 59, 737–742. [Google Scholar] [CrossRef]
| Developed Packaging Materials Containing Nisin | Antimicrobial Action | References |
|---|---|---|
| PVA-AHSG films incorporated nisin | tested on Listeria innocua—inhibition zones increasing with nisin concentration in films | [79] |
| nisin-incorporated triaxial fibers | excellent biocidal activities for up to 5 days | [80] |
| BOPP/LDPE coated with NC and nisin | inhibited the growth of Listeria monocytogenes by 94% | [81] |
| PEO nanofibers containing nisin-loaded poly-g-glutamic acid/chitosan | great antimicrobial activity; effective against Listeria monocytogenes on cheese, with no alteration of sensorial properties | [82] |
| nisin grafted magnetic graphene oxide nanohybrids | long term antibacterial activity and stability (after 6 months of storage) against Bacillus subtilis and Staphylococcus aureus | [83] |
| chitosan-coated nisin-silica liposome | sustained antibacterial activity against Listeria monocytogenes; no effect on the sensory properties of cheddar cheese | [84] |
| starch/halloysite/nisin based films | films with 2 g/100 g nisin significantly reduced the initial bacterial load of minas frescal cheese after 4 days; films with 6 g/100 g nisin completely inhibited the development of Listeria monocytogenes | [85] |
| bilayer film based on chitosan, cellulose, and nisin (500 and 1000 g/mL) | films with great antimicrobial properties; the one containing 1000 g/mL inhibited completely the development of Listeria monocytogenes on the surface of ultra-filter (UF) white cheese after storage at 4 °C for 14 days | [86] |
| nisin-incorporated cellulose films | presented antimicrobial activity against Staphylococcus aureus, Listeria monocytogenes, Alicyclobacillus acidoterrestris, and Bacillus cereus | [87] |
| nisin and ε-PL with chitosan coating | decreased the growth of yeast and mold, total viable counts, total coliforms count, Staphylococcus aureus, and Pseudomonas spp. in fresh-cut carrots | [88] |
| N-succinyl chitosan-based films with nisin and Perilla essential oil | good antioxidant and antibacterial activity against Staphylococcus aureus, Escherichia coli, Salmonella enteritidis, and Pseudomonas tolaasii | [89] |
| novel films with chitosan/nano/SiO2/nisin films | used as coatings for cantaloupes—reduced the yeast and mold counts | [90] |
| chitosan coating plus SiO2 nanoparticles and nisin | the coating containing nisin inhibited the microbial populations for molds/yeast and mesophilics on blueberries | [91] |
| chitosan/silica nanoparticle/nisin films | coated blueberries with chitosan/silica nanoparticle/nisin films reported the lowest microbial contamination counts | [92] |
| nisin in polylactide and polylactide/PEG blends | good antimicrobial activity against Micrococcus luteus | [93] |
| starch/PBAT films containing nisin (2%) and ε-PL (1%) | efficiently inhibited the development of Staphylococcus aureus and Escherichia coli | [94] |
| PBAT incorporated with nisin | inhibitory effect on Listeria monocytogenes | [95] |
| nisin anchored on oxidized cellulose film | good antimicrobial activity against Alicyclobacillus acidoterrestris DSM 3922T | [96] |
| incorporated nisin and ε-polylysine into corn starch phosphate/nanocrystalline cellulose-based films | antimicrobial activity against gram-positive bacteria (Staphylococcus aureus) and gram-negative bacteria (Escherichia coli) | [97] |
| nisin loaded chi-tosan/PVA film | the concentration of Staphylococcus aureus decreased from 100% to 11.65% when nisin content increased from 0 to 10% | [98] |
| nisin-incorporated chitosan films | effective against Listeria monocytogenes | [99] |
| nisin-containing bacterial cellulose film | great inhibitory effect on gram-positive bacteria (Staphylococcus aureus ATCC 6538) | [100] |
| encapsulated nisin in silica | great antibacterial activity against both gram-positive and gram-negative bacteria, especially against Escherichia coli | [101] |
| chitosan-PLA composite film with nisin and EDTA | high antibacterial activity | [52] |
| starch/PVA/nisin films | Films containing nisin showed an inhibitory effect against Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella Choleraesuis, and Escherichia coli | [102] |
| Product | Material | Results | References |
|---|---|---|---|
| Meat and meat products | |||
| minced pork meat | gelatin-based films incorporated with nisin and catechin | retarded lipid oxidation and microbial growth | [115] |
| fresh pork loin | coatings based on nisin/gallic acid/chitosan | preserved product color, had lower lipid and protein oxidation, the lowest TBARS value, and presented a synergistic bactericidal effect | [116] |
| pork meat | nisin, EDTA, and PBAT/TPS blends | inhibited lipid degradation, microbial growth, stabilized meat redness, delayed discoloration | [117] |
| Yao meat | nanoemulsion incorporated with nisin, star anise essential oil, and polylysine | no effect on sample moisture content for 20 days, maintaining good color and odor, the extension of shelf-life was from 8 to 16 days | [118] |
| sliced ham | Poly(hidroxybutyrate) (PHB)/poly(caprolactone) (PCL)/nisin films | effective against Lactobacillus plantarum CRL691, prolonged shelf-life | [108] |
| dry-cured Iberian ham | Chitosan-based films containing nisin and/or rice bran in combination with high-pressure processing (HPP) | reduced the population of L. monocytogenes by 6 log CFU/g | [53] |
| ready-to-eat ham | cellulose nanofibrils/nisin nanohybrid films | complete inhibition of Listeria monocytogenes during storage for 7 days at 4 °C | [119] |
| Vienna sausage | collagen casting impregnated with nisin | extended shelf-life for at least 90 days at 4 °C and 49 days at 10 °C | [120] |
| raw turkey breast slices | pullulan films containing lauric arginate and nisin Z | reduced Salmonella typhimurium, Salmonella enteritidis Staphylococcus au-reus and Listeria monocytogenes Scott A load during cold storage | [121] |
| turkey bologna | coatings containing nisin applied on two types of materials used as substrates | inhibition of Listeria monocytogenes during the entire period of testing | [77] |
| minced beef | combinations of lysozyme 500 U/mL (L), nisin 1000 IU/mL (N), EDTA nanoparticles 5 mM (E), and ZnO nanoparticles 12 mM (Z) | the synergistic antimicrobial effect observed for LNZ and LNEZ, which efficiently inhibited Escherichia coli O157:H7, Listeria monocytogenes, and Bacillus cereus during storage al 4 °C for 15 days | [122] |
| beef | nanocoatings based on bacterial cellulose nanocrystals and nisin-loaded bacterial cellulose nanocrystals | no alteration of sensorial and physicochemical properties, reduced bacterial growth | [123] |
| yellow croaker | chitosan combined with nisin | nisin’s presence led to better quality parameters, controlling total viable count growth, and maintaining color and sensory acceptability | [124] |
| seer fish steaks | the coating formed from gel dispersion of seer fish incorporated with nisin combined with gamma irradiation | shelf-life prolonging from 7 days to 34–42 days during cold storage | [125] |
| fish fillets | nisin and curcumin nanomats | acceptable sensory attributes by the tenth day of storage compared to control, shelf-life extended to 12 days | [126] |
| fish fillets | nisin-loaded PVA-based nanofibers with polyethylene | delayed TMB and LAB development by 31% and 38%, respectively, better sensorial attributes compared to control samples | [127] |
| fish balls | zein incorporated nisin (54.4 AU/cm2) or nisin/EDTA 568 µg/cm2 | The microbial count of Escherichia coli, Enterobacter aerogenes, and Citrobacter freundii decreased from 3.19 ± 0.03 log CFU/g for uncoated fish balls to less than 1 log unit for coated food products; the control recorded a microbial count of 6.08 ± 0.23 log CFU/g | [64] |
| bigeye snapper and tiger prawn slices | thermoplastic starch/PBAT film coated with gelatin with lauric arginate alone or in combination with nisin | in combination with nisin, reduced Salmonella Typhimurium ATCC 14,028 and Vibrio parahaemolyticus after 28 days at 4 °C and Salmonella Typhimurium after 60 days at −20 °C; reduced Vibrio parahaemolyticus on frozen samples after 14 days (bigeye snapper) and 21 days (tiger prawn) | [128] |
| Penaeus vannamei shrimp | chitosan/sodium alginate/nisin preservatives | Lower pH, TVB-N, TVC, and freeness values, decreased the predominant microbial load significantly, longer shelf-life | [129] |
| fish fillets | chitosan-PLA composite added with nisin and EDTA | significant reduction in coliform, mesophile, and spoilage bacteria loads and of the total volatile basic nitrogen content during storage at 25 °C and 4 °C | [52] |
| Dairy products | |||
| minas frescal cheese | nanocomposite films based on starch/halloysite/nisin | films with 2 g/100 g nisin significantly reduced the bacterial count after 4 days, while films with 6 g/100 g nisin entirely inhibited bacterial growth | [85] |
| UF cheese | bilayer film based on chitosan, cellulose, and nisin | films containing 1000 µg/mL totally inactivated Listeria monocytogenes after storage for 14 days at 4 °C | [86] |
| cheese | nanoparticles based on nisin-loaded poly-γ-glutamic acid/chitosan contained in PEO nanofibers | good antibacterial activity against Listeria monocytogenes, no effect on the sensory attributes | [82] |
| kasar cheese slices | whey protein isolate films incorporated with nisin | microbial inactivation against Salmonella Enteritidis and Listeria monocytogenes | [133] |
| Gouda cheese | an edible coating based on tapioca starch and glycerol, containing nisin and natamycin | no effect on physicochemical parameters during the ripening process, a better barrier against external contamination | [134] |
| sliced mozzarella cheese | films based on hydroxypropylmethylcellulose (HPMC) incorporated with nisin Z | nisin presented antimicrobial activity Staphylococcus aureus, Listeria innocua, and Salmonella enterica; films with 10% wt. were effective in mesophilic microorganisms’ inhibition during 8 days of storage | [135] |
| fresh cheese | Nisin-loaded amaranth protein isolate/pullulan nanofibers | complete inactivation of microorganisms after 142 h for Salmonella Typhimurium, 120 h for Listeria monocytogenes, and 170 h for Leuconostoc mesenteroides | [136] |
| Port Salut cheese | edible films containing nisin and natamycin | inhibited the growth of yeasts and molds, controlled the growth of product’s own psychrotrophic bacteria, inhibited superficial contamination of a mixed culture of Listeria innocua and Saccharomyces cerevisiae | [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, E.E.; Miteluț, A.C.; Râpă, M.; Popescu, P.A.; Drăghici, M.C.; Geicu-Cristea, M.; Popa, M.E. Antimicrobial Active Packaging Containing Nisin for Preservation of Products of Animal Origin: An Overview. Foods 2022, 11, 3820. https://doi.org/10.3390/foods11233820
Popa EE, Miteluț AC, Râpă M, Popescu PA, Drăghici MC, Geicu-Cristea M, Popa ME. Antimicrobial Active Packaging Containing Nisin for Preservation of Products of Animal Origin: An Overview. Foods. 2022; 11(23):3820. https://doi.org/10.3390/foods11233820
Chicago/Turabian StylePopa, Elisabeta Elena, Amalia Carmen Miteluț, Maria Râpă, Paul Alexandru Popescu, Mihaela Cristina Drăghici, Mihaela Geicu-Cristea, and Mona Elena Popa. 2022. "Antimicrobial Active Packaging Containing Nisin for Preservation of Products of Animal Origin: An Overview" Foods 11, no. 23: 3820. https://doi.org/10.3390/foods11233820






