Sustainable Environmental Assessment of Waste-to-Energy Practices: Co-Pyrolysis of Food Waste and Discarded Meal Boxes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Test Device and Operating Parameters
2.3. Analysis of the Thermodynamics Model
2.4. Plotting and Statistical Analysis
3. Results and Discussion
3.1. Characterization of MS and PP
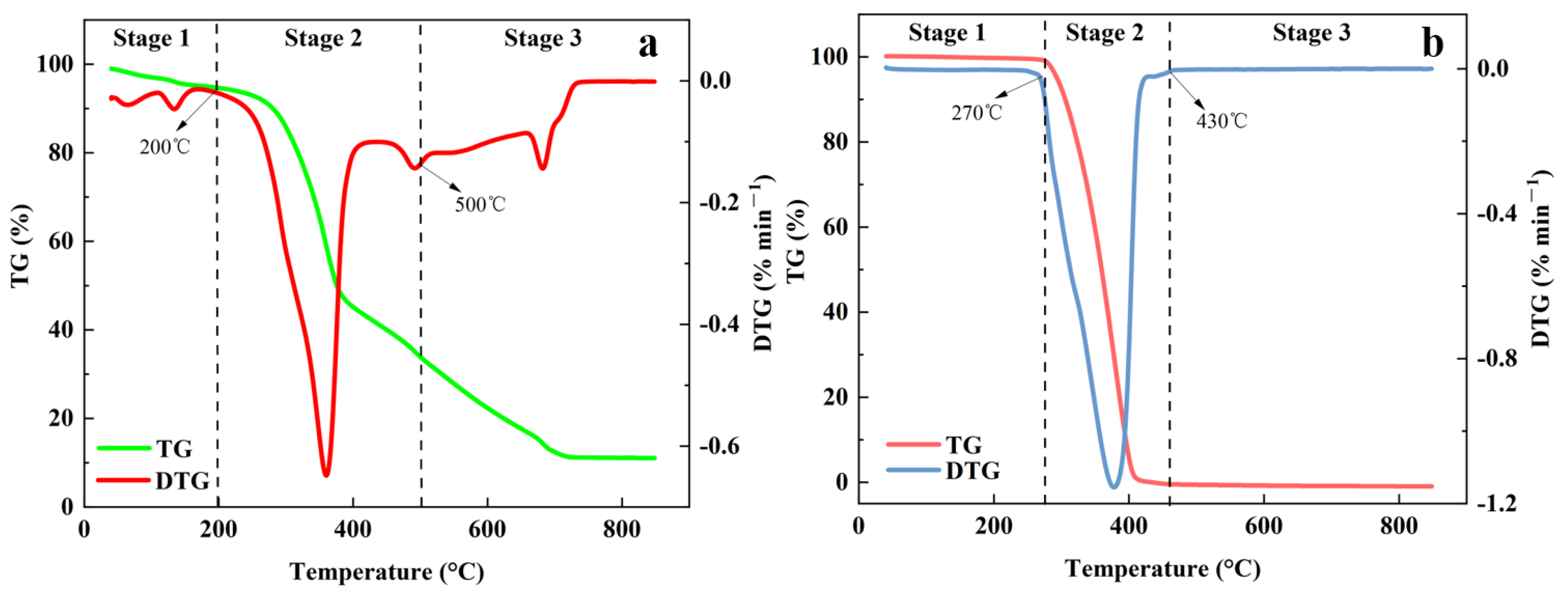
3.2. Separate Pyrolysis of MS and PP
3.3. Co-Pyrolysis of SP11
3.4. Thermogravimetric Characteristics and Kinetic Analysis
3.4.1. TG-DTG Curves at Different Heating Rates
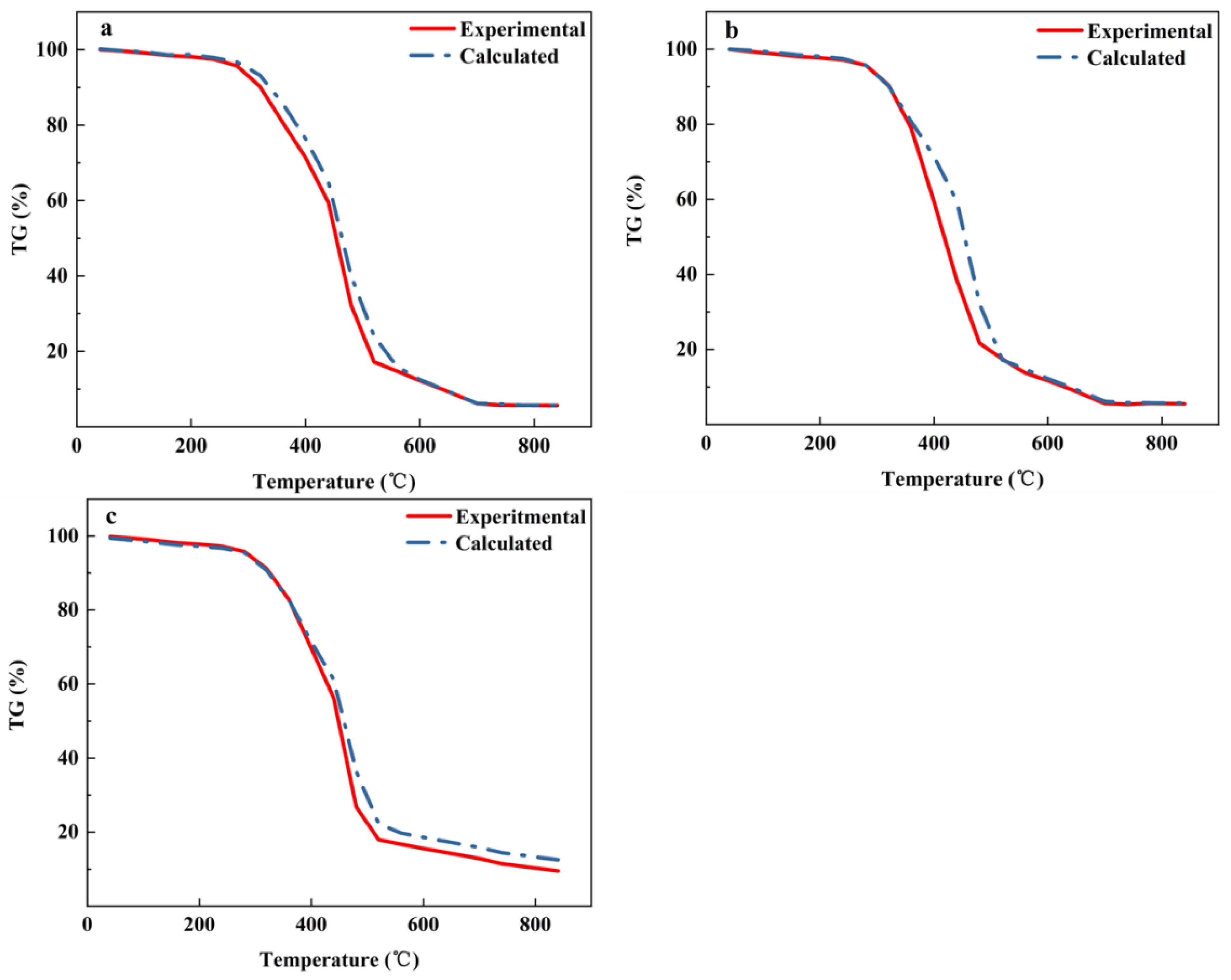
3.4.2. Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MS | mushroom sticks | t | time |
| PP | polypropylene | E | activation energy |
| SP11 | mixture of MS and PP | f(α) | reaction mechanism function |
| TG-FTIR | thermogravimetric analyzer coupled with Fourier transform infrared spectroscopy | A | pre-exponential factor |
| WCal | theoretical weight loss of the sample | T | reaction temperature |
| xMS | ratio of MS in the mixed sample | R | universal gas constant (8.314 J/(mol·K)) |
| WPP | weight loss of PP at corresponding temperatures | m0 | the initial mass (g) of the sample |
| WMS | weight loss of MS at corresponding temperatures | mt | the sample mass (g) at the time point of the reaction t |
| α | mass conversion rate | TG | thermal weight curve |
| DS | Dunaliella salina | DTG | differential thermal weight curve |
| SW | solid waste | LVC | low volatile coal |
References
- Janssens, K.; Lambrechts, W.; van Osch, A.; Semeijn, J. How Consumer Behavior in Daily Food Provisioning Affects Food Waste at Household Level in The Netherlands. Foods 2019, 8, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Cruz-Martins, N.; Dhanjal, D.; Singh, R.; Chopra, C.; Verma, R.; Abd-Elsalam, K.; et al. Potential Usage of Edible Mushrooms and Their Residues to Retrieve Valuable Supplies for Industrial Applications. J. Fungi 2021, 7, 427. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, J.; Li, H.; Hu, R.; Yao, X.; Liu, Y.; Zhou, Y.; Lyu, T. Towards high-quality biodiesel production from microalgae using original and anaerobically-digested livestock wastewater. Chemosphere 2021, 273, 128578. [Google Scholar] [CrossRef]
- Li, G.; Hao, Y.; Yang, T.; Wu, J.; Xu, F.; Li, L.; Wang, B.; Li, M.; Zhao, N.; Wang, N.; et al. Air pollutant emissions from sludge-bituminous briquettes as a potential household energy source. Case Stud. Therm. Eng. 2022, 37, 102251. [Google Scholar] [CrossRef]
- Doria, E.; Boncompagni, E.; Marra, A.; Dossena, M.; Verri, M.; Buonocore, D. Polyphenols Extraction From Vegetable Wastes Using a Green and Sustainable Method. Front. Sustain. Food Syst. 2021, 5, 342. [Google Scholar] [CrossRef]
- Digman, B.; Kim, D.-S. Review: Alternative energy from food processing wastes. Environ. Prog. 2008, 27, 524–537. [Google Scholar] [CrossRef]
- Principato, L.; Ruini, L.; Guidi, M.; Secondi, L. Adopting the circular economy approach on food loss and waste: The case of Italian pasta production. Resour. Conserv. Recycl. 2019, 144, 82–89. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, A.; Sridhar, K.; Sharma, M.; Singh, T.P.; Kumar, S. Development and quality characteristics of functional Kulfi fortified with microencapsulated betalains. Int. J. Food Sci. Technol. 2021, 56, 5362–5370. [Google Scholar] [CrossRef]
- Jalalipour, H.; Jaafarzadeh, N.; Morscheck, G.; Narra, S.; Nelles, M. Potential of Producing Compost from Source-Separated Municipal Organic Waste (A Case Study in Shiraz, Iran). Sustainability 2020, 12, 9704. [Google Scholar] [CrossRef]
- Zhu, Q.; Jia, R.; Lin, X. Accelerate the Turnover of Agricultural Products Funds to Promote the Development of Agricultural and Rural Econom. In Proceedings of the International Conference on Arts, Management, Education and Innovation, Seoul, Republic of Korea, 12–14 January 2019. [Google Scholar]
- Lou, Z.; Sun, Y.; Bian, S.; Baig, S.A.; Hu, B.; Xu, X. Nutrient conservation during spent mushroom compost application using spent mushroom substrate derived biochar. Chemosphere 2017, 169, 23–31. [Google Scholar] [CrossRef] [PubMed]
- China Edible Fungi Association. Available online: https://www.cefa.org.cn/web/index.html (accessed on 7 December 2021).
- Chen, G.; Yu, H.; Lin, F.; Zhang, Z.; Yan, B.; Song, Y. Utilization of edible fungi residues towards synthesis of high-performance porous carbon for effective sorption of Cl-VOCs. Sci. Total Environ. 2020, 727, 138475. [Google Scholar] [CrossRef] [PubMed]
- Niego, A.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K. Macrofungi as a Nutraceutical Source: Promising Bioactive Compounds and Market Value. J. Fungi 2021, 7, 397. [Google Scholar] [CrossRef]
- Castanho, N.; de Oliveira, R.; Batista, B.; Freire, B.; Lange, C.; Lopes, A.; Jozala, A.; Grotto, D. Comparative Study on Lead and Copper Biosorption Using Three Bioproducts from Edible Mushrooms Residues. J. Fungi 2021, 7, 441. [Google Scholar] [CrossRef] [PubMed]
- Bhoi, P.; Ouedraogo, A.; Soloiu, V.; Quirino, R. Recent advances on catalysts for improving hydrocarbon compounds in bio-oil of biomass catalytic pyrolysis. Renew. Sustain. Energy Rev. 2020, 121, 109676. [Google Scholar] [CrossRef]
- Hanafi, F.H.M.; Rezania, S.; Taib, S.M.; Din, M.F.; Yamauchi, M.; Sakamoto, M.; Hara, H.; Park, J.; Ebrahimi, S.S. Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): An overview. J. Mater. Cycles Waste Manag. 2018, 20, 1383–1396. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Di Bartolo, A.; Infurna, G.; Dintcheva, N. A Review of Bioplastics and Their Adoption in the Circular Economy. Polymers 2021, 13, 1229. [Google Scholar] [CrossRef]
- Rahman, N.; Ishitsuka, K.; Piedvache, A.; Tanaka, H.; Murayama, N.; Morisaki, N. Convenience Food Options and Adequacy of Nutrient Intake among School Children during the COVID-19 Pandemic. Nutrients 2022, 14, 630. [Google Scholar] [CrossRef] [PubMed]
- Ngan, N.T.; Khoi, B.H. Optimal Choice for Online Shopping in COVID-19 Pandemic. Qual. Access Success 2022, 23, 143–149. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- National Bureau of Statistics. 2021. Available online: http://www.stats.gov.cn (accessed on 5 May 2021).
- China National Resources Recycling Association. Available online: http://www.crra.com.cn (accessed on 28 July 2022).
- De León, D.; Hernández, A.; Marzoa, S. Diseño de planta y equipo para la revalorización de residuos sólidos. Mem. Investig. En Ing. 2020, 18, 25–33. [Google Scholar]
- Lee, A.; Liew, M.S. Tertiary recycling of plastics waste: An analysis of feedstock, chemical and biological degradation methods. J. Mater. Cycles Waste Manag. 2020, 23, 32–43. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Zhang, L. Recent Progress in the Chemical Upcycling of Plastic Wastes. ChemSusChem 2021, 14, 4137–4151. [Google Scholar] [CrossRef]
- Hassan, H.; Lim, J.; Hameed, B. Recent progress on biomass co-pyrolysis conversion into high-quality bio-oil. Bioresour. Technol. 2016, 221, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Lee, S.; Choi, H.; Lin, K.-Y.; Lee, J. Single-Use Disposable Waste Upcycling via Thermochemical Conversion Pathway. Polymers 2021, 13, 2617. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Li, H.; Xu, J.; Nyambura, S.M.; Wang, J.; Li, C.; Zhu, X.; Feng, X.; Wang, Y. Food waste pyrolysis by traditional heating and microwave heating: A review. Fuel 2022, 324, 124574. [Google Scholar] [CrossRef]
- Dewangan, A.; Pradhan, D.; Singh, R. Co-pyrolysis of sugarcane bagasse and low-density polyethylene: Influence of plastic on pyrolysis product yield. Fuel 2016, 185, 508–516. [Google Scholar] [CrossRef]
- Akancha; Kumari, N.; Singh, R. Co-pyrolysis of waste polypropylene and rice bran wax- production of biofuel and its characterization. J. Energy Inst. 2019, 92, 933–946. [Google Scholar] [CrossRef]
- Li, G.; Bai, X.; Huo, S.; Huang, Z. Fast pyrolysis of LERDADEs for renewable biofuels. IET Renew. Power Gener. 2020, 14, 959–967. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Pan, M.; Hao, Y.; Hu, R.; Xiao, W.; Li, G.; Lyu, T. Valorisation of microalgae residues after lipid extraction: Pyrolysis characteristics for biofuel production. Biochem. Eng. J. 2022, 179, 108330. [Google Scholar] [CrossRef]
- Madhu, P.; Vidhya, L.; Vinodha, S.; Wilson, S.; Sekar, S.; Patil, P.P.; Kaliappan, S.; Prabhakar, S. Co-pyrolysis of Hardwood Combined with Industrial Pressed Oil Cake and Agricultural Residues for Enhanced Bio-Oil Production. J. Chem. 2022, 2022, 9884766. [Google Scholar] [CrossRef]
- Inayat, A.; Rocha-Meneses, L.; Ghenai, C.; Abdallah, M.; Shanableh, A.; Al-Ali, K.; Alghfeli, A.; Alsuwaidi, R. Co-pyrolysis for bio-oil production via fixed bed reactor using date seeds and plastic waste as biomass. Case Stud. Therm. Eng. 2022, 31, 101841. [Google Scholar] [CrossRef]
- Ansari, K.B.; Hassan, S.Z.; Bhoi, R.; Ahmad, E. Co-pyrolysis of biomass and plastic wastes: A review on reactants synergy, catalyst impact, process parameter, hydrocarbon fuel potential, COVID-19. J. Environ. Chem. Eng. 2021, 9, 106436. [Google Scholar] [CrossRef]
- Sanahuja-Parejo, O.; Veses, A.; Navarro, M.; López, J.; Murillo, R.; Callén, M.; García, T. Drop-in biofuels from the co-pyrolysis of grape seeds and polystyrene. Chem. Eng. J. 2019, 377, 120246. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Hu, R.; Wang, N.; Yang, T.; Xu, F.; Li, J.; Wu, J.; Huang, Z.; Pan, M.; Lyu, T. Cultivation of microalgae in adjusted wastewater to enhance biofuel production and reduce environmental impact: Pyrolysis performances and life cycle assessment. J. Clean. Prod. 2022, 355, 131768. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Chia, W.Y.; Cheah, W.Y.; Munawaroh, H.S.H.; Ong, W.-J. Abatement of hazardous materials and biomass waste via pyrolysis and co-pyrolysis for environmental sustainability and circular economy. Environ. Pollut. 2021, 278, 116836. [Google Scholar] [CrossRef] [PubMed]
- Özsin, G.; Pütün, A.E. TGA/MS/FT-IR study for kinetic evaluation and evolved gas analysis of a biomass/PVC co-pyrolysis process. Energy Convers. Manag. 2019, 182, 143–153. [Google Scholar] [CrossRef]
- Oyedun, A.O.; Tee, C.Z.; Hanson, S.; Hui, C.W. Thermogravimetric analysis of the pyrolysis characteristics and kinetics of plastics and biomass blends. Fuel Process. Technol. 2014, 128, 471–481. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren; Université Paris Ouest Nanterre La Défense: Nanterre, France, 1889. [Google Scholar]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Yerrayya, A.; Nagababu, G.; Guduru, R.K.; Kumar, T.H. Recovery of renewable aromatic and aliphatic hydrocarbon resources from microwave pyrolysis/co-pyrolysis of agro-residues and plastics wastes. Bioresour. Technol. 2020, 318, 124277. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zheng, N.; An, Q.; Sun, S.; Wang, S.; Li, X.; Li, P.; Hua, X.; Dong, D.; Zhao, C.; et al. The effect of carbonization temperature on the capacity and mechanisms of Cd(II)-Pb(II) mix-ions adsorption by wood ear mushroom sticks derived biochar. Ecotoxicol. Environ. Saf. 2022, 239, 113646. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, S.; Cong, K.; Li, Q.; Zhang, Y. Insight into synergistic effects of biomass-polypropylene co-pyrolysis using representative biomass constituents. Bioresour. Technol. 2020, 307, 123243. [Google Scholar] [CrossRef] [PubMed]
- Banyasz, J.L.; Li, S.; Lyons-Hart, J.L.; Shafer, K.H. Cellulose pyrolysis: The kinetics of hydroxyacetaldehyde evolution. J. Anal. Appl. Pyrolysis 2001, 57, 223–248. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Liu, G.; He, Y.; Chen, C.; Liu, X.; Li, G.; Gu, Y.; Zhao, Y.; He, Y. Mixed heavy metal removal from wastewater by using discarded mushroom-stick biochar: Adsorption properties and mechanisms. Environ. Sci. Process. Impacts 2019, 21, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, P.F.; Van Heemst, J.D.; Guthrie, E.A.; Hatcher, P.G. Laser micropyrolysis GC–MS of lignin. J. Anal. Appl. Pyrolysis 2002, 62, 365–373. [Google Scholar] [CrossRef]
- Yang, Y.; Gilbert, A.; Xu, C.C. Production of bio-crude from forestry waste by hydro-liquefaction in sub-/super-critical methanol. Environ. Energy Eng. 2009, 3, 807–819. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Chen, W.-H. Pyrolysis characteristics and kinetics of microalgae via thermogravimetric analysis (TGA): A state-of-the-art review. Bioresour. Technol. 2017, 246, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wu, C.; Onwudili, J.A.; Meng, A.; Zhang, Y.; Williams, P.T. Influence of process conditions on the formation of 2–4 ring polycyclic aromatic hydrocarbons from the pyrolysis of polyvinyl chloride. Fuel Process. Technol. 2016, 144, 299–304. [Google Scholar] [CrossRef]
- Dong, C.-Q.; Zhang, Z.-F.; Lu, Q.; Yang, Y.-P. Characteristics and mechanism study of analytical fast pyrolysis of poplar wood. Energy Convers. Manag. 2012, 57, 49–59. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; Dong, D.; Gao, G.; Zhang, S.; Wang, Y.; Xiang, J.; Hu, S.; Mortaza, G.; Hu, X. Co-pyrolysis of cellulose/lignin and sawdust: Influence of secondary condensation of the volatiles on characteristics of biochar. Energy 2021, 226, 120442. [Google Scholar] [CrossRef]
- Galiwango, E.; Gabbar, H.A. Synergistic interactions, kinetic and thermodynamic analysis of co-pyrolysis of municipal paper and polypropylene waste. Waste Manag. 2022, 146, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Azizi, K.; Moraveji, M.K.; Najafabadi, H.A. Characteristics and kinetics study of simultaneous pyrolysis of microalgae Chlorella vulgaris, wood and polypropylene through TGA. Bioresour. Technol. 2017, 243, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yin, Y.; Li, S.; Ma, H.; Gao, R.; Yin, Y. Effects of Biochar Feedstock and Pyrolysis Temperature on Soil Organic Matter Mineralization and Microbial Community Structures of Forest Soils. Front. Environ. Sci. 2021, 9, 313. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S.; Meng, H.; Wu, Z.; Zhao, J. Synergistic effect on thermal behavior and char morphology analysis during co-pyrolysis of paulownia wood blended with different plastics waste. Appl. Therm. Eng. 2017, 111, 834–846. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, W.; Kookana, R.; Katayama, A. Characteristics of biochar and its application in remediation of contaminated soil. J. Biosci. Bioeng. 2013, 116, 653–659. [Google Scholar] [CrossRef] [PubMed]
- De Tender, C.A.; Debode, J.; Vandecasteele, B.; D’Hose, T.; Cremelie, P.; Haegeman, A.; Ruttink, T.; Dawyndt, P.; Maes, M. Biological, physicochemical and plant health responses in lettuce and strawberry in soil or peat amended with biochar. Appl. Soil Ecol. 2016, 107, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, L.; Liu, Y.; Ren, X.; Chen, J.; Levendis, Y.A. Sulfur and Nitrogen Release From Co-Pyrolysis of Coal and Biomass Under Oxidative and Non-Oxidative Conditions. J. Energy Resour. Technol. 2021, 143, 61304. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Yang, H.; Xia, M.; Li, K.; Chen, X.; Chen, H. Co-pyrolysis of lignocellulosic biomass and microalgae: Products characteristics and interaction effect. Bioresour. Technol. 2017, 245, 860–868. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S.; Kucukbayrak, S. Effect of heating rate on the pyrolysis yields of rapeseed. Renew. Energy 2006, 31, 803–810. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Liu, A.; Wang, L.; Yu, G. Co-pyrolysis characteristic of biomass and bituminous coal. Bioresour. Technol. 2015, 179, 414–420. [Google Scholar] [CrossRef]
- Chen, C.; Ma, X.; He, Y. Co-pyrolysis characteristics of microalgae Chlorella vulgaris and coal through TGA. Bioresour. Technol. 2012, 117, 264–273. [Google Scholar] [CrossRef]
- Viotto, R.S.; Maia, A.A.D.; Yamaji, F.M.; De Morais, L.C. Thermogravimetric investigation of spent shiitake substrate to solid biofuel. Can. J. Chem. Eng. 2018, 96, 845–854. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Chen, J.; Xie, W.; Kuo, J.; Lu, X.; Chang, K.; Wen, S.; Sun, G.; Cai, H.; et al. Combustion behaviors of spent mushroom substrate using TG-MS and TG-FTIR: Thermal conversion, kinetic, thermodynamic and emission analyses. Bioresour. Technol. 2018, 266, 389–397. [Google Scholar] [CrossRef]
- Li, G.; Hao, Y.; Yang, T.; Xiao, W.; Pan, M.; Huo, S.; Lyu, T. Enhancing Bioenergy Production from the Raw and Defatted Microalgal Biomass Using Wastewater as the Cultivation Medium. Bioengineering 2022, 9, 637. [Google Scholar] [CrossRef]
- He, W.; Yin, G.; Zhao, Y.; Zhang, L.; Xu, S.; Huang, T.; Chang, L.; Lu, H. Interactions between free radicals during co-pyrolysis of lignite and biomass. Fuel 2021, 302, 121098. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y.; Huang, Q.; Cai, J. Thermogravimetric characteristics and kinetic of plastic and biomass blends co-pyrolysis. Fuel Process. Technol. 2006, 87, 963–969. [Google Scholar] [CrossRef]
- Nisar, J.; Ali, F.; Malana, M.A.; Ali, G.; Iqbal, M.; Shah, A.; Bhatti, I.A.; Khan, T.A.; Rashid, U. Kinetics of the pyrolysis of cobalt-impregnated sesame stalk biomass. Biomass Convers. Biorefin. 2019, 10, 1179–1187. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Chen, S.; Wu, J. Catalytic co-pyrolysis of lignocellulosic biomass with polymers: A critical review. Green Chem. 2016, 18, 4145–4169. [Google Scholar] [CrossRef]
- Burra, K.; Gupta, A. Kinetics of synergistic effects in co-pyrolysis of biomass with plastic wastes. Appl. Energy 2018, 220, 408–418. [Google Scholar] [CrossRef]
- Gai, C.; Dong, Y.; Zhang, T. The kinetic analysis of the pyrolysis of agricultural residue under non-isothermal conditions. Bioresour. Technol. 2013, 127, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, Y.; Wu, K.; Chen, Y.; Hu, H.; Yang, M. Study on pyrolytic kinetics and behavior: The co-pyrolysis of microalgae and polypropylene. Bioresour. Technol. 2015, 192, 522–528. [Google Scholar] [CrossRef]
- Hu, Q.; Tang, Z.; Yao, D.; Yang, H.; Shao, J.; Chen, H. Thermal behavior, kinetics and gas evolution characteristics for the co-pyrolysis of real-world plastic and tyre wastes. J. Clean. Prod. 2020, 260, 121102. [Google Scholar] [CrossRef]
- Zhou, L.; Luo, T.; Huang, Q. Co-pyrolysis characteristics and kinetics of coal and plastic blends. Energy Convers. Manag. 2009, 50, 705–710. [Google Scholar] [CrossRef]
- Vo, T.A.; Tran, Q.K.; Ly, H.V.; Kwon, B.; Hwang, H.T.; Kim, J.; Kim, S.-S. Co-pyrolysis of lignocellulosic biomass and plastics: A comprehensive study on pyrolysis kinetics and characteristics. J. Anal. Appl. Pyrolysis 2022, 163, 105464. [Google Scholar] [CrossRef]




| Sample | Elemental Analysis (%) | ||||
|---|---|---|---|---|---|
| C | H | O | N | S | |
| MS | 37.73 | 5.38 | 53.59 | 2.09 | 0.18 |
| PP | 81.65 | 16.71 | 0.94 | 0.00 | 0.37 |
| Sample | Heating Rate (°C·min−1) | Temperature Range (°C) | Activation Energy (E) (kJ·mol−1) | Frequency Factor (A) (mol−1) | Correlation Index (R2) |
|---|---|---|---|---|---|
| 10 | 200–500 | 42.7 | 246.65 | 0.971 | |
| MS | 20 | 245–401 | 43 | 472.12 | 0.978 |
| 30 | 249–408 | 47.4 | 1901.80 | 0.986 | |
| 10 | 260–431 | 107.7 | 1.8 × 108 | 0.991 | |
| PP | 20 | 300–465 | 141.4 | 2.6 × 1010 | 0.996 |
| 30 | 340–490 | 150.3 | 4.1 × 1010 | 0.996 | |
| SP11 | 10 | 230–520 | 43.3 | 103.58 | 0.980 |
| 20 | 261–521 | 45.1 | 303.37 | 0.982 | |
| 30 | 270–520 | 44.41 | 377.91 | 0.983 |
| Sample | Temperature Range (°C) | Activation Energy (E) (kJ·mol−1) | Frequency Factor (A) (mol−1) | Correlation Index (R) | Reference |
|---|---|---|---|---|---|
| 250–348 | 60.44 | 1.43 × 104 | 0.988 | [48] | |
| SP-PP | 440–520 | 129.29 | 5.68 × 108 | 0.919 | |
| - | - | - | - | ||
| PP-D. tertiolecta (6:4) | - | - | - | - | [77] |
| - | 142.9 | 2.68 × 1013 | - | ||
| - | - | - | - | ||
| PP-Tyre | 319–378 | 70.08 | 1.85 × 104 | 0.998 | [78] |
| 378–428 | 52.08 | 5.19 × 102 | 0.997 | ||
| 428–488 | 138.04 | 2.94 × 109 | 0.971 | ||
| DS-PP | 224–295 | 49.8 | 2.6 × 102 | 0.980 | [27] |
| 295–345 | 45.7 | 1.0 × 103 | 0.980 | ||
| 446–504 | 160.1 | 1.8 × 1011 | 0.960 | ||
| SW-PP (3:7) | 286–405 | 128 | 1.77 × 1010 | 0.978 | [72] |
| 405–461 | 249 | 1.26 × 1018 | 0.976 | ||
| 461–525 | 426 | 1.19 × 1029 | 0.982 | ||
| 95%LVC-PP (1:5) | 191–399 | 35.7 | 351 | 0.945 | [79] |
| 399–474 | 242.4 | 3.0 × 1017 | 0.988 | ||
| 474–516 | 558.8 | 7.7 × 1032 | 0.999 | ||
| 516–617 | 198.4 | 9.5 × 1011 | 0.968 | ||
| Bamboo-PP (4:1) | - | - | - | - | [80] |
| - | 213.81 | 2.87 × 1017 | - | ||
| - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Yang, T.; Xiao, W.; Wu, J.; Xu, F.; Li, L.; Gao, F.; Huang, Z. Sustainable Environmental Assessment of Waste-to-Energy Practices: Co-Pyrolysis of Food Waste and Discarded Meal Boxes. Foods 2022, 11, 3840. https://doi.org/10.3390/foods11233840
Li G, Yang T, Xiao W, Wu J, Xu F, Li L, Gao F, Huang Z. Sustainable Environmental Assessment of Waste-to-Energy Practices: Co-Pyrolysis of Food Waste and Discarded Meal Boxes. Foods. 2022; 11(23):3840. https://doi.org/10.3390/foods11233840
Chicago/Turabian StyleLi, Gang, Tenglun Yang, Wenbo Xiao, Jiahui Wu, Fuzhuo Xu, Lianliang Li, Fei Gao, and Zhigang Huang. 2022. "Sustainable Environmental Assessment of Waste-to-Energy Practices: Co-Pyrolysis of Food Waste and Discarded Meal Boxes" Foods 11, no. 23: 3840. https://doi.org/10.3390/foods11233840






