In Vitro Effect of Flavonoids on Basophils Degranulation and Intestinal Epithelial Barrier Damage Induced by ω-5 Gliadin-Derived Peptide
Abstract
:1. Introduction
2. Materials and Method
2.1. In Vitro Simulation of Gastrointestinal Digestion of Gliadin in Infants and Young Children
2.2. Tricine-SDS-PAGE and RP-HPLC Analysis
2.3. Screening and Synthesis of ω-5 Gliadin-Derived Peptides
2.4. Establishment of Serum Pools in Children with Wheat Allergy
2.5. Cell Culture and Viability Testing
2.6. Mediator Release Assay from KU812 Cells
2.7. Evaluation of the Effects of Four Flavonoids against the Reduction of Cell Viability Induced by ω-5 Gliadin-Derived Peptide
2.8. Establishment of Caco-2 Cell Monolayer Model
2.9. Effects of Flavonoids on Secretion of IL-6 and IL-8 of Caco-2 Cells Induced by ω-5 Gliadin-Derived Peptide
2.10. Effects of Flavonoids on Tight Junction Damage of Caco-2 Cells Induced by ω-5 Gliadin-Derived Peptide
2.11. Statistical Analysis
3. Results
3.1. Identification of Gastrointestinal Digestion Products of Gliadin
3.2. Selected and Synthesized of ω-5 Gliadin-Derived Peptide
3.3. Effect of Peptides and Flavonoids on KU812 Cell Viability
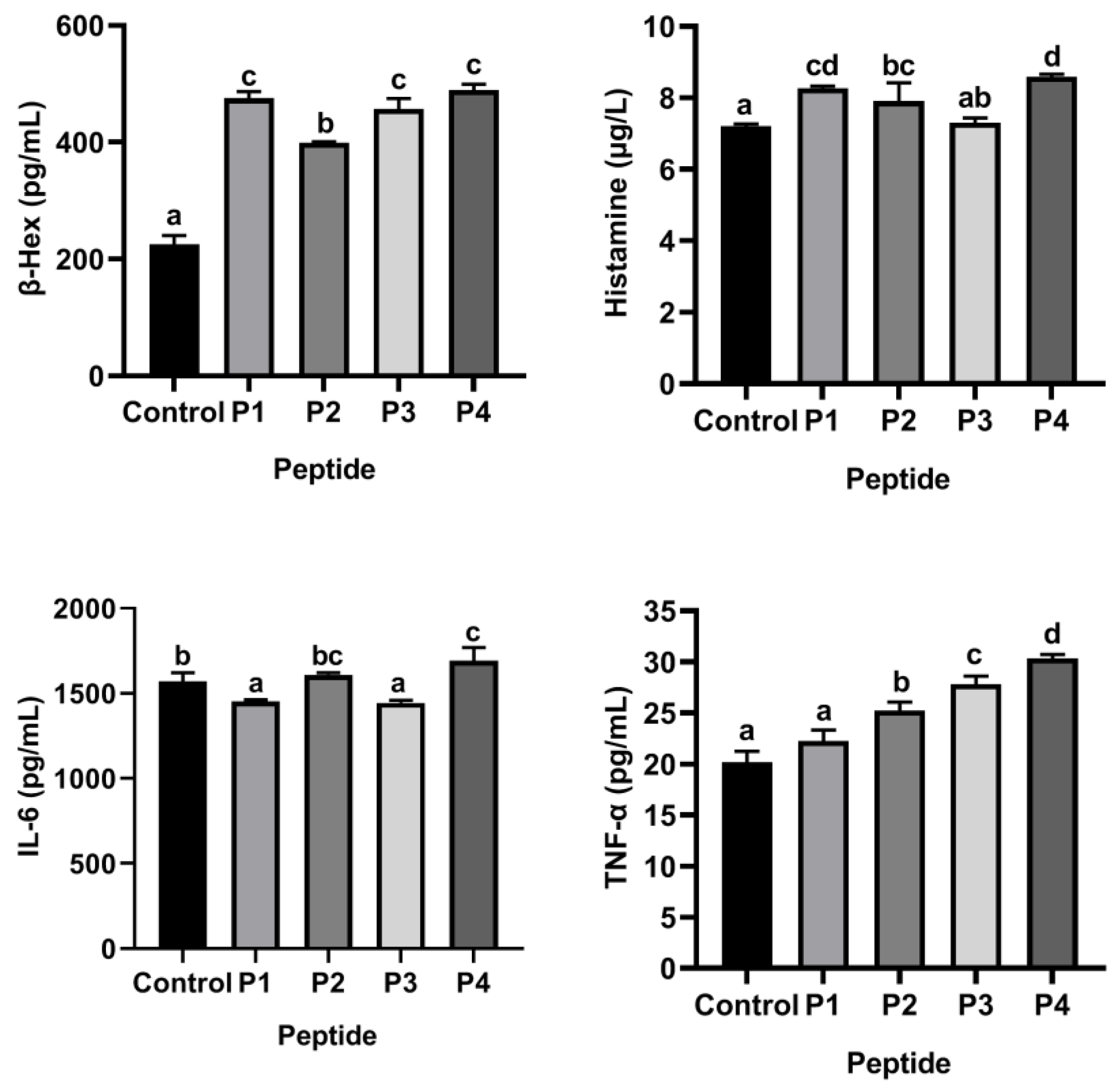
3.4. Selected ω-5 Gliadin-Derived Peptides with Strong Allergenicity Based on KU812 Cell Degranulation Model
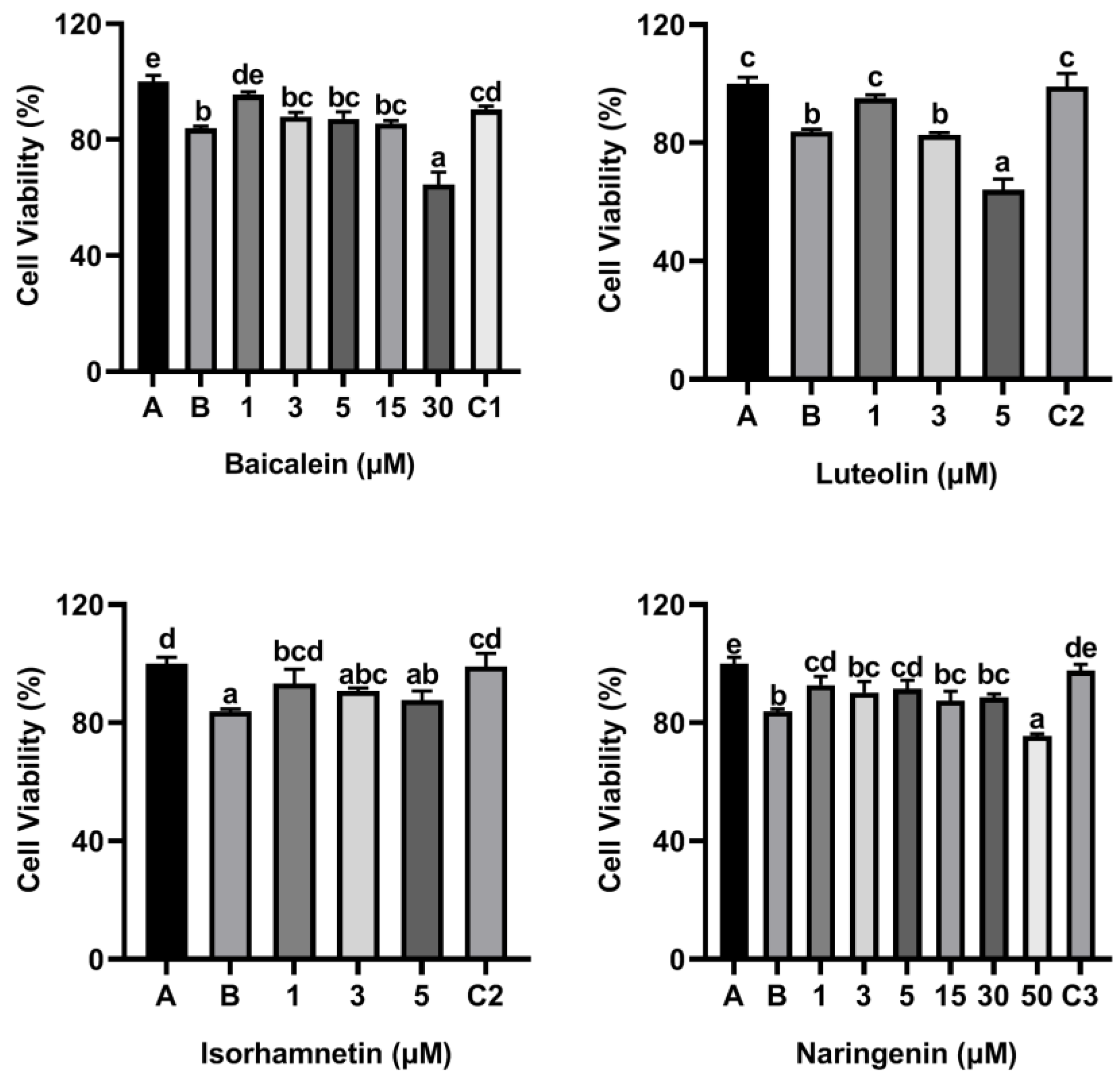
3.5. Flavonoids Isorhamnetin, Naringenin, Luteolin and Baicalein Attenuated Cytotoxicity of ω-5 Gliadin-Derived Peptide P4 to KU812 Cells
3.6. Flavonoids Inhibited the Release of Inflammatory Mediators from KU812 Cells Induced by ω-5 Gliadin-Derived Peptide P4
3.7. Effect of Peptide P4 and Flavonoids on Caco-2 Cell Viability
3.8. Flavonoids Isorhamnetin, Naringenin, Luteolin and Baicalein Attenuated Peptide P4-Induced Cytotoxicity in Caco-2 Cells
3.9. Flavonoids Inhibited the Release of IL-6 and IL-8 from Caco-2 Cell Monolayers Induced by ω-5 Gliadin-Derived Peptide P4
3.10. Flavonoids Improved Intestinal Epithelial Tight Junction Damage Induced by ω-5 Gliadin-Derived Peptide
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ricci, G.; Andreozzi, L.; Cipriani, F.; Giannetti, A.; Gallucci, M.; Caffarelli, C. Wheat allergy in children: A comprehensive update. Medicina 2019, 55, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabanillas, B. Gluten-related disorders: Celiac disease, wheat allergy, and nonceliac gluten sensitivity. Crit. Rev. Food Sci. Nutr. 2020, 60, 2606–2621. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A. Wheat allergy: Diagnosis and management. J. Asthma Allergy 2016, 9, 13–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quirce, S.; Boyano-Martínez, T.; Díaz-Perales, A. Clinical presentation, allergens, and management of wheat allergy. Expert Rev. Clin. Immunol. 2016, 12, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Palosuo, K.; Varjonen, E.; Kekki, O.M.; Klemola, T.; Kalkkinen, N.; Alenius, H.; Reunala, T. Wheat omega-5 gliadin is a major allergen in children with immediate allergy to ingested wheat. J. Allergy Clin. Immunol. 2001, 108, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Futamura, M.; Borres, M.P.; Takaoka, Y.; Dahlstrom, J.; Sakamoto, T.; Tanaka, A.; Kohno, K.; Matsuo, H.; Morita, E. IgE antibodies to omega-5 gliadin associate with immediate symptoms on oral wheat challenge in Japanese children. Allergy 2008, 63, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, N.; Sjölander, S.; Baar, A.; Berthold, M.; Pahr, S.; Vrtala, S.; Valenta, R.; Morita, E.; Hedlin, G.; Borres, M.P.; et al. Wheat allergy in children evaluated with challenge and IgE antibodies to wheat components. Pediatr. Allergy Immunol. 2015, 26, 119–125. [Google Scholar] [CrossRef]
- Matsuo, H.; Morita, E.; Tatham, A.S.; Morimoto, K.; Horikawa, T.; Osuna, H.; Ikezawa, Z.; Kaneko, S.; Kohno, K.; Dekio, S. Identification of the IgE-binding epitope in omega-5 gliadin, a major allergen in wheat-dependent exercise-induced anaphylaxis. J. Biol. Chem. 2004, 279, 12135–12140. [Google Scholar] [CrossRef] [Green Version]
- Battais, F.; Mothes, T.; Moneret-Vautrin, D.A.; Pineau, F.; Kanny, G.; Popineau, Y.; Bodinier, M.; Denery-Papini, S. Identification of IgE-binding epitopes on gliadins for patients with food allergy to wheat. Allergy 2005, 60, 815–821. [Google Scholar] [CrossRef]
- Matsuo, H.; Kohno, K.; Morita, E. Molecular cloning, recombinant expression and IgE-binding epitope of omega-5 gliadin, a major allergen in wheat-dependent exercise-induced anaphylaxis. FEBS J. 2005, 272, 4431–4438. [Google Scholar] [CrossRef]
- Denery-Papini, S.; Bodinier, M.; Pineau, F.; Triballeau, S.; Tranquet, O.; Adel-Patient, K.; Moneret-Vautrin, D.A.; Bakan, B.; Marion, D.; Mothes, T.; et al. Immunoglobulin-E-binding epitopes of wheat allergens in patients with food allergy to wheat and in mice experimentally sensitized to wheat proteins. Clin. Exp. Allergy 2011, 41, 1478–1492. [Google Scholar] [CrossRef] [PubMed]
- Pacharn, P.; Vichyanond, P. Immunotherapy for IgE-mediated wheat allergy. Hum. Vaccin. Immunother. 2017, 13, 2462–2466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wang, Z.Z.; Geliebter, J.; Tiwari, R.; Li, X.M. Traditional Chinese medicine for food allergy and eczema. Ann. Allergy Asthma Immunol. 2021, 126, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.; González-Santander, J.L.; Lara, A.; Torrens, F. Classification of flavonoid compounds by using entropy of information theory. Phytochemistry 2013, 93, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Amić, D.; Davidović-Amić, D.; Beslo, D.; Rastija, V.; Lucić, B.; Trinajstić, N. SAR and QSAR of the antioxidant activity of flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef] [PubMed]
- Boersma, B.J.; D’Alessandro, T.; Benton, M.R.; Kirk, M.; Wilson, L.S.; Prasain, J.; Botting, N.P.; Barnes, S.; Darley-Usmar, V.M.; Patel, R.P. Neutrophil myeloperoxidase chlorinates and nitrates soy isoflavones and enhances their antioxidant properties. Free Radic. Biol. Med. 2003, 35, 1417–1430. [Google Scholar] [CrossRef]
- Tuñón, M.J.; García-Mediavilla, M.V.; Sánchez-Campos, S.; González-Gallego, J. Potential of flavonoids as anti-inflammatory agents: Modulation of pro-inflammatory gene expression and signal transduction pathways. Curr. Drug Metab. 2009, 10, 256–271. [Google Scholar] [CrossRef]
- Jucá, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; Mesquita, D.D.S.; Barriga, J.R.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef]
- Matsuda, H.; Nakamura, S.; Yoshikawa, M. Degranulation inhibitors from medicinal plants in antigen-stimulated rat basophilic leukemia (RBL-2H3) cells. Chem. Pharm. Bull. 2016, 64, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T. Flavonoids for allergic diseases: Present evidence and future perspective. Curr. Pharm. Des. 2014, 20, 879–885. [Google Scholar] [CrossRef]
- Wang, J.L.; Quan, Q.; Ji, R.; Guo, X.Y.; Zhang, J.M.; Li, X.; Liu, Y.G. Isorhamnetin suppresses PANC-1 pancreatic cancer cell proliferation through S phase arrest. Biomed. Pharmacother. 2018, 108, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Jafarinia, M.; Sadat Hosseini, M.; Kasiri, N.; Fazel, N.; Fathi, F.; Ganjalikhani Hakemi, M.; Eskandari, N. Quercetin with the potential effect on allergic diseases. Allergy Asthma Clin. Immunol. 2020, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.J.; Shin, H.S.; See, H.J.; Jung, S.Y.; Kwon, D.A.; Shon, D.H. Baicalein induces CD4(+)Foxp3(+) T cells and enhances intestinal barrier function in a mouse model of food allergy. Sci. Rep. 2016, 6, 32225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shishehbor, F.; Behroo, L.; Ghafouriyan Broujerdnia, M.; Namjoyan, F.; Latifi, S.M. Quercetin effectively quells peanut-induced anaphylactic reactions in the peanut sensitized rats. Iran. J. Allergy Asthma Immunol. 2010, 9, 27–34. [Google Scholar]
- Anvari, S.; Miller, J.; Yeh, C.Y.; Davis, C.M. IgE-Mediated Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 244–260. [Google Scholar] [CrossRef] [Green Version]
- Chi, D.S.; Lin, T.C.; Hall, K.; Ha, T.; Li, C.; Wu, Z.D.; Soike, T.; Krishnaswamy, G. Enhanced effects of cigarette smoke extract on inflammatory cytokine expression in IL-1β-activated human mast cells were inhibited by Baicalein via regulation of the NF-κB pathway. Clin. Mol. Allergy 2012, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- Xie, T.; Yuan, J.; Mei, L.; Li, P.; Pan, R. Luteolin suppresses TNF-α-induced inflammatory injury and senescence of nucleus pulposus cells via the Sirt6/NF-κB pathway. Exp. Ther. Med. 2022, 24, 469. [Google Scholar] [CrossRef]
- Weng, Z.; Patel, A.B.; Panagiotidou, S.; Theoharides, T.C. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J. Allergy Clin. Immunol. 2015, 135, 1044–1052.e5. [Google Scholar] [CrossRef] [Green Version]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef]
- Murata, K.; Takano, S.; Masuda, M.; Iinuma, M.; Matsuda, H. Anti-degranulating activity in rat basophil leukemia RBL-2H3 cells of flavanone glycosides and their aglycones in citrus fruits. J. Nat. Med. 2013, 67, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Dupont, D.; Mandalari, G.; Molle, D.; Jardin, J.; Léonil, J.; Faulks, R.M.; Wickham, M.S.; Mills, E.N.; Mackie, A.R. Comparative resistance of food proteins to adult and infant in vitro digestion models. Mol. Nutr. Food Res. 2010, 54, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Jun, L.; Yong, W.; Juanli, Y.; Jin, Y.; Zhongliang, W.; Jinyan, G.; Hongbing, C. Characterization of bacillus cereus AFA01 capable of degrading gluten and celiac-immunotoxic peptides. Foods 2021, 10, 1725. [Google Scholar]
- Denery Papini, S.; Bodinier, M.; Larré, C.; Brossard, C.; Pineau, F.; Triballeau, S.; Pietri, M.; Battais, F.; Mothes, T.; Paty, E.; et al. Allergy to deamidated gluten in patients tolerant to wheat: Specific epitopes linked to deamidation. Allergy 2012, 67, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, G.; Di Stasio, L.; Fierro, O.; Picariello, G.; Venezia, A.; Gazza, L.; Ferranti, P.; Mamone, G. Protective effects of ID331 Triticum monococcum gliadin on in vitro models of the intestinal epithelium. Food Chem. 2016, 212, 537–542. [Google Scholar] [CrossRef]
- Che, S.Y.; Yuan, J.W.; Zhang, L.; Ruan, Z.; Sun, X.M.; Lu, H. Puerarin prevents epithelial tight junction dysfunction induced by ethanol in Caco-2 cell mode. J. Funct. Foods 2020, 73, 104079. [Google Scholar] [CrossRef]
- Shan, L.; Molberg, Ø.; Parrot, I.; Hausch, F.; Filiz, F.; Gray, G.M.; Sollid, L.M.; Khosla, C. Structural basis for gluten intolerance in celiac sprue. Science 2002, 297, 2275–2279. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, D.D.; Pawankar, R.; Ackerman, S.J.; Akin, C.; Clayton, F.; Falcone, F.H.; Gleich, G.J.; Irani, A.M.; Johansson, M.W.; Klion, A.D.; et al. Biomarkers of the involvement of mast cells, basophils and eosinophils in asthma and allergic diseases. World Allergy Organ. J. 2016, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Sahid, M.N.A.; Kiyoi, T. Mast cell activation markers for in vitro study. J. Immunoass. Immunochem. 2020, 41, 778–816. [Google Scholar] [CrossRef] [PubMed]
- Morishita, Y.; Ikeda, K.; Matsuno, H.; Ito, H.; Tai, A. Identification of degranulation inhibitors from rooibos (Aspalathus linearis) tea in rat basophilic leukaemia cells. Nat. Prod. Res. 2019, 33, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Van De Walle, J.; Hendrickx, A.; Romier, B.; Larondelle, Y.; Schneider, Y.J. Inflammatory parameters in Caco-2 cells: Effect of stimuli nature, concentration, combination and cell differentiation. Toxicol. In Vitro 2010, 24, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Sonnier, D.I.; Bailey, S.R.; Schuster, R.M.; Lentsch, A.B.; Pritts, T.A. TNF-α induces vectorial secretion of IL-8 in Caco-2 cells. J. Gastrointest. Surg. 2010, 14, 1592–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susewind, J.; de Souza Carvalho-Wodarz, C.; Repnik, U.; Collnot, E.M.; Schneider-Daum, N.; Griffiths, G.W.; Lehr, C.M. A 3D co-culture of three human cell lines to model the inflamed intestinal mucosa for safety testing of nanomaterials. Nanotoxicology 2016, 10, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Che, S.; Ruan, Z.; Song, L.; Tang, R.; Zhang, L. Regulatory effects of flavonoids luteolin on BDE-209-induced intestinal epithelial barrier damage in Caco-2 cell monolayer model. Food Chem. Toxicol. 2021, 150, 112098. [Google Scholar] [CrossRef]
- Yuan, J.; Che, S.; Zhang, L.; Ruan, Z. Reparative effects of ethanol-induced intestinal barrier injury by flavonoid luteolin via MAPK/NF-κB/MLCK and Nrf2 signaling pathways. J. Agric. Food Chem. 2021, 69, 4101–4110. [Google Scholar] [CrossRef]
- Liu, X.; Sun, R.; Li, Z.; Xiao, R.; Lv, P.; Sun, X.; Olson, M.A.; Gong, Y. Luteolin alleviates non-alcoholic fatty liver disease in rats via restoration of intestinal mucosal barrier damage and microbiota imbalance involving in gut-liver axis. Arch. Biochem. Biophys. 2021, 711, 109019. [Google Scholar] [CrossRef]
- Noda, S.; Tanabe, S.; Suzuki, T. Naringenin enhances intestinal barrier function through the expression and cytoskeletal association of tight junction proteins in Caco-2 cells. Mol. Nutr. Food Res. 2013, 57, 2019–2028. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P.; Sharma, J.; Dixit, A. Flavonoids modulate tight junction barrier functions in hyperglycemic human intestinal Caco-2 cells. Nutrition 2020, 78, 110792. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Shigeshiro, M.; Kodama, M.; Tanabe, S.; Suzuki, T. Supplemental naringenin prevents intestinal barrier defects and inflammation in colitic mice. J. Nutr. 2013, 143, 827–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.; Lee, J.; Park, S.; Kim, J.S.; Shim, S.; Lee, S.B.; Han, S.H.; Myung, H.; Kim, H.; Jang, W.S.; et al. Baicalein mitigates radiation-induced enteritis by improving endothelial dysfunction. Front. Pharmacol. 2019, 10, 892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: A review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Bonilla-Rosso, G.; Kwong Chung, C.K.C.; Bäriswyl, L.; Rodriguez, M.P.; Kim, B.S.; Engel, P.; Noti, M. High dietary fat intake induces a microbiota signature that promotes food allergy. J. Allergy Clin. Immunol. 2019, 144, 157–170.e8. [Google Scholar] [CrossRef] [Green Version]
- Pu, P.; Zheng, X.; Jiao, L.; Chen, L.; Yang, H.; Zhang, Y.; Liang, G. Six flavonoids inhibit the antigenicity of β-lactoglobulin by noncovalent interactions: A spectroscopic and molecular docking study. Food Chem. 2021, 339, 128106. [Google Scholar] [CrossRef]
- Wang, T.; Chen, W.; Shao, Y.; Liu, J.; Tu, Z. Ultrasound improved the non-covalent interaction of β-Lactoglobulin with luteolin: Regulating human intestinal microbiota and conformational epitopes reduced allergy Risks. Foods 2022, 11, 988. [Google Scholar] [CrossRef]











| Allergen | Biochemical Name | Molecular Weight (Kda) | Allergen Epitope |
|---|---|---|---|
| Tri a 19 | ω-5 gliadin | 49~55 | QQX1PX2QQ (X1 = L, F, S, I, Y; X2 = Q, E, G) |
| Peptide | Sequence | the Number of Epitopes |
|---|---|---|
| P1: AA374–404 |  | 3 |
| P2: AA160–189 |  | 2 |
| P3: AA252–271 |  | 2 |
| P4: AA253–279 |  | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Zhang, R.; Liu, Y.; Gao, J.; Wu, Y.; Tu, C.; Chen, H.; Yuan, J. In Vitro Effect of Flavonoids on Basophils Degranulation and Intestinal Epithelial Barrier Damage Induced by ω-5 Gliadin-Derived Peptide. Foods 2022, 11, 3857. https://doi.org/10.3390/foods11233857
Wu S, Zhang R, Liu Y, Gao J, Wu Y, Tu C, Chen H, Yuan J. In Vitro Effect of Flavonoids on Basophils Degranulation and Intestinal Epithelial Barrier Damage Induced by ω-5 Gliadin-Derived Peptide. Foods. 2022; 11(23):3857. https://doi.org/10.3390/foods11233857
Chicago/Turabian StyleWu, Shuangshuang, Ranran Zhang, Yaran Liu, Jinyan Gao, Yong Wu, Changchun Tu, Hongbing Chen, and Juanli Yuan. 2022. "In Vitro Effect of Flavonoids on Basophils Degranulation and Intestinal Epithelial Barrier Damage Induced by ω-5 Gliadin-Derived Peptide" Foods 11, no. 23: 3857. https://doi.org/10.3390/foods11233857





