Emulgels Structured with Dietary Fiber for Food Uses: A Rheological Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Single Phases
2.2. Preparation of Emulgels
2.3. Rheological Characterization
2.4. Microscopy Analysis
2.5. Interfacial Characterization
2.6. Rheological Modelling
3. Results and Discussion
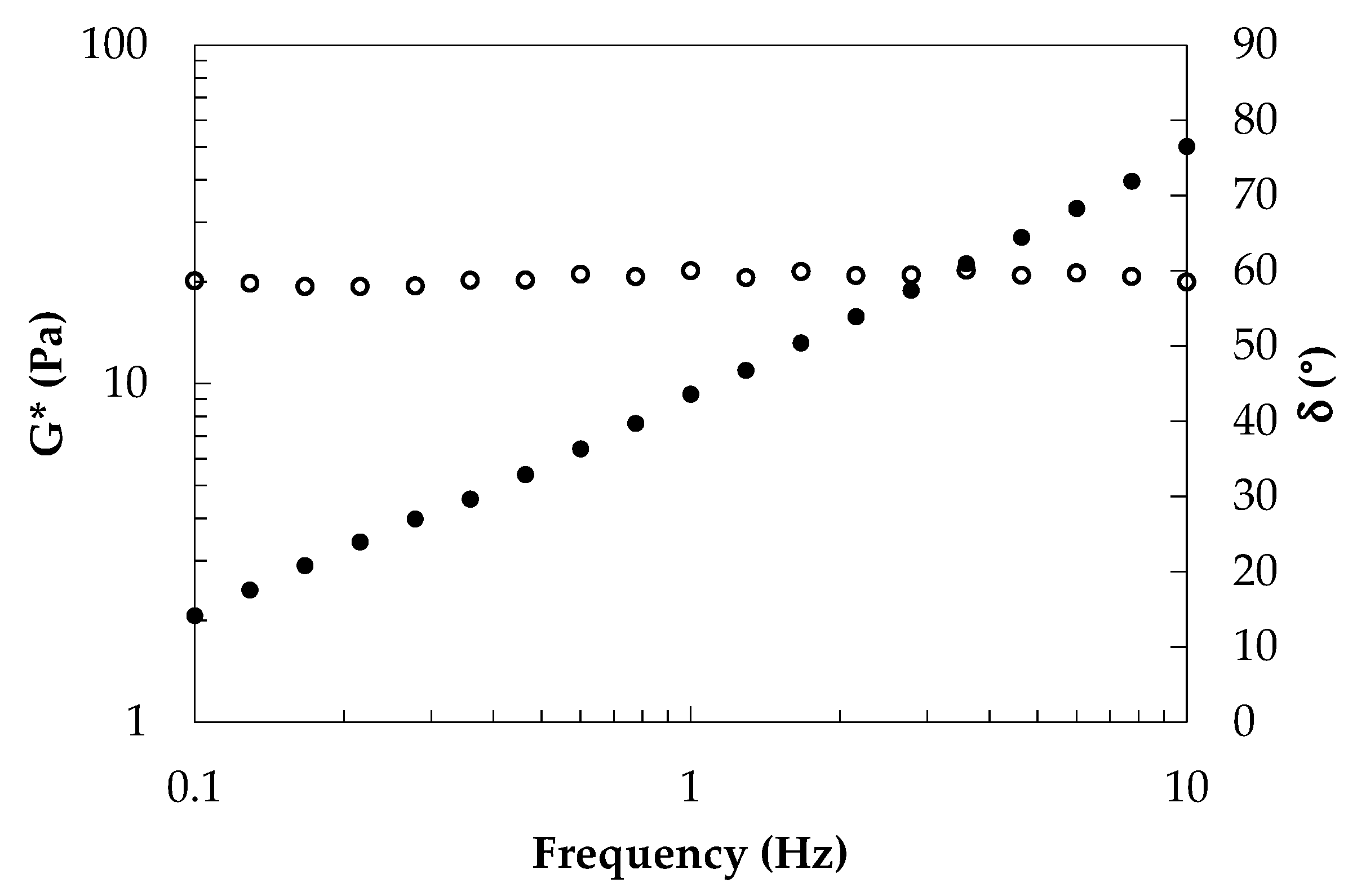
3.1. Rheological Characterization of Raw Phases
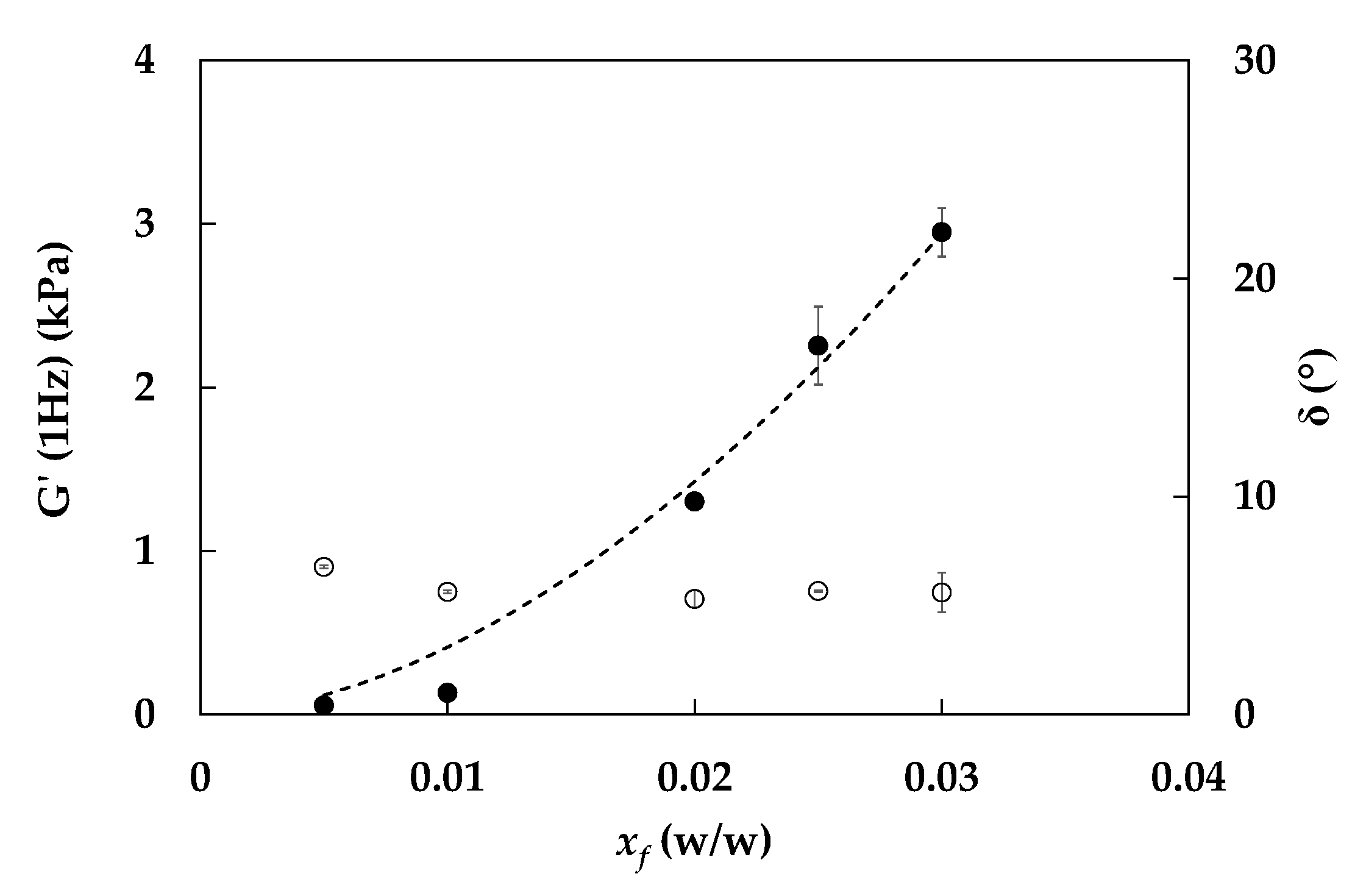
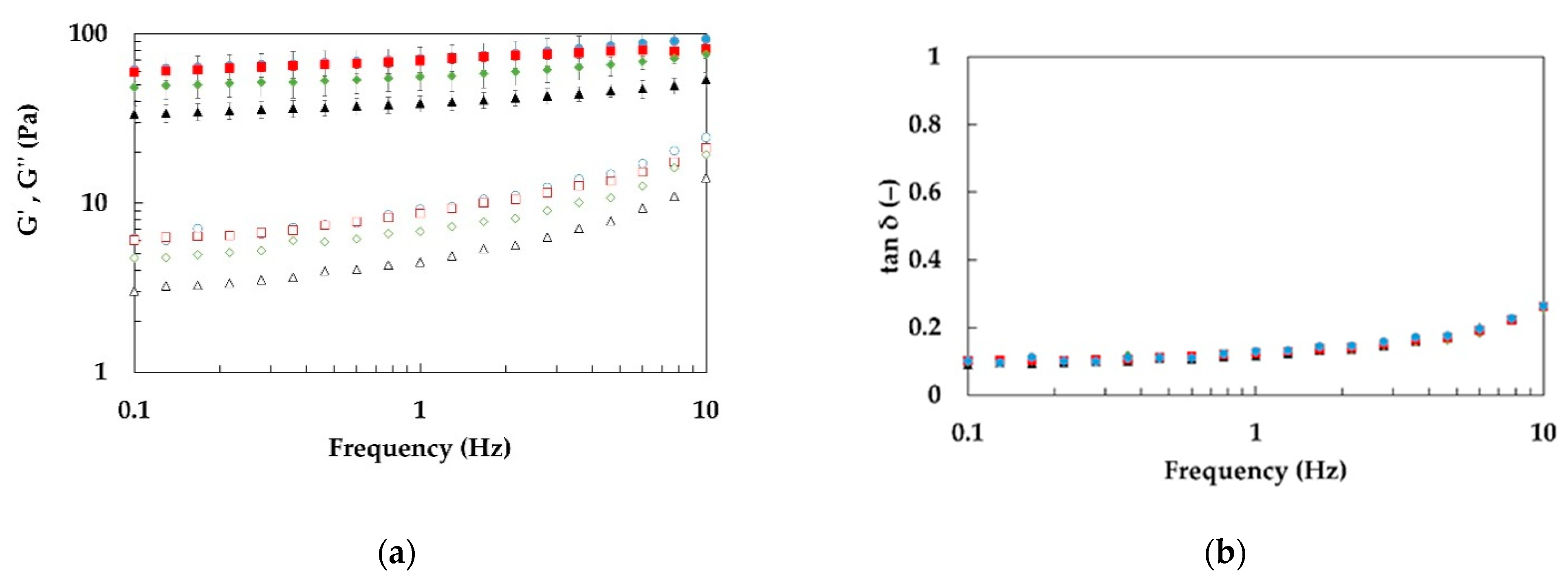
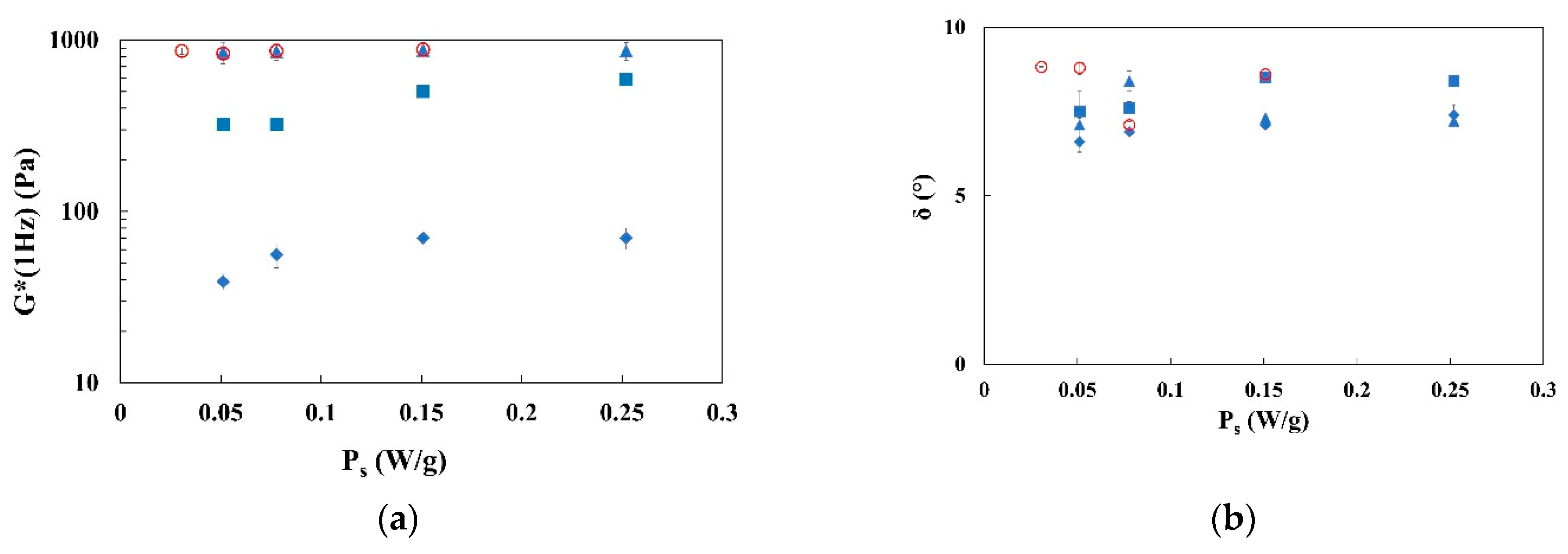
3.2. Rheological Characterization of Emulgels: Effect of Process and Fiber Concentrations
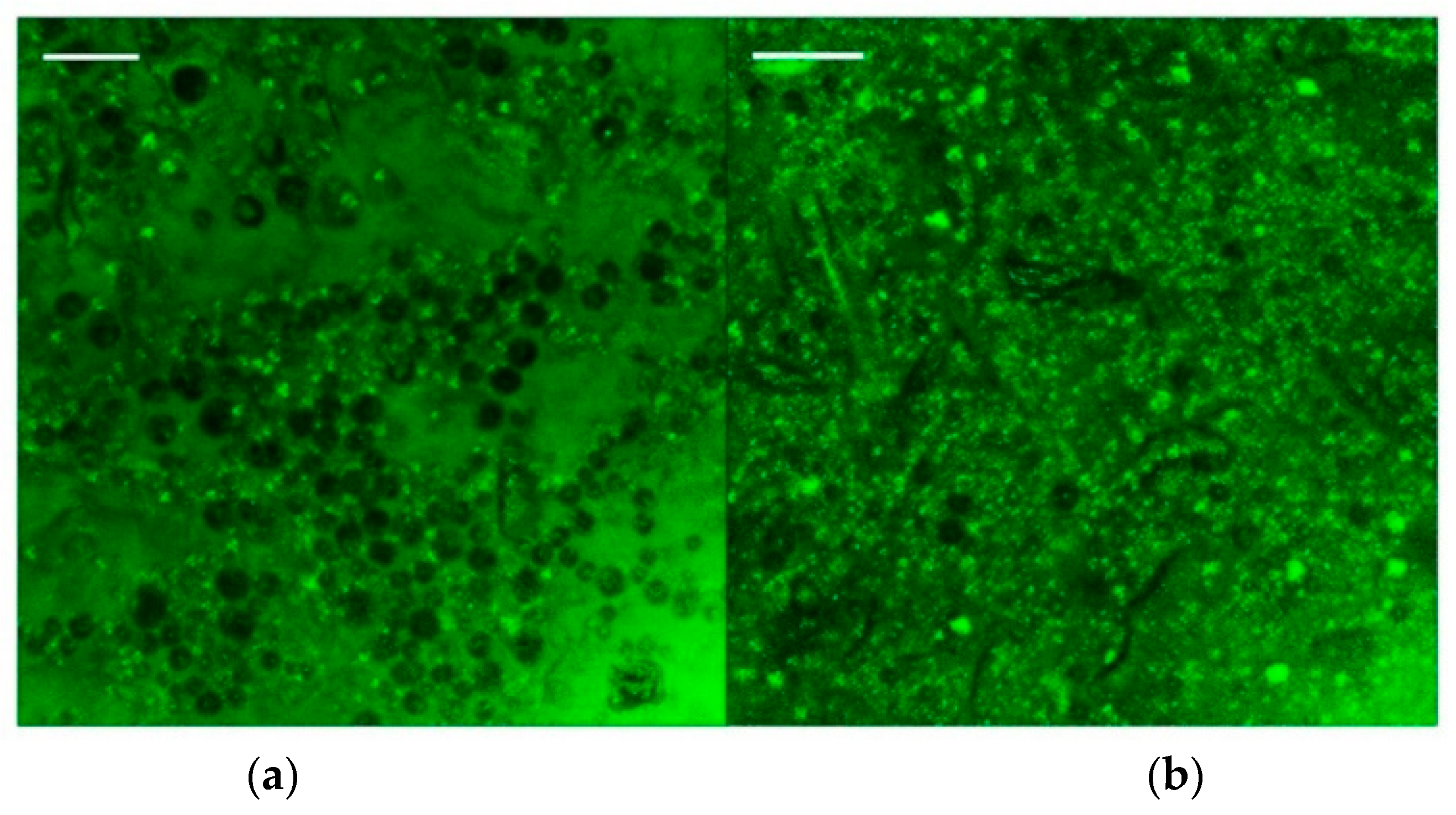
3.3. Microstructural Analysis of Emulgels
3.4. Interfacial Properties
3.5. Rheological Characterization and Modelling of Emulgels
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, C.; McClements, D.J. Application of Advanced Emulsion Technology in the Food Industry: A Review and Critical Evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Geremias-Andrade, I.M.; Souki, N.P.B.G.; Moraes, I.C.F.; Pinho, S.C. Rheology of Emulsion-Filled Gels Applied to the Development of Food Materials. Gels 2016, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Biswal, D.; Uvanesh, K.; Srivastava, A.K.; Bhattacharya, M.K.; Paramanik, K.; Pal, K. Modulating the Properties of Sunflower Oil Based Novel Emulgels Using Castor Oil Fatty Acid Ester: Prospects for Topical Antimicrobial Drug Delivery. Colloids Surf. B Biointerfaces 2015, 128, 155–164. [Google Scholar] [CrossRef]
- Shakeel, A.; Farooq, U.; Gabriele, D.; Marangoni, A.G.; Lupi, F.R. Bigels and Multi-Component Organogels: An Overview from Rheological Perspective. Food Hydrocoll. 2021, 111, 106190. [Google Scholar] [CrossRef]
- Rahmani-Neishaboor, E.; Jallili, R.; Hartwell, R.; Leung, V.; Carr, N.; Ghahary, A. Topical Application of a Film-Forming Emulgel Dressing That Controls the Release of Stratifin and Acetylsalicylic Acid and Improves/Prevents Hypertrophic Scarring. Wound Repair Regen. 2013, 21, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Teaima, M.H.; Badawi, N.M.; Attia, D.A.; El-Nabarawi, M.A.; Elmazar, M.M.; Mousa, S.A. Efficacy of Pomegranate Extract Loaded Solid Lipid Nanoparticles Transdermal Emulgel against Ehrlich Ascites Carcinoma. Nanomedicine 2022, 39, 102466. [Google Scholar] [CrossRef]
- Lupi, F.R.; Gabriele, D.; Seta, L.; Baldino, N.; de Cindio, B.; Marino, R. Rheological Investigation of Pectin-Based Emulsion Gels for Pharmaceutical and Cosmetic Uses. Rheol. Acta 2015, 54, 41–52. [Google Scholar] [CrossRef]
- Ajazuddin; Alexander, A. Khichariya, A.; Gupta, S.; Patel, R.J.; Giri, T.K.; Tripathi, D.K. Recent Expansions in an Emergent Novel Drug Delivery Technology: Emulgel. J. Control. Release 2013, 171, 122–132. [Google Scholar] [CrossRef]
- Roullet, M.; Clegg, P.S.; Frith, W.J. Rheology of Protein-Stabilised Emulsion Gels Envisioned as Composite Networks. 2-Framework for the Study of Emulsion Gels. J. Colloid Interface Sci. 2021, 594, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Lupi, F.R.; Shakeel, A.; Baldino, N.; Gabriele, D. Chapter 23-Rheology of Food Bigel System. In Advances in Food Rheology and Its Applications, 2nd ed.; Ahmed, J., Basu, S., Eds.; Woodhead Publishing: Soston, UK, 2023; pp. 689–706. ISBN 978-0-12-823983-4. [Google Scholar]
- Chen, X.-W.; Fu, S.-Y.; Hou, J.-J.; Guo, J.; Wang, J.-M.; Yang, X.-Q. Zein Based Oil-in-Glycerol Emulgels Enriched with β-Carotene as Margarine Alternatives. Food Chem. 2016, 211, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Nasirpour-Tabrizi, P.; Azadmard-Damirchi, S.; Hesari, J.; Khakbaz Heshmati, M.; Savage, G.P. Rheological and Physicochemical Properties of Novel Low-Fat Emulgels Containing Flaxseed Oil as a Rich Source of ω-3 Fatty Acids. LWT 2020, 133, 110107. [Google Scholar] [CrossRef]
- Oppong, D.; Panpipat, W.; Cheong, L.-Z.; Chaijan, M. Rice Flour-Emulgel as a Bifunctional Ingredient, Stabiliser-Cryoprotactant, for Formulation of Healthier Frozen Fish Nugget. LWT 2022, 159, 113241. [Google Scholar] [CrossRef]
- Oliver, L.; Wieck, L.; Scholten, E. Influence of Matrix Inhomogeneity on the Rheological Properties of Emulsion-Filled Gels. Food Hydrocoll. 2016, 52, 116–125. [Google Scholar] [CrossRef]
- Oliver, L.; Berndsen, L.; van Aken, G.A.; Scholten, E. Influence of Droplet Clustering on the Rheological Properties of Emulsion-Filled Gels. Food Hydrocoll. 2015, 50, 74–83. [Google Scholar] [CrossRef]
- Oliver, L.; Scholten, E.; van Aken, G.A. Effect of Fat Hardness on Large Deformation Rheology of Emulsion-Filled Gels. Food Hydrocoll. 2015, 43, 299–310. [Google Scholar] [CrossRef]
- Khalesi, H.; Emadzadeh, B.; Kadkhodaee, R.; Fang, Y. Effect of Persian Gum on Whey Protein Concentrate Cold-Set Emulsion Gel: Structure and Rheology Study. Int. J. Biol. Macromol. 2019, 125, 17–26. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, B.; Sun, Z.; Liu, T.; Cai, Y.; Huang, L.; Deng, X.; Zhao, M.; Zhao, Q. A Novel Preparation Strategy of Emulsion Gel Solely Stabilized by Alkaline Assisted Steam-Cooking Treated Insoluble Soybean Fiber. Food Hydrocoll. 2022, 129, 107646. [Google Scholar] [CrossRef]
- dos Santos, M.; Ozaki, M.M.; Ribeiro, W.O.; Paglarini, C.d.S.; Vidal, V.A.S.; Campagnol, P.C.B.; Pollonio, M.A.R. Emulsion Gels Based on Pork Skin and Dietary Fibers as Animal Fat Replacers in Meat Emulsions: An Adding Value Strategy to Byproducts. LWT 2020, 120, 108895. [Google Scholar] [CrossRef]
- Morales-Medina, R.; Dong, D.; Schalow, S.; Drusch, S. Impact of Microfluidization on the Microstructure and Functional Properties of Pea Hull Fibre. Food Hydrocoll. 2020, 103, 105660. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary Fibre in Gastrointestinal Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Szafrańska, J.O.; Muszyński, S.; Tomasevic, I.; Sołowiej, B.G. The Influence of Dietary Fibers on Physicochemical Properties of Acid Casein Processed Cheese Sauces Obtained with Whey Proteins and Coconut Oil or Anhydrous Milk Fat. Foods 2021, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority Dietary Reference Values for Nutrients Summary Report. EFSA Support. Publ. 2017, 14, e15121E. [CrossRef]
- Szafrańska, J.O.; Sołowiej, B.G. Effect of Different Fibres on Texture, Rheological and Sensory Properties of Acid Casein Processed Cheese Sauces. Int. J. Food Sci. Technol. 2020, 55, 1971–1979. [Google Scholar] [CrossRef]
- Lundberg, B.; Pan, X.; White, A.; Chau, H.; Hotchkiss, A. Rheology and Composition of Citrus Fiber. J. Food Eng. 2014, 125, 97–104. [Google Scholar] [CrossRef]
- Yildiz, E.; Ozcan, T. Functional and Textural Properties of Vegetable-Fibre Enriched Yoghurt. Int. J. Dairy Technol. 2019, 72, 199–207. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Marangoni, A.G. Effect of Matrix Architecture on the Elastic Behavior of an Emulsion-Filled Polymer Gel. Food Hydrocoll. 2021, 119, 106875. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Marangoni, A.G. The Influence of Network Architecture on the Large Deformation and Fracture Behavior of Emulsion-Filled Gelatin Gels. Food Struct. 2021, 29, 100193. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Nicholson, R.A.; Barbut, S.; Marangoni, A.G. Considerations for Readdressing Theoretical Descriptions of Particle-Reinforced Composite Food Gels. Food Res. Int. 2019, 122, 209–221. [Google Scholar] [CrossRef]
- Lorenzo, G.; Zaritzky, N.; Califano, A. Rheological Analysis of Emulsion-Filled Gels Based on High Acyl Gellan Gum. Food Hydrocoll. 2013, 30, 672–680. [Google Scholar] [CrossRef]
- Rosa, P.; Sala, G.; van Vliet, T.; van de Velde, F. Cold Gelation of Whey Protein Emulsions. J. Texture Stud. 2006, 37, 516–537. [Google Scholar] [CrossRef]
- Genovese, D.B. Shear Rheology of Hard-Sphere, Dispersed, and Aggregated Suspensions, and Filler-Matrix Composites. Adv. Colloid Interface Sci. 2012, 171–172, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bruno, E.; Lupi, F.R.; Martin-Piñero, M.J.; Girimonte, R.; Baldino, N.; Muñoz, J.; Gabriele, D. Influence of Different Dispersing Systems on Rheological and Microstructural Properties of Citrus Fiber Suspensions. LWT 2021, 152, 112270. [Google Scholar] [CrossRef]
- Seta, L.; Baldino, N.; Gabriele, D.; Lupi, F.R.; de Cindio, B. The Influence of Carrageenan on Interfacial Properties and Short-Term Stability of Milk Whey Proteins Emulsions. Food Hydrocoll. 2013, 32, 373–382. [Google Scholar] [CrossRef]
- Biresaw, G.; Liu, Z.S.; Erhan, S.Z. Investigation of the Surface Properties of Polymeric Soaps Obtained by Ring-Opening Polymerization of Epoxidized Soybean Oil. J. Appl. Polym. Sci. 2008, 108, 1976–1985. [Google Scholar] [CrossRef]
- Seta, L.; Baldino, N.; Gabriele, D.; Lupi, F.R.; de Cindio, B. The Effect of Surfactant Type on the Rheology of Ovalbumin Layers at the Air/Water and Oil/Water Interfaces. Food Hydrocoll. 2012, 29, 247–257. [Google Scholar] [CrossRef]
- Baldino, N.; Mileti, O.; Lupi, F.R.; Gabriele, D. Rheological Surface Properties of Commercial Citrus Pectins at Different PH and Concentration. LWT 2018, 93, 124–130. [Google Scholar] [CrossRef]
- Mileti, O.; Baldino, N.; Carmona, J.A.; Lupi, F.R.; Muñoz, J.; Gabriele, D. Shear and Dilatational Rheological Properties of Vegetable Proteins at the Air/Water Interface. Food Hydrocoll. 2022, 126, 107472. [Google Scholar] [CrossRef]
- Lupi, F.R.; Puoci, F.; Bruno, E.; Baldino, N.; Marino, R.; Gabriele, D. The Effects of Process Conditions on Rheological Properties of Functional Citrus Fibre Suspensions. Food Bioprod. Process. 2020, 121, 54–64. [Google Scholar] [CrossRef]
- Lupi, F.R.; Franco, G.; Baldino, N.; Gabriele, D. The Effect of Operating Conditions on the Physicochemical Characteristics of Whey Protein–Based Systems. Rheol. Acta 2020, 59, 227–238. [Google Scholar] [CrossRef]
- Shih, W.-H.; Shih, W.Y.; Kim, S.-I.; Liu, J.; Aksay, I.A. Scaling Behavior of the Elastic Properties of Colloidal Gels. Phys. Rev. A 1990, 42, 4772–4779. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Marangoni, A.G. Modeling the Rheological Properties and Structure of Colloidal Fat Crystal Networks. Trends Food Sci. Technol. 2007, 18, 474–483. [Google Scholar] [CrossRef]
- Narine, S.S.; Marangoni, A.G. Fractal Nature of Fat Crystal Networks. Phys. Rev. E 1999, 59, 1908–1920. [Google Scholar] [CrossRef]
- Lapasin, R.; Grassi, M.; Pricl, S. Rheological Modeling of Fractal and Dense Suspensions. Chem. Eng. J. Biochem. Eng. J. 1996, 64, 99–106. [Google Scholar] [CrossRef][Green Version]
- Lupi, F.R.; Gabriele, D.; Greco, V.; Baldino, N.; Seta, L.; de Cindio, B. A Rheological Characterisation of an Olive Oil/Fatty Alcohols Organogel. Food Res. Int. 2013, 51, 510–517. [Google Scholar] [CrossRef]
- Muthukumar, M. Screening Effect on Viscoelasticity near the Gel Point. Macromolecules 1989, 22, 4656–4658. [Google Scholar] [CrossRef]
- Palierne, J.F. Linear Rheology of Viscoelastic Emulsions with Interfacial Tension. Rheol. Acta 1990, 29, 204–214. [Google Scholar] [CrossRef]
- van Aken, G.A.; Oliver, L.; Scholten, E. Rheological Effect of Particle Clustering in Gelled Dispersions. Food Hydrocoll. 2015, 48, 102–109. [Google Scholar] [CrossRef]
- Pakseresht, S.; Mazaheri Tehrani, M. Advances in Multi-Component Supramolecular Oleogels- a Review. Food Rev. Int. 2022, 38, 760–782. [Google Scholar] [CrossRef]
- Gabriele, D.; de Cindio, B.; D’Antona, P. A Weak Gel Model for Foods. Rheol. Acta 2001, 40, 120–127. [Google Scholar] [CrossRef]
- Qi, J.; Song, L.; Zeng, W.; Liao, J. Citrus Fiber for the Stabilization of O/W Emulsion through Combination of Pickering Effect and Fiber-Based Network. Food Chem. 2021, 343, 128523. [Google Scholar] [CrossRef] [PubMed]
- Wallecan, J.R.P.; Mccrae, C.H.; Debon, S.J.J.; Dong, J.; Mazoyer, J. Emulsifying and Stabilizing Properties of Functionalized Orange Pulp Fibers. Food Hydrocoll. 2015, 47, 115–123. [Google Scholar] [CrossRef]
- Fainerman, V.B.; Aksenenko, E.V.; Kovalchuk, V.I.; Mucic, N.; Javadi, A.; Liggieri, L.; Ravera, F.; Loglio, G.; Makievski, A.V.; Schneck, E.; et al. New View of the Adsorption of Surfactants at Water/Alkane Interfaces—Competitive and Cooperative Effects of Surfactant and Alkane Molecules. Adv. Colloid Interface Sci. 2020, 279, 102143. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Emulsion Gels: The Structuring of Soft Solids with Protein-Stabilized Oil Droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]


| ID | Ps(W/g) | Ω (rpm) | t (s) | Fiber Fraction (w/w) | G* (1 Hz) (Pa) | δ (1 Hz) (°) | D (−) |
|---|---|---|---|---|---|---|---|
| H_0.5 | 0.151 | 8000 | 300 | 0.005 | 57 ± 2 a | 6.77 ± 0.06 a | 2.42 ± 0.01 a |
| H_1 | 0.01 | 140 ±10 b | 5.75 ± 0.09 b | 2.444 ± 0.002 b | |||
| H_2 | 0.02 | 1310 ± 50 c | 5.3 ± 0.3 b | 2.453 ± 0.005 b | |||
| H_2.5 | 0.025 | 2300 ± 200 d | 5.66 ± 0.05 b | 2.438 ± 0.003 ab | |||
| H_3 | 0.03 | 3000 ± 100 e | 5.57 ± 0.09 b | 2.446 ± 0.007 b |
| Isoenergy E1s = 22.6 J/g | ||||||
|---|---|---|---|---|---|---|
| ID | Ps (W/g) | Ω (rpm) | t (s) | Fiber Fraction (w/w) | G* (1 Hz) (Pa) | δ (1 Hz) (°) |
| E1.1.3.20 | 0.031 | 4000 | 738 | 0.03 | 860 ± 40 a | 8.82 ± 0.02 a |
| E1.2.3.20 | 0.051 | 5000 | 442 | 0.03 | 840 ± 60 a | 8.2 ± 0.2 a |
| E1.3.3.20 | 0.078 | 6000 | 290 | 0.03 | 860 ± 30 a | 7.1 ± 0.1 b |
| E1.4.3.20 | 0.151 | 8000 | 150 | 0.03 | 880 ± 90 a | 8.6 ± 0.3 a |
| Isoenergy E2s = 45.2 J/g | ||||||
| ID | Ps (W/g) | Ω (rpm) | t (s) | Fiber fraction (w/w) | G* (1 Hz) (Pa) | δ (1 Hz) (°) |
| E2.1.1.20 | 0.051 | 5000 | 886 | 0.01 | 39 ± 4 a | 6.6 ± 0.3 a |
| E2.2.1.20 | 0.078 | 6000 | 580 | 0.01 | 56 ± 9 ab | 6.9 ± 0.1 ab |
| E2.3.1.20 | 0.151 | 8000 | 300 | 0.01 | 70 ± 5 b | 7.1 ± 0.1 ab |
| E2.4.1.20 | 0.252 | 10,000 | 179 | 0.01 | 70 ± 10 b | 7.4 ± 0.3 b |
| E2.1.2.20 | 0.051 | 5000 | 886 | 0.02 | 323 ± 5 a | 7.5 ± 0.6 a |
| E2.2.2.20 | 0.078 | 6000 | 580 | 0.02 | 323 ± 2 a | 7.7 ± 0.2 ab |
| E2.3.2.20 | 0.151 | 8000 | 300 | 0.02 | 500 ± 30 b | 8.51 ± 0.01 b |
| E2.4.2.20 | 0.252 | 10,000 | 179 | 0.02 | 590 ± 40 c | 8.4 ± 0.1 ab |
| E2.1.3.20 | 0.051 | 5000 | 886 | 0.03 | 850 ± 120 a | 7.1 ± 0.2 b |
| E2.2.3.20 | 0.078 | 6000 | 580 | 0.03 | 860 ± 90 a | 8.4 ± 0.3 a |
| E2.3.3.20 | 0.151 | 8000 | 300 | 0.03 | 870 ± 70 a | 7.3 ± 0.1 b |
| E2.4.3.20 | 0.252 | 10,000 | 179 | 0.03 | 860 ± 100 a | 7.2 ± 0.1 b |
| ID | Fiber Fraction (w/w) | Oil Weight Fraction (% w/w) | Oil Volume Fraction (% v/v) | G* (1 Hz) (Pa) | δ (1 Hz) (°) |
|---|---|---|---|---|---|
| E2.3.3.10 | 0.03 | 10 | 10.8 | 1600 ± 100 a | 7.6 ± 0.2 a |
| E2.3.3.15 | 15 | 16.1 | 1200 ± 60 b | 7.9 ± 0.1 a | |
| E2.3.3.20 | 20 | 21.4 | 870 ± 70 c | 7.3 ± 0.1 a | |
| E2.3.3.30 | 30 | 31.8 | 433 ± 30 d | 7.6 ± 0.2 a | |
| E2.3.3.35 | 35 | 36.9 | 327 ± 20 d | 8.0 ± 0.3 a | |
| E2.3.3.40 | 40 | 42.0 | 340 ± 20 d | 7.3 ± 0.3 a | |
| E2.3.0_5.20 | 0.005 | 20 | 21.4 | 43 ± 3 e | 9.8 ± 0.4 e |
| E2.3.1.20 | 0.01 | 70 ± 5 e | 7.1 ± 0.1 f | ||
| E2.3.2.20 | 0.02 | 501 ± 35 f | 8.5 ± 0.1 g | ||
| E2.3.2_5.20 | 0.025 | 830 ± 50 g | 8.4 ± 0.5 g | ||
| E2.3.3.20 | 0.3 | 870 ± 70 g | 7.3 ± 0.1 f |
| Lecithin Fraction (w/w) | Fiber Fraction (w/w) | γ (mN/m) |
|---|---|---|
| - | - | 23.0 ± 0.1 |
| - | 0.01 | 16.0 ± 0.2 |
| 10−9 | - | 23.0 ± 0.1 |
| 0.0001 | - | 9.43 ± 0.07 |
| 0.001 | - | 3.9 ± 0.1 |
| 0.005 | - | 1.43 ± 0.05 |
| 0.005 | 0.005 | 1.72 ± 0.03 |
| 0.005 | 0.01 | 1.4 ± 0.1 |
| 0.005 | 0.015 | 1.40 ± 0.08 |
| 0.01 | - | 1.40 ± 0.07 |
| 0.02 | - | 1.49 ± 0.08 |
| ID | ds (μm) | σs (μm) |
|---|---|---|
| E2.3.1.20 | 7.1 ± 0.8 a | 6 ± 1 a |
| E2.3.3.20 | 6.5 ± 0.3 a | 4.3 ± 0.1 ab |
| E2.3.3.30 | 5.5 ± 0.6 a | 3.1 ± 0.5 b |
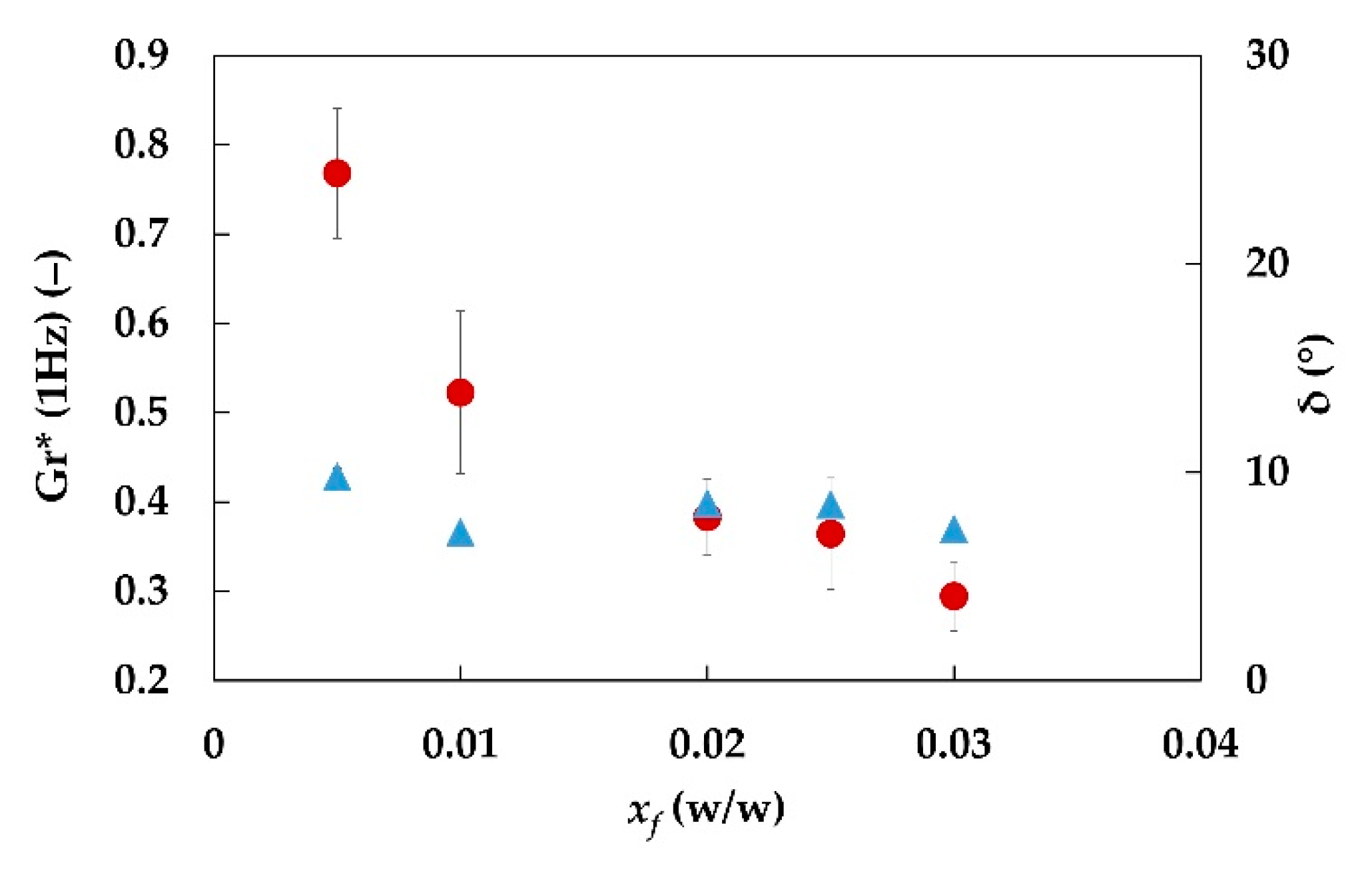
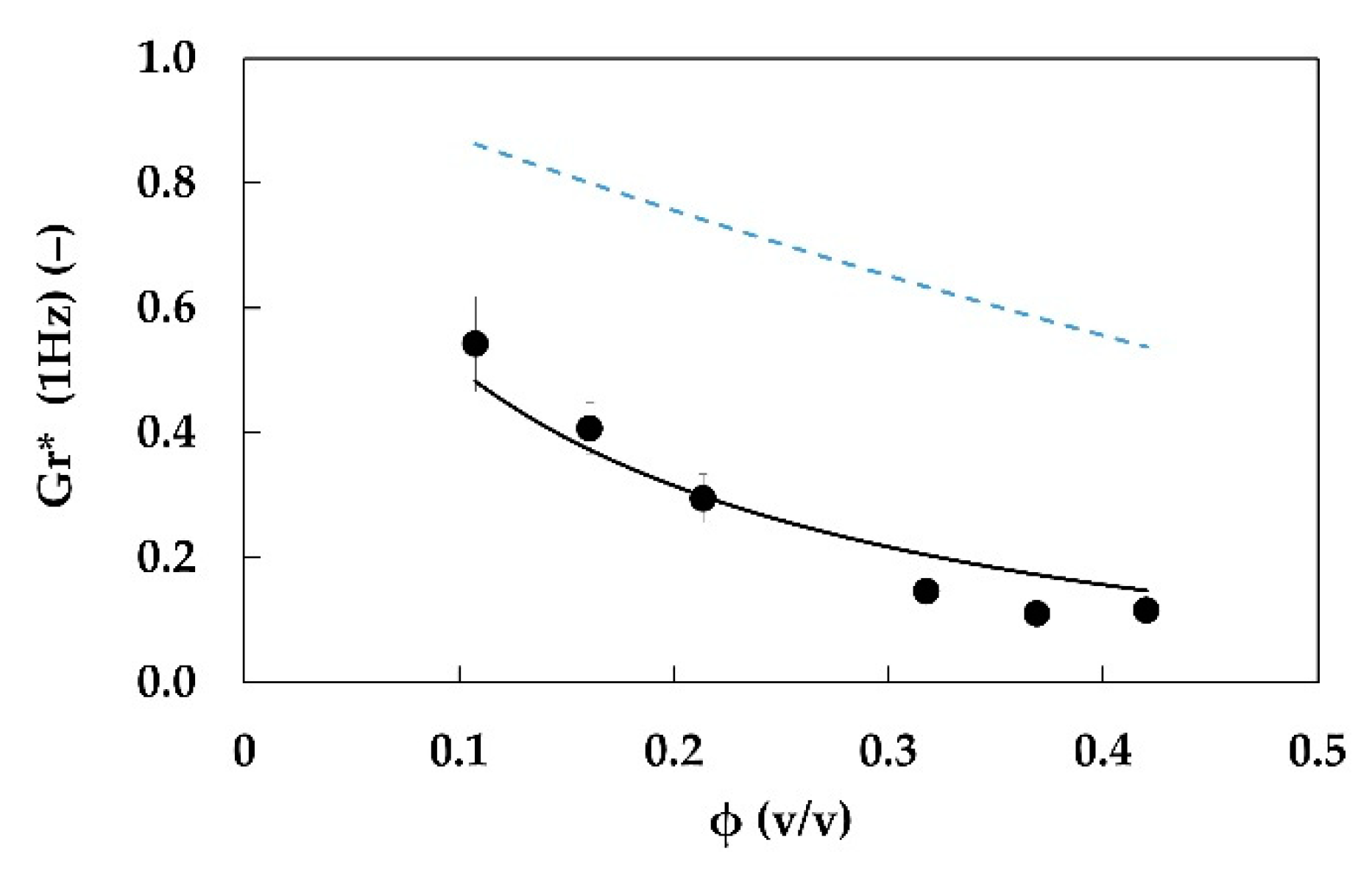
| Sample ID | xf (‒) | Gr*exp (‒) | M (‒) | H (‒) | Gr*mod (‒) | Error (%) |
|---|---|---|---|---|---|---|
| E2.3.0_5.20 | 0.005 | 0.6 ± 0.1 | 0.19 | 0.190 | <0 | - |
| E2.3.1.20 | 0.01 | 0.52 ± 0.08 | 0.081 | 0.084 | 2.35 | 300% |
| E2.3.2.20 | 0.02 | 0.38 ± 0.03 | 0.0084 | ‒0.208 | 0.37 | ‒4% |
| E2.3.2_5.20 | 0.025 | 0.36 ± 0.05 | 0.0048 | ‒0.253 | 0.32 | ‒14% |
| E2.3.3.20 | 0.03 | 0.29 ± 0.04 | 0.0037 | ‒0.270 | 0.30 | 1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, E.; Lupi, F.R.; Mammolenti, D.; Mileti, O.; Baldino, N.; Gabriele, D. Emulgels Structured with Dietary Fiber for Food Uses: A Rheological Model. Foods 2022, 11, 3866. https://doi.org/10.3390/foods11233866
Bruno E, Lupi FR, Mammolenti D, Mileti O, Baldino N, Gabriele D. Emulgels Structured with Dietary Fiber for Food Uses: A Rheological Model. Foods. 2022; 11(23):3866. https://doi.org/10.3390/foods11233866
Chicago/Turabian StyleBruno, Elisabetta, Francesca Romana Lupi, Domenico Mammolenti, Olga Mileti, Noemi Baldino, and Domenico Gabriele. 2022. "Emulgels Structured with Dietary Fiber for Food Uses: A Rheological Model" Foods 11, no. 23: 3866. https://doi.org/10.3390/foods11233866
APA StyleBruno, E., Lupi, F. R., Mammolenti, D., Mileti, O., Baldino, N., & Gabriele, D. (2022). Emulgels Structured with Dietary Fiber for Food Uses: A Rheological Model. Foods, 11(23), 3866. https://doi.org/10.3390/foods11233866







