Insights into Protective Effects of Different Synbiotic Microcapsules on the Survival of Lactiplantibacillus plantarum by Electrospraying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Preparation of Polymer Solutions
2.4. Production of Microcapsules by Coaxial Electrospraying
2.5. Encapsulation Efficiency
2.6. Survival Rate of Encapsulated L. plantarum GIM1.648 Exposed to Simulated Digestive System
2.7. Characterization of Microcapsules
2.8. Tolerance of Encapsulated L. plantarum GIM1.648 to Adverse Conditions
2.8.1. Heat Treatment
2.8.2. Freeze Drying
2.8.3. Storage Study
2.9. Statistical Analysis
3. Results and Discussion
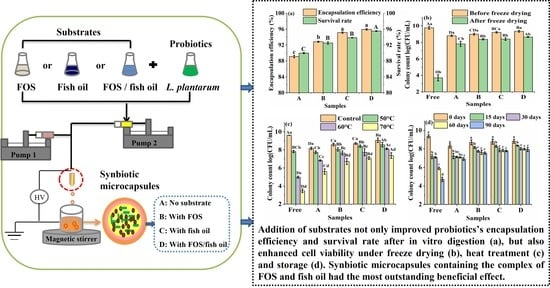
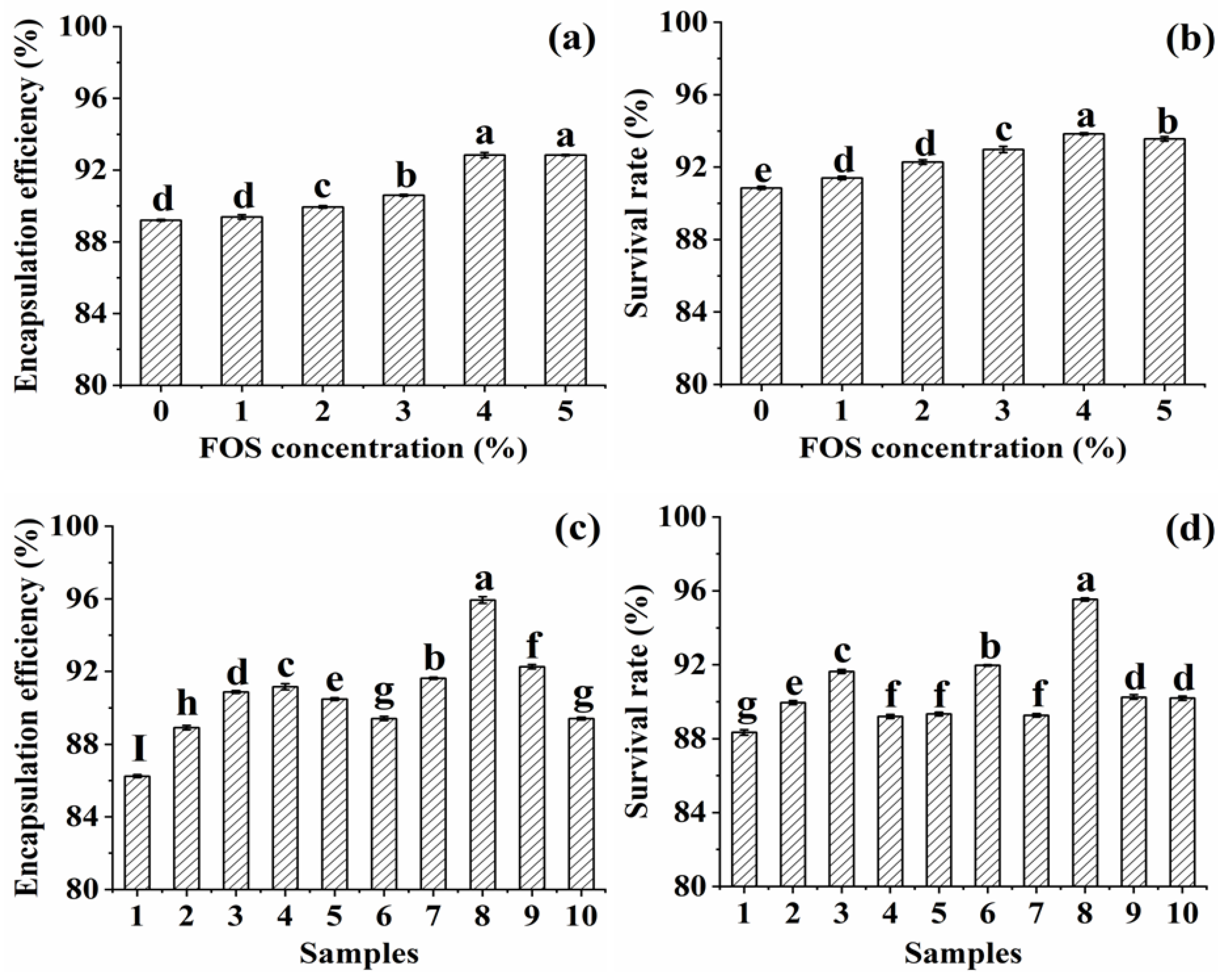
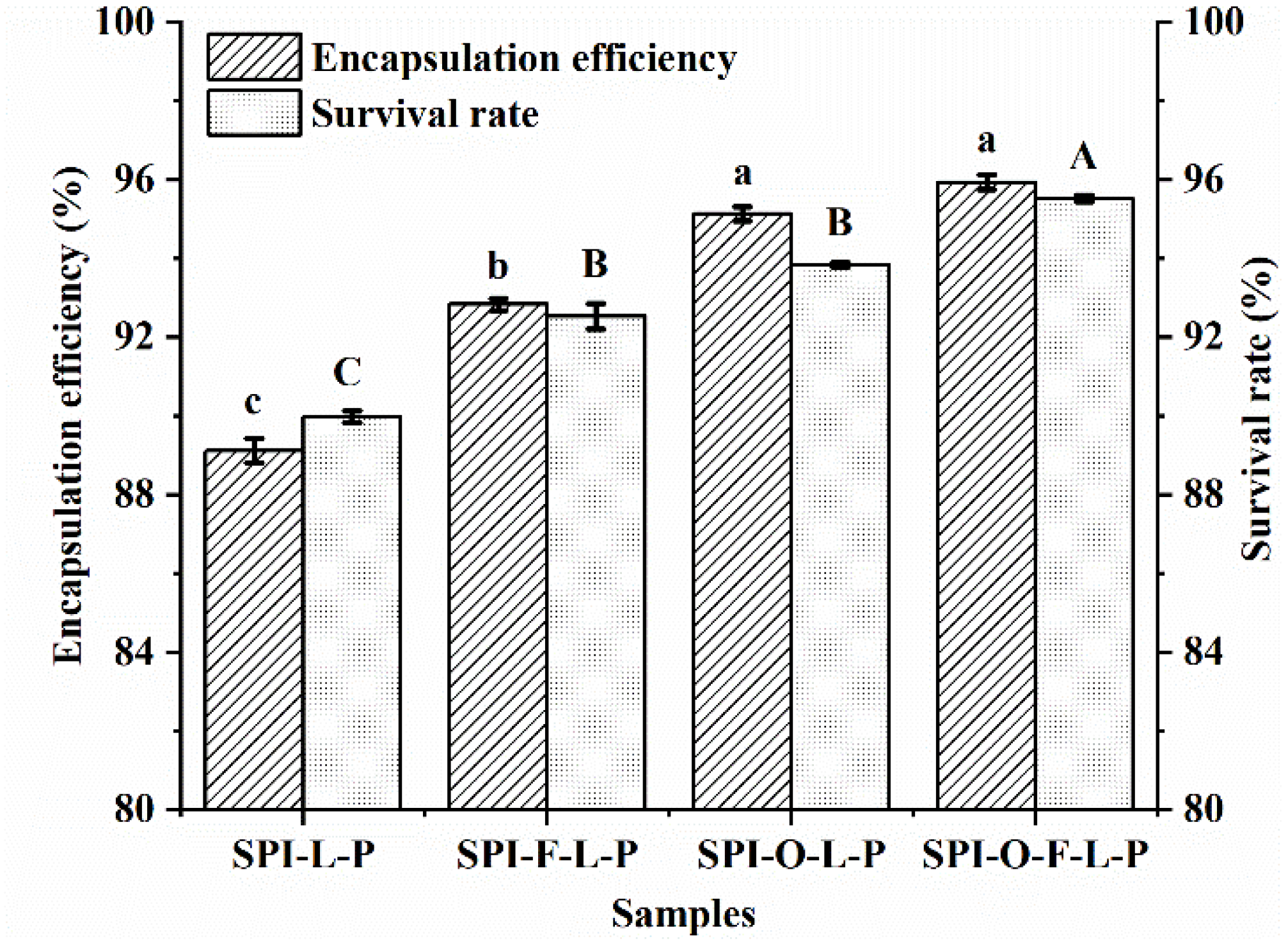
3.1. Effects of Synbiotic Microcapsules Containing Different Substrates on the Encapsulation Efficiency and Survival Rate of L. plantarum GIM1.648
3.2. Characterization of Microcapsules
3.2.1. SEM
3.2.2. FTIR
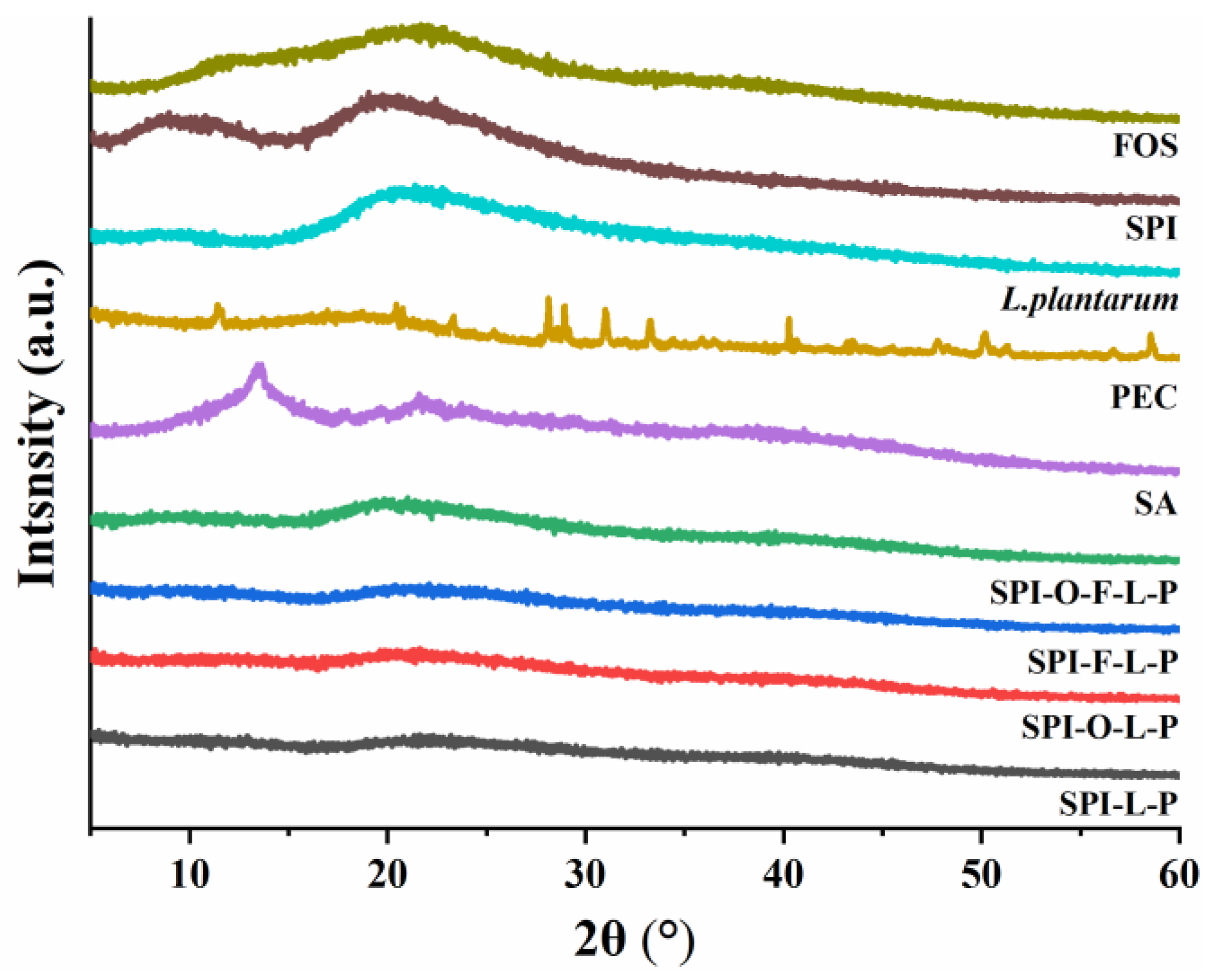
3.2.3. XRD
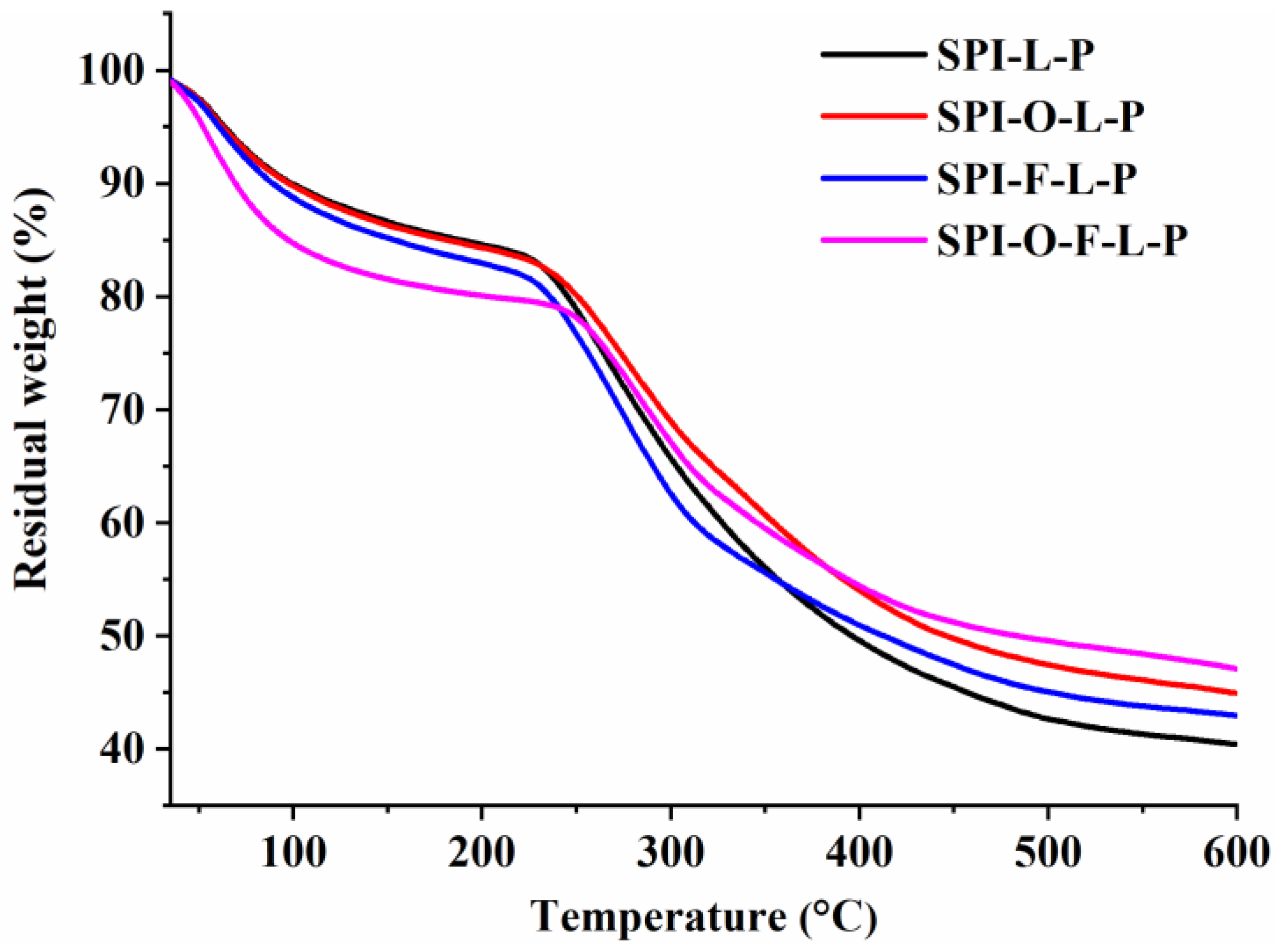
3.2.4. TGA
3.3. Tolerance of Encapsulated L. plantarum GIM1.648 to Adverse Conditions
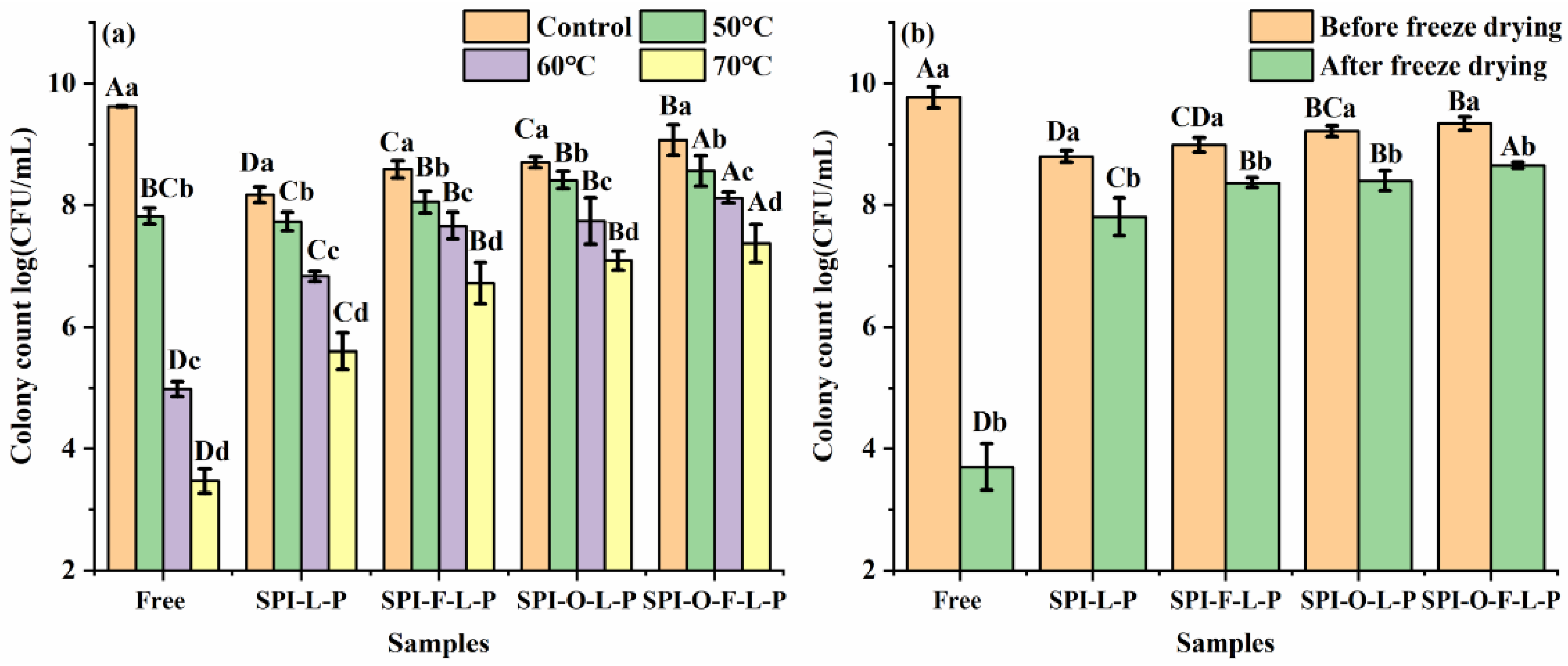
3.3.1. Heat Treatment
3.3.2. Freeze Drying Treatment
3.3.3. Storage Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morelli, L. Probiotics: Definition and Taxonomy 10 Years after the FAO/WHO Guidelines. In Probiotic Bacteria and Their Effect on Human Health and Well-Being; Guarino, A., Quigley, E.M.M., Walker, W.A., Eds.; Karger: Basel, Switzerland, 2013; Volume 107, pp. 1–8. [Google Scholar]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.W. Advances in colonic drug delivery. Drugs 2005, 65, 1991–2007. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Dimitrijevic, M.; Aleksic, A.; Neffe-Skocinska, K.; Zielinska, D.; Kolozyn-Krajewska, D.; Sharifi-Rad, J.; Stojanovic-Radic, Z.; Prabu, S.M.; Rodrigues, C.F.; et al. Human microbiome and homeostasis: Insights into the key role of prebiotics, probiotics, and symbiotics. Crit. Rev. Food Sci. Nutr. 2021, 61, 1415–1428. [Google Scholar] [CrossRef]
- Amidon, S.; Brown, J.E.; Dave, V.S. Colon-Targeted Oral Drug Delivery Systems: Design Trends and Approaches. Aaps Pharmscitech 2015, 16, 731–741. [Google Scholar] [CrossRef]
- Ta, L.P.; Bujna, E.; Kun, S.; Charalampopoulos, D.; Khutoryanskiy, V.V. Electrosprayed mucoadhesive alginate-chitosan microcapsules for gastrointestinal delivery of probiotics. Int. J. Pharm. 2021, 597, 11. [Google Scholar] [CrossRef]
- Jaworek, A.; Sobczyk, A.T. Electrospraying route to nanotechnology: An overview. J. Electrost. 2008, 66, 197–219. [Google Scholar] [CrossRef]
- Mendes, A.C.; Chronakis, I.S. Electrohydrodynamic encapsulation of probiotics: A review. Food Hydrocoll. 2021, 117, 9. [Google Scholar] [CrossRef]
- dos Passos, F.R.; Maestre, K.L.; da Silva, B.F.; Rodrigues, A.C.; Triques, C.C.; Garcia, H.A.; Fagundes-Klen, M.R.; da Silva, E.A.; Fiorese, M.L. Production of a synbiotic composed of galacto-oligosaccharides and Saccharomyces boulardii using enzymatic-fermentative method. Food Chem. 2021, 353, 8. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Cavalcante, R.B.; Telli, G.S.; Tachibana, L.; Dias, D.D.; Oshiro, E.; Natori, M.M.; da Silva, W.F.; Ranzani-Paiva, M.J. Probiotics, Prebiotics and Synbiotics for Nile tilapia: Growth performance and protection against Aeromonas hydrophila infection. Aquac. Rep. 2020, 17, 8. [Google Scholar] [CrossRef]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luca, L.; Oroian, M. Influence of Different Prebiotics on Viability of Lactobacillus casei, Lactobacillus plantarum and Lactobacillus rhamnosus Encapsulated in Alginate Microcapsules. Foods 2021, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Poletto, G.; Raddatz, G.C.; Cichoski, A.J.; Zepka, L.Q.; Lopes, E.J.; Barin, J.S.; Wagner, R.; de Menezes, C.R. Study of viability and storage stability of Lactobacillus acidophillus when encapsulated with the prebiotics rice bran, inulin and Hi-maize. Food Hydrocoll. 2019, 95, 238–244. [Google Scholar] [CrossRef]
- Liao, N.; Luo, B.L.; Gao, J.; Li, X.J.; Zhao, Z.X.; Zhang, Y.; Ni, Y.Q.; Tian, F.W. Oligosaccharides as co-encapsulating agents: Effect on oral Lactobacillus fermentum survival in a simulated gastrointestinal tract. Biotechnol. Lett. 2019, 41, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eratte, D.; Dowling, K.; Barrow, C.J.; Adhikari, B. Recent advances in the microencapsulation of omega-3 oil and probiotic bacteria through complex coacervation: A review. Trends Food Sci. Technol. 2018, 71, 121–131. [Google Scholar] [CrossRef]
- Huang, R.M.; Feng, K.; Li, S.F.; Zong, M.H.; Wu, H.; Han, S.Y. Enhanced survival of probiotics in the electrosprayed microcapsule by addition of fish oil. J. Food Eng. 2021, 307, 9. [Google Scholar] [CrossRef]
- Feng, K.; Huang, R.M.; Wu, R.Q.; Wei, Y.S.; Zong, M.H.; Linhardt, R.J.; Wu, H. A novel route for double-layered encapsulation of probiotics with improved viability under adverse conditions. Food Chem. 2020, 310, 7. [Google Scholar] [CrossRef]
- Rajam, R.; Karthik, P.; Parthasarathi, S.; Joseph, G.S.; Anandharamakrishnan, C. Effect of whey protein-alginate wall systems on survival of microencapsulated Lactobacillus plantarum in simulated gastrointestinal conditions. J. Funct. Foods 2012, 4, 891–898. [Google Scholar] [CrossRef]
- Feng, K.; Zhai, M.Y.; Zhang, Y.; Linhardt, R.J.; Zong, M.H.; Li, L.; Wu, H. Improved Viability and Thermal Stability of the Probiotics Encapsulated in a Novel Electrospun Fiber Mat. J. Agric. Food Chem. 2018, 66, 10890–10897. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.M.; Tian, H.F.; Luo, X.G.; Liu, S.L. Encapsulation of Lactobacillus plantarum in cellulose based microgel with controlled release behavior and increased long-term storage stability. Carbohydr. Polym. 2019, 223, 7. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Saeed, M.; Pasha, I.; Shahid, M. A prebiotic-based biopolymeric encapsulation system for improved survival of Lactobacillus rhamnosus. Food Biosci. 2020, 37, 12. [Google Scholar] [CrossRef]
- Kumherova, M.; Vesela, K.; Jokesova, K.; Klojdova, I.; Horackova, S. Influence of co-encapsulation of Bifidobacterium animalis subsp. lactis Bb12 with inulin and ascorbic acid on its viability. Czech J. Food Sci. 2020, 38, 57–62. [Google Scholar] [CrossRef]
- Peredo, A.G.; Beristain, C.I.; Pascual, L.A.; Azuara, E.; Jimenez, M. The effect of prebiotics on the viability of encapsulated probiotic bacteria. Lwt-Food Sci. Technol. 2016, 73, 191–196. [Google Scholar] [CrossRef]
- Silva, K.C.G.; Cezarino, E.C.; Michelon, M.; Sato, A.C.K. Symbiotic microencapsulation to enhance Lactobacillus acidophilus survival. Lwt-Food Sci. Technol. 2018, 89, 503–509. [Google Scholar] [CrossRef]
- Vaziri, A.S.; Alemzadeh, I.; Vossoughi, M.; Khorasani, A.C. Co-microencapsulation of Lactobacillus plantarum and DHA fatty acid in alginate-pectin-gelatin biocomposites. Carbohydr. Polym. 2018, 199, 266–275. [Google Scholar] [CrossRef]
- Premjit, Y.; Mitra, J. Optimization of Electrospray-Assisted Microencapsulation of Probiotics (Leuconostoc lactis) in Soy Protein Isolate-Oil Particles Using Box-Behnken Experimental Design. Food Bioprocess Technol. 2021, 14, 1712–1729. [Google Scholar] [CrossRef]
- Shah, A.; Gani, A.; Ahmad, M.; Ashwar, B.A.; Masoodi, F.A. beta-Glucan as an encapsulating agent: Effect on probiotic survival in simulated gastrointestinal tract. Int. J. Biol. Macromol. 2016, 82, 217–222. [Google Scholar] [CrossRef]
- Ashwar, B.A.; Gani, A.; Gani, A.; Shah, A.; Masoodi, F.A. Production of RS4 from rice starch and its utilization as an encapsulating agent for targeted delivery of probiotics. Food Chem. 2018, 239, 287–294. [Google Scholar] [CrossRef]
- Li, F.; Wang, H.; Mei, X. Preparation and characterization of phytosterol-loaded microcapsules based on the complex coacervation. J. Food Eng. 2021, 311, 110728. [Google Scholar] [CrossRef]
- Rathna, G.V.N.; Birajdar, M.S.; Bhagwani, M.; Paul, V.L. Studies on fabrication, characterization, and metal extraction using metal chelating nonwoven nanofiber mats of poly(vinyl alcohol) and sodium alginate blends. Polym. Eng. Sci. 2013, 53, 321–333. [Google Scholar] [CrossRef]
- Wang, M.W.; Yang, J.; Li, M.; Wang, Y.F.; Wu, H.; Xiong, L.; Sun, Q.J. Enhanced viability of layer-by-layer encapsulated Lactobacillus pentosus using chitosan and sodium phytate. Food Chem. 2019, 285, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.C.; Stanton, C.; Fitzgerald, G.F.; Daly, C.; Ross, R.P. Anhydrobiotics: The challenges of drying probiotic cultures. Food Chem. 2008, 106, 1406–1416. [Google Scholar] [CrossRef]
- Guedes, J.D.; Pimentel, T.C.; Diniz-Silva, H.T.; Almeida, E.T.D.; Tavares, J.F.; de Souza, E.L.; Garcia, E.F.; Magnani, M. Protective effects of beta-glucan extracted from spent brewer yeast during freeze-drying, storage and exposure to simulated gastrointestinal conditions of probiotic lactobacilli. Lwt-Food Sci. Technol. 2019, 116, 9. [Google Scholar] [CrossRef]
- Succi, M.; Sorrentino, E.; Renzo, T.D.; Tremonte, P.; Reale, A.; Tipaldi, L.; Pannella, G.; Russo, A.; Coppola, R. Lactic Acid Bacteria in Pharmaceutical Formulations: Presence and Viability of “Healthy Microorganisms”. J. Pharm.Nutr. Nutr. Sci. Sci. 2014, 4, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Mu, R.J.; Yuan, Y.; Wang, L.; Ni, Y.S.; Li, M.F.; Chen, H.B.; Pang, J. Microencapsulation of Lactobacillus acidophilus with konjac glucomannan hydrogel. Food Hydrocoll. 2018, 76, 42–48. [Google Scholar] [CrossRef]
- Eratte, D.; Wang, B.; Dowling, K.; Barrow, C.J.; Adhikari, B. Survival and fermentation activity of probiotic bacteria and oxidative stability of omega-3 oil in co-microcapsules during storage. J. Funct. Foods 2016, 23, 485–496. [Google Scholar] [CrossRef]
- Eratte, D.; Gengenbach, T.R.; Dowling, K.; Barrow, C.J.; Adhikari, B. Survival, oxidative stability, and surface characteristics of spray dried co-microcapsules containing omega-3 fatty acids and probiotic bacteria. Dry. Technol. 2016, 34, 1926–1935. [Google Scholar] [CrossRef]


| Storage at 4 °C | |||||
|---|---|---|---|---|---|
| Time (Days) | Free | SPI-L-P | SPI-F-L-P | SPI-O-L-P | SPI-O-F-L-P |
| 0 | 9.36 ± 0.17 aA | 8.32 ± 0.23 aC | 8.70 ± 0.27 aBC | 8.77 ± 0.27 aB | 8.82 ± 0.22 aB |
| 15 | 7.15 ± 0.21 bB | 7.22 ± 0.28 bB | 8.15 ± 0.11 bA | 8.29 ± 0.12 bA | 8.36 ± 0.06 bA |
| 30 | 7.08 ± 0.08 bC | 7.13 ± 0.08 bcC | 7.74 ± 0.11 cB | 7.88 ± 0.19 cAB | 8.01 ± 0.14 cA |
| 60 | 5.89 ± 0.14 cD | 7.08 ± 0.06 bcC | 7.63 ± 0.23 cB | 7.82 ± 0.13 cAB | 7.98 ± 0.09 cA |
| 90 | 4.70 ± 0.24 dD | 6.90 ± 0.15 cC | 7.54 ± 0.13 cB | 7.77 ± 0.08 cAB | 7.92 ± 0.21 cA |
| Storage at 25 °C | |||||
| Time (Days) | Free | SPI-L-P | SPI-F-L-P | SPI-O-L-P | SPI-O-F-L-P |
| 0 | 9.36 ± 0.17 aA | 8.32 ± 0.23 aC | 8.70 ± 0.27 aBC | 8.77 ± 0.27 aB | 8.82 ± 0.22 aB |
| 15 | 6.89 ± 0.16 bB | 7.08 ± 0.31 bB | 7.44 ± 0.08 bA | 7.77 ± 0.19 bA | 7.78 ± 0.26 bA |
| 30 | 5.75 ± 0.10 cC | 6.17 ± 0.16 cBC | 6.38 ± 0.21 cA | 6.04 ± 0.18 cB | 6.27 ± 0.27 cA |
| 60 | 4.86 ± 0.10 dC | 5.75 ± 0.21 dB | 6.09 ± 0.07 dA | 5.81 ± 0.13 cB | 6.13 ± 0.09 cA |
| 90 | 2.49 ± 0.09 eC | 5.38 ± 0.11 eB | 5.73 ± 0.13 eA | 5.32 ± 0.15 dB | 5.68 ± 0.12 dA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.-F.; Feng, K.; Huang, R.-M.; Wei, Y.-S.; Wu, H. Insights into Protective Effects of Different Synbiotic Microcapsules on the Survival of Lactiplantibacillus plantarum by Electrospraying. Foods 2022, 11, 3872. https://doi.org/10.3390/foods11233872
Li S-F, Feng K, Huang R-M, Wei Y-S, Wu H. Insights into Protective Effects of Different Synbiotic Microcapsules on the Survival of Lactiplantibacillus plantarum by Electrospraying. Foods. 2022; 11(23):3872. https://doi.org/10.3390/foods11233872
Chicago/Turabian StyleLi, Shu-Fang, Kun Feng, Ru-Meng Huang, Yun-Shan Wei, and Hong Wu. 2022. "Insights into Protective Effects of Different Synbiotic Microcapsules on the Survival of Lactiplantibacillus plantarum by Electrospraying" Foods 11, no. 23: 3872. https://doi.org/10.3390/foods11233872
APA StyleLi, S. -F., Feng, K., Huang, R. -M., Wei, Y. -S., & Wu, H. (2022). Insights into Protective Effects of Different Synbiotic Microcapsules on the Survival of Lactiplantibacillus plantarum by Electrospraying. Foods, 11(23), 3872. https://doi.org/10.3390/foods11233872