The Effects of Soluble Dietary Fibers on Glycemic Response: An Overview and Futures Perspectives
Abstract
:1. Introduction
| SDF | Main Polymer | Main Monomers | Linkage | Viscosity | Source | Reference |
|---|---|---|---|---|---|---|
| Alginate | Mannuronic acid and guluronic acid | β-(1,4) D-mannuronic acid or α-(1,4) L-guluronic acid | Viscous | Cell walls from brown seaweed (Phaeophyceae) | Garcia-Cruz et al., 2008 [10] | |
| Arabinoxylan | Arabinose and xylose | β-D-(1,4) xylose | Viscous | Cereal grains | Elleuch et al., 2011 [11] | |
| Psyllium | Arabinoxylan | Arabinose and xylose | β-(1,4) linked D-xylopyranosyl residues (Polymer of arabinoxylans with 1,4 and 1,3 linkages) | Viscous | Plantago ovata (seeds) | Fischer et al., 2004 [12] |
| β-glucan | Glucose | β-(1,4) and β-(1,3) glucose | Viscous | Oat, barley, yeast, algae | Elleuch et al., 2011 [11] | |
| Galactomannan | Mannose and galactose | β-(1,4) mannopyranose, β-(1,6) D-galactopyranosyl | Viscous | Seed gums from leguminous plants and microbial sources (yeast and fungi) | Srivastava; Kapoor, 2005 [13] | |
| Guar gum | Galactomannan | Mannose and galactose | β-(1,4) linked D-mannopyranosyl units with α-(1,6)-linked D-galactopyranosyl residues as side chains | Viscous | Cyamopsis tetragonolobus shrub | Mudgill et al., 2018 [14] |
| Inulin | Fructose, glucose | β-(2,1) D fructosyl-fructose | Non-viscous | Chicory (root), onion, garlic | Elleuch et al., 2011 [11] | |
| Konjak gum | Glucomannan | Glucose e mannose | β-(1,4) D-glucose and D-mannose, β-(1,6)-glucosyl | Viscous | Amorphophallus konja (tubérculo) | Chua et al., 2010 [15] |
| Pectin | Galacturonic acid and rhamnose, | α-(1,4) linked D-galacturonic acid, (1,2) linked L-rhamnose | Viscous | Citrus peel, apple pomace, sugar beet pulp | Voragen et al., 2009 [16] | |
| Pullulan | Glucose | three α-1,4-linked glucose polymerized by α-1,6 linkages on the terminal glucose, resulting in a stair-step structure | Low viscosity | Secreted by the fungus Aureobasidium pullulans | Wolf et al., 2003 [17] | |
| Resistant maltodextrin (RD) | Dextrose | α-(1,4) D-glucose, α-(1,6) D-glucose | Low viscosity | Heat and enzymatic treatment of starch | Elleuch et al., 2011 [11] | |
| Soluble corn fiber | RD | Dextrose, fructose | α-(1,4) glucose α-(1,6) D-glucose | Low viscosity | Product from enzymatic hydrolysis of cornstarch | Harrison; Hoffman, 2007 [18] |
| Nutriose | RD | Dextrose | α-(1,4) glucose α-(1,6) D-glucose and α-1,6 and (or) β-1,6; a-1,2 and (or) β-1,2; α-1,3 and (or) β-1,3; and β-1,4 | Non-viscous | RD obtained from wheat, maize, or other edible starch | Hobden et al., 2021 [19] Li et al., 2010 [20] |
| Polydextrose | Glucose | α- and β-(1,2) (1,3) (1,4) (1,6) D-glucose, with a predominance of α and β (1.6) | Viscous | PDX is a highly branched, randomly bonded synthetic glucose polymer | do Carmo et al., 2016 [21] | |
| Xanthan gum | Glucose, mannose, glucuronic acid | β-(1–4) D-glucose | Viscous | Fermentation by Xanthomonas campestris | Bhat et al., 2022 [22] |
Mechanisms for Reducing the Glycemic Response
2. Methodology
3. Human Studies with Dietary Fibers and Glycemic Response
3.1. Alginate
3.2. Arabinoxylan and Psyllium (Arabinoxylan Source)
3.3. Arabinoxylan (AX) and β-glucan
3.4. β-glucan
3.5. Pleurotus spp.
3.6. Guar Gum and Galactomannans
3.7. Konjac Gum (Glucomannan)
3.8. Pectin
3.9. Pullulan
3.10. Resistant Dextrins
3.11. Xanthan Gum
3.12. Soluble Dietary Fibers (Various)
4. Glycemic Response to Bread
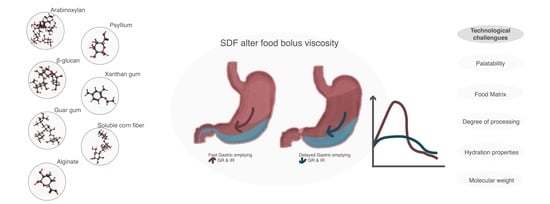
5. Technological Aspects and Challenges
6. General Considerations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- CAC. Codex Alimentarius Commission Guidelines on Nutrition Labelling CAC/GL 2—1985 as Last Amended 2021; Codex Alimentarius Commission. Joint FAO/WHO Food Standards Programme; FAO: Rome, Italy, 2021. [Google Scholar]
- Menezes, E.; Giuntini, E.; Dan, M.; Hoffmann Sarda, F.; Lajolo, F. Codex dietary fibre definition—Justification for inclusion of carbohydrates from 3 to 9 degrees of polymerisation. Food Chem. 2013, 140, 581–585. [Google Scholar] [CrossRef]
- Tungland, B.C.; Meyer, D. Nondigestible oligo- and polysaccharides (dietary fiber): Their physiology and role in human health and food. Compr. Rev. Food Sci. Food Saf. 2002, 1, 90–109. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.; Ryden, P.; Edwards, C.H.; Grundy, M.M.-L. Plant cell walls: Impact on nutrient bioaccessibility and digestibility. Foods 2020, 9, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, M.; Dou, B.; Pugh, J.E.; Lett, A.M.; Frost, G.S. The impact of starchy food structure on postprandial glycemic response and appetite: A systematic review with meta-analysis of randomized crossover trials. Am. J. Clin. Nutr. 2021, 114, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Grundy, M.M.-L.; Edwards, C.H.; Mackie, A.R.; Gidley, M.J.; Butterworth, P.J.; Ellis, P.R. Re-evaluation of the mechanisms of dietary fibre and implications for macronutrient bioaccessibility, digestion and postprandial metabolism. Br. J. Nutr. 2016, 116, 816–833. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Standards of medical care in diabetes—2022 abridged for primary care providers. Clin. Diabetes 2022, 40, 10–38. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Koh, E.S.; Kim, J.E. Lowering breakfast glycemic index and glycemic load attenuates postprandial glycemic response: A systematically searched meta-analysis of randomized controlled trials. Nutrition 2020, 71, 110634. [Google Scholar] [CrossRef]
- Fardet, A.; Leenhardt, F.; Lioger, D.; Scalbert, A.; Rémésy, C. Parameters controlling the glycaemic response to breads. Nutr. Res. Rev. 2006, 19, 18–25. [Google Scholar] [CrossRef]
- Garcia-Cruz, C.H.; Foggetti, U.; da Silva, A.N. Bacterial alginate: Technological aspects, characteristics and production. Quím. Nova 2008, 31, 1800–1806. [Google Scholar] [CrossRef] [Green Version]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Fischer, M.H.; Yu, N.; Gray, G.R.; Ralph, J.; Anderson, L.; Marlett, J.A. The gel-forming polysaccharide of psyllium husk (Plantago Ovata Forsk). Carbohydr. Res. 2004, 339, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Kapoor, V.P. Seed galactomannans: An overview. Chem. Biodivers. 2005, 2, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D. Partially Hydrolyzed Guar Gum: Preparation and Properties|SpringerLink. Available online: https://link.springer.com/chapter/10.1007/978-3-319-94625-2_20 (accessed on 22 June 2022).
- Chua, M.; Baldwin, T.C.; Hocking, T.J.; Chan, K. Traditional uses and potential health benefits of Amorphophallus konjac K. Koch ex N.E.Br. J. Ethnopharmacol. 2010, 128, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Wolf, B.W.; Garleb, K.A.; Choe, Y.S.; Humphrey, P.M.; Maki, K.C. Pullulan is a slowly digested carbohydrate in humans. J. Nutr. 2003, 133, 1051–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamaker, B.R.; Venktachalam, M.; Zhang, G.; Keshavarzian, A.; Rose, D.J. Slowly Digesting Starch and Fermentable Fiber Patent. U.S. Patent 8,557,274, 15 October 2013. [Google Scholar]
- Hobden, M.R.; Commane, D.M.; Guérin-Deremaux, L.; Wils, D.; Thabuis, C.; Martin-Morales, A.; Wolfram, S.; Dìaz, A.; Collins, S.; Morais, I.; et al. Impact of dietary supplementation with resistant dextrin (NUTRIOSE®) on satiety, glycaemia, and related endpoints, in healthy adults. Eur. J. Nutr. 2021, 60, 4635–4643. [Google Scholar] [CrossRef]
- Li, S.; Guerin-Deremaux, L.; Pochat, M.; Wils, D.; Reifer, C.; Miller, L.E. NUTRIOSE dietary fiber supplementation improves insulin resistance and determinants of metabolic syndrome in overweight men: A double-blind, randomized, placebo-controlled study. Appl. Physiol. Nutr. Metab. 2010, 35, 773–782. [Google Scholar] [CrossRef]
- do Carmo, M.M.R.; Walker, J.C.L.; Novello, D.; Caselato, V.M.; Sgarbieri, V.C.; Ouwehand, A.C.; Andreollo, N.A.; Hiane, P.A.; Dos Santos, E.F. Polydextrose: Physiological function, and effects on health. Nutrients 2016, 8, 553. [Google Scholar] [CrossRef]
- Bhat, I.M.; Wani, S.M.; Mir, S.A.; Masoodi, F.A. Advances in xanthan gum production, modifications and its applications. Biocatal. Agric. Biotechnol. 2022, 42, 102328. [Google Scholar] [CrossRef]
- Mahmood, N. A review of α-amylase inhibitors on weight loss and glycemic control in pathological state such as obesity and diabetes. Comp. Clin. Pathol. 2016, 25, 1253–1264. [Google Scholar] [CrossRef]
- Balisteiro, D.M.; de Araujo, R.L.; Giacaglia, L.R.; Genovese, M.I. Effect of clarified brazilian native fruit juices on postprandial glycemia in healthy subjects. Food Res. Int. 2017, 100, 196–203. [Google Scholar] [CrossRef]
- Qin, W.; Sun, L.; Miao, M.; Zhang, G. Plant-sourced intrinsic dietary fiber: Physical structure and health function. Trends Food Sci. Technol. 2021, 118, 341–355. [Google Scholar] [CrossRef]
- Silva, F.M.; Kramer, C.K.; de Almeida, J.C.; Steemburgo, T.; Gross, J.L.; Azevedo, M.J. Fiber intake and glycemic control in patients with type 2 diabetes mellitus: A systematic review with meta-analysis of randomized controlled trials. Nutr. Rev. 2013, 71, 790–801. [Google Scholar] [CrossRef]
- Cassidy, Y.M.; McSorley, E.M.; Allsopp, P.J. Effect of soluble dietary fibre on postprandial blood glucose response and its potential as a functional food ingredient. J. Funct. Foods 2018, 46, 423–439. [Google Scholar] [CrossRef]
- Tosh, S.M.; Brummer, Y.; Miller, S.S.; Regand, A.; Defelice, C.; Duss, R.; Wolever, T.M.S.; Wood, P.J. Processing affects the physicochemical properties of β-glucan in oat bran cereal. J. Agric. Food Chem. 2010, 58, 7723–7730. [Google Scholar] [CrossRef]
- Cesbron-Lavau, G.; Goux, A.; Atkinson, F.; Meynier, A.; Vinoy, S. Deep dive into the effects of food processing on limiting starch digestibility and lowering the glycemic response. Nutrients 2021, 13, 381. [Google Scholar] [CrossRef]
- McRorie, J.W.; McKeown, N.M. Understanding the physics of functional fibers in the gastrointestinal tract: An evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, D.J.; Wolever, T.M.; Leeds, A.R.; Gassull, M.A.; Haisman, P.; Dilawari, J.; Goff, D.V.; Metz, G.L.; Alberti, K.G. Dietary fibres, fibre analogues, and glucose tolerance: Importance of viscosity. Br. Med. J. 1978, 1, 1392–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dall’Alba, V.; Silva, F.M.; Antonio, J.P.; Steemburgo, T.; Royer, C.P.; Almeida, J.C.; Gross, J.L.; Azevedo, M.J. Improvement of the metabolic syndrome profile by soluble fibre–guar gum–in patients with type 2 diabetes: A randomised clinical trial. Br. J. Nutr. 2013, 110, 1601–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abutair, A.S.; Naser, I.A.; Hamed, A.T. Soluble fibers from psyllium improve glycemic response and body weight among diabetes type 2 patients (randomized control trial). Nutr. J. 2016, 15, 86. [Google Scholar] [CrossRef]
- Soltanian, N.; Janghorbani, M. Effect of flaxseed or psyllium vs. placebo on management of constipation, weight, glycemia, and lipids: A randomized trial in constipated patients with type 2 diabetes. Clin. Nutr. ESPEN 2019, 29, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Feinglos, M.N.; Gibb, R.D.; Ramsey, D.L.; Surwit, R.S.; McRorie, J.W. Psyllium improves glycemic control in patients with type-2 diabetes mellitus. Bioact. Carbohydr. Diet. Fibre 2013, 1, 156–161. [Google Scholar] [CrossRef]
- Karthikeyan, J.S.; Salvi, D.; Corradini, M.G.; Ludescher, R.D.; Karwe, M.V. Effect of bolus viscosity on carbohydrate digestion and glucose absorption processes: An in vitro study. Phys. Fluids 2019, 31, 111905. [Google Scholar] [CrossRef]
- McRorie, J.W. Evidence-based approach to fiber supplements and clinically meaningful health benefits, part 1: What to look for and how to recommend an effective fiber therapy. Nutr. Today 2015, 50, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Maljaars, P.W.J.; Peters, H.P.F.; Mela, D.J.; Masclee, A.A.M. Ileal brake: A sensible food target for appetite control. A review. Physiol. Behav. 2008, 95, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, N.; Suzuki, Y.; Urashima, M.; Nakayoshi, T.; Tsuboi, K.; Tanishima, Y.; Hanyu, N.; Kashiwagi, H. Effect of gelatinization on gastric emptying and absorption. Hepatogastroenterology 2008, 55, 1843–1845. [Google Scholar] [PubMed]
- Kendall, C.W.C.; Esfahani, A.; Hoffman, A.J.; Evans, A.; Sanders, L.M.; Josse, A.R.; Vidgen, E.; Potter, S.M. Effect of novel maize-based dietary fibers on postprandial glycemia and insulinemia. J. Am. Coll. Nutr. 2008, 27, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Konings, E.; Schoffelen, P.F.; Stegen, J.; Blaak, E.E. Effect of polydextrose and soluble maize fibre on energy metabolism, metabolic profile and appetite control in overweight men and women. Br. J. Nutr. 2014, 111, 111–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Salhy, M.; Ystad, S.O.; Mazzawi, T.; Gundersen, D. Dietary fiber in irritable bowel syndrome (review). Int. J. Mol. Med. 2017, 40, 607–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, C.; Swanson, K.S.; Fahey, G.C.; Garleb, K.A. Perspective: Physiologic importance of short-chain fatty acids from nondigestible carbohydrate fermentation. Adv. Nutr. Int. Rev. J. 2019, 10, 576–589. [Google Scholar] [CrossRef]
- Lehmann, A.; Hornby, P.J. Intestinal SGLT1 in metabolic health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G887–G898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Yang, H.; Huang, H.; Zhang, C.; Zuo, H.-X.; Xu, P.; Niu, Y.-M.; Wu, S.-S. Inulin-type fructans supplementation improves glycemic control for the prediabetes and type 2 diabetes populations: Results from a GRADE-assessed systematic review and dose-response meta-analysis of 33 randomized controlled trials. J. Transl. Med. 2019, 17, 410. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, D.; Goff, H.D.; Berengut, S.; Kubant, R.; Anderson, G.H. Effect of sodium alginate addition to chocolate milk on glycemia, insulin, appetite and food intake in healthy adult men. Eur. J. Clin. Nutr. 2014, 68, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Harden, C.J.; Craig Richardson, J.; Dettmar, P.W.; Corfe, B.M.; Paxman, J.R. An ionic-gelling alginate drink attenuates postprandial glycaemia in males. J. Funct. Foods 2012, 4, 122–128. [Google Scholar] [CrossRef]
- Georg Jensen, M.; Kristensen, M.; Belza, A.; Knudsen, J.C.; Astrup, A. Acute effect of alginate-based preload on satiety feelings, energy intake, and gastric emptying rate in healthy subjects. Obesity 2012, 20, 1851–1858. [Google Scholar] [CrossRef]
- Torsdottir, I.; Alpsten, M.; Holm, G.; Sandberg, A.S.; Tölli, J. A small dose of soluble alginate-fiber affects postprandial glycemia and gastric emptying in humans with diabetes. J. Nutr. 1991, 121, 795–799. [Google Scholar] [CrossRef]
- Kato, T.; Idota, Y.; Shiragami, K.; Koike, M.; Nishibori, F.; Tomokane, M.; Seki, T.; Itabashi, K.; Hakoda, K.; Takahashi, H.; et al. Randomized, double-blind, crossover clinical trial of the effect of calcium alginate in noodles on postprandial blood glucose level. Biol. Pharm. Bull. 2018, 41, 1367–1371. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Wang, Y.; Shafer, R.; Winn, N.C.; Kanaley, J.A.; Vardhanabhuti, B. Glycemic effects following the consumption of mixed soy protein isolate and alginate beverages in healthy adults. Food Funct. 2019, 10, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.X.; Walker, K.Z.; Muir, J.G.; Mascara, T.; O’Dea, K. Arabinoxylan fiber, a byproduct of wheat flour processing, reduces the postprandial glucose response in normoglycemic subjects. Am. J. Clin. Nutr. 2000, 71, 1123–1128. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.X.; Walker, K.Z.; Muir, J.G.; O’Dea, K. Arabinoxylan fibre improves metabolic control in people with type II diabetes. Eur. J. Clin. Nutr. 2004, 58, 621–628. [Google Scholar] [CrossRef]
- Möhlig, M.; Koebnick, C.; Weickert, M.O.; Lueder, W.; Otto, B.; Steiniger, J.; Twilfert, M.; Meuser, F.; Pfeiffer, A.F.H.; Zunft, H.J. Arabinoxylan-enriched meal increases serum ghrelin levels in healthy humans. Horm. Metab. Res. 2005, 37, 303–308. [Google Scholar] [CrossRef]
- Noureddin, S.; Mohsen, J.; Payman, A. Effects of psyllium vs. placebo on constipation, weight, glycemia, and lipids: A randomized trial in patients with type 2 diabetes and chronic constipation. Complement. Ther. Med. 2018, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.L.; Otto, B.; Reich, S.-C.; Weickert, M.O.; Steiniger, J.; Machowetz, A.; Rudovich, N.N.; Möhlig, M.; Katz, N.; Speth, M.; et al. Arabinoxylan consumption decreases postprandial serum glucose, serum insulin and plasma total ghrelin response in subjects with impaired glucose tolerance. Eur. J. Clin. Nutr. 2007, 61, 334–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, A.L.; Steiniger, J.; Reich, S.C.; Weickert, M.O.; Harsch, I.; Machowetz, A.; Mohlig, M.; Spranger, J.; Rudovich, N.N.; Meuser, F.; et al. Arabinoxylan fibre consumption improved glucose metabolism, but did not affect serum adipokines in subjects with impaired glucose tolerance. Horm. Metab. Res. 2006, 38, 761–766. [Google Scholar] [CrossRef]
- Gibb, R.D.; McRorie, J.W.; Russell, D.A.; Hasselblad, V.; D’Alessio, D.A. Psyllium fiber improves glycemic control proportional to loss of glycemic control: A meta-analysis of data in euglycemic subjects, patients at risk of type 2 diabetes mellitus, and patients being treated for type 2 diabetes mellitus. Am. J. Clin. Nutr. 2015, 102, 1604–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fratelli, C.; Muniz, D.G.; Santos, F.G.; Capriles, V.D. Modelling the effects of psyllium and water in gluten-free bread: An approach to improve the bread quality and glycemic response. J. Funct. Foods 2018, 42, 339–345. [Google Scholar] [CrossRef]
- Santos, F.G.; Aguiar, E.V.; Rosell, C.M.; Capriles, V.D. Potential of chickpea and psyllium in gluten-free breadmaking: Assessing bread’s quality, sensory acceptability, and glycemic and satiety indexes. Food Hydrocoll. 2021, 113, 106487. [Google Scholar] [CrossRef]
- Johansson, L.; Tuomainen, P.; Ylinen, M.; Ekholm, P.; Virkki, L. Structural analysis of water-soluble and -insoluble β-glucans of whole-grain oats and barley. Carbohydr. Polym. 2004, 58, 267–274. [Google Scholar] [CrossRef]
- Hartvigsen, M.L.; Gregersen, S.; Lærke, H.N.; Holst, J.J.; Bach Knudsen, K.E.; Hermansen, K. Effects of concentrated arabinoxylan and β-glucan compared with refined wheat and whole grain rye on glucose and appetite in subjects with the metabolic syndrome: A randomized study. Eur. J. Clin. Nutr. 2014, 68, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Hartvigsen, M.L.; Lærke, H.N.; Overgaard, A.; Holst, J.J.; Bach Knudsen, K.E.; Hermansen, K. Postprandial effects of test meals including concentrated arabinoxylan and whole grain rye in subjects with the metabolic syndrome: A randomised study. Eur. J. Clin. Nutr. 2014, 68, 567–574. [Google Scholar] [CrossRef]
- Liljeberg, H.G.; Granfeldt, Y.E.; Björck, I.M. products based on a high fiber barley genotype, but not on common barley or oats, lower postprandial glucose and insulin responses in healthy humans. J. Nutr. 1996, 126, 458–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Östman, E.; Rossi, E.; Larsson, H.; Brighenti, F.; Björck, I. Glucose and insulin responses in healthy men to barley bread with different levels of (1→3;1→4)-β-glucans; predictions using fluidity measurements of in vitro enzyme digests. J. Cereal Sci. 2006, 43, 230–235. [Google Scholar] [CrossRef]
- Granfeldt, Y.; Hagander, B.; Björck, I. Metabolic responses to starch in oat and wheat products. on the importance of food structure, incomplete gelatinization or presence of viscous dietary fibre. Eur. J. Clin. Nutr. 1995, 49, 189–199. [Google Scholar] [PubMed]
- Cavallero, A.; Empilli, S.; Brighenti, F.; Stanca, A.M. High (1→3,1→4)-β-glucan barley fractions in bread making and their effects on human glycemic response. J. Cereal Sci. 2002, 36, 59–66. [Google Scholar] [CrossRef]
- Regand, A.; Chowdhury, Z.; Tosh, S.M.; Wolever, T.M.S.; Wood, P. The molecular weight, solubility and viscosity of oat beta-glucan affect human glycemic response by modifying starch digestibility. Food Chem. 2011, 129, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Kwong, M.G.Y.; Wolever, T.M.S.; Brummer, Y.; Tosh, S.M. Increasing the viscosity of oat β-glucan beverages by reducing solution volume does not reduce glycaemic responses. Br. J. Nutr. 2013, 110, 1465–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaremba, S.M.M.; Gow, I.F.; Drummond, S.; McCluskey, J.T.; Steinert, R.E. Effects of oat β-glucan consumption at breakfast on ad libitum eating, appetite, glycemia, insulinemia and glp-1 concentrations in healthy subjects. Appetite 2018, 128, 197–204. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Mattila, O.; Rosa-Sibakov, N.; Tosh, S.M.; Jenkins, A.L.; Ezatagha, A.; Duss, R.; Steinert, R.E. Effect of varying molecular weight of oat β-glucan taken just before eating on postprandial glycemic response in healthy humans. Nutrients 2020, 12, 2275. [Google Scholar] [CrossRef]
- Ames, N.; Malunga, L.N.; Mollard, R.; Johnson, J.; Chu, Y.; Thandapilly, S.J. Effect of processing on oat β-glucan viscosity, postprandial glycemic response and subjective measures of appetite. Food Funct. 2021, 12, 3672–3679. [Google Scholar] [CrossRef]
- Bozbulut, R.; Şanlıer, N.; Döğer, E.; Bideci, A.; Çamurdan, O.; Cinaz, P. The effect of beta-glucan supplementation on glycemic control and variability in adolescents with type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 2020, 169, 108464. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Raederstorff, D.; Wolever, T.M.S. Effect of consuming oat bran mixed in water before a meal on glycemic responses in healthy humans—A pilot study. Nutrients 2016, 8, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolever, T.M.S.; Tosh, S.M.; Spruill, S.E.; Jenkins, A.L.; Ezatagha, A.; Duss, R.; Johnson, J.; Chu, Y.; Steinert, R.E. Increasing oat β-glucan viscosity in a breakfast meal slows gastric emptying and reduces glycemic and insulinemic responses but has no effect on appetite, food intake, or plasma ghrelin and PYY responses in healthy humans: A randomized, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2020, 111, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Peressini, D.; Cavarape, A.; Brennan, M.A.; Gao, J.; Brennan, C.S. Viscoelastic properties of durum wheat doughs enriched with soluble dietary fibres in relation to pasta-making performance and glycaemic response of spaghetti. Food Hydrocoll. 2020, 102, 105613. [Google Scholar] [CrossRef]
- Telle-Hansen, V.H.; Gaundal, L.; Høgvard, B.; Ulven, S.M.; Holven, K.B.; Byfuglien, M.G.; Måge, I.; Knutsen, S.H.; Ballance, S.; Rieder, A.; et al. A three-day intervention with granola containing cereal beta-glucan improves glycemic response and changes the gut microbiota in healthy individuals: A crossover study. Front. Nutr. 2022, 9, 796362. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Míčková, K.; Jablonský, I.; Sluková, M.; Čopíková, J. Mushrooms of genus pleurotus as a source of dietary fibres and glucans for food supplements. Czech J. Food Sci. 2009, 26, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, R.P.; Chopra, A.; Lavekar, G.S.; Padhi, M.M.; Srikanth, N.; Ota, S.; Jain, S. Effect of oyster mushroom on glycemia, lipid profile and quality of life in type 2 diabetic patients. Aust. J. Med. Herbal. 2010, 22, 50–54. [Google Scholar]
- Jayasuriya, W.J.A.B.N.; Wanigatunge, C.A.; Fernando, G.H.; Abeytunga, D.T.U.; Suresh, T.S. Hypoglycaemic activity of culinary Pleurotus ostreatus and P. Cystidiosus mushrooms in healthy volunteers and type 2 diabetic patients on diet control and the possible mechanisms of action. Phytother. Res. PTR 2015, 29, 303–309. [Google Scholar] [CrossRef]
- Ng, S.H.; Robert, S.D.; Wan Ahmad, W.A.N.; Wan Ishak, W.R. Incorporation of dietary fibre-rich oyster mushroom (Pleurotus sajor-caju) powder improves postprandial glycaemic response by interfering with starch granule structure and starch digestibility of biscuit. Food Chem. 2017, 227, 358–368. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, M.; Rahman, T.; Kakon, A.; Hoque, N.; Akhtaruzzaman, M.; Begum, M.; Choudhuri, M.; Hossain, M. Effects of Pleurotus ostreatus on blood pressure and glycemic status of hypertensive diabetic male volunteers. Bangladesh J. Med. Biochem. 2013, 6, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Kleftaki, S.-A.; Simati, S.; Amerikanou, C.; Gioxari, A.; Tzavara, C.; Zervakis, G.I.; Kalogeropoulos, N.; Kokkinos, A.; Kaliora, A.C. Pleurotus eryngii improves postprandial glycaemia, hunger and fullness perception, and enhances ghrelin suppression in people with metabolically unhealthy obesity. Pharmacol. Res. 2022, 175, 105979. [Google Scholar] [CrossRef]
- Ishak, W.R.W.W. Does oyster mushroom (Pleurotus sajorcaju) powder addition improves nutrient composition, sensory acceptability and glycaemic index (GI) of flatbread (Tortilla)? Kuwait J. Sci. 2021, 48, 1–13. [Google Scholar] [CrossRef]
- Ellis, P.R.; Apling, E.C.; Leeds, A.R.; Bolster, N.R. Guar bread: Acceptability and efficacy combined. Studies on blood glucose, serum insulin and satiety in normal subjects. Br. J. Nutr. 1981, 46, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Galali, Y.; Rees, G.; Kuri, V. The impact of waxy wheat flour, inulin and guar gum on post-prandial glycaemic and satiety indices, sensory attributes and shelf life of tandoori and pita breads. Appl. Sci. 2022, 12, 3355. [Google Scholar] [CrossRef]
- Roberts, K.T. The physiological and rheological effects of foods supplemented with guar gum. Food Res. Int. 2011, 44, 1109–1114. [Google Scholar] [CrossRef]
- Chearskul, S.; Sangurai, S.; Nitiyanant, W.; Kriengsinyos, W.; Kooptiwut, S.; Harindhanavudhi, T. Glycemic and lipid responses to glucomannan in thais with type 2 diabetes mellitus. J. Med. Assoc. Thail. 2007, 90, 2150–2157. [Google Scholar]
- Cheang, K.-U.; Chen, C.-M.; Oliver Chen, C.-Y.; Liang, F.-Y.; Shih, C.-K.; Li, S.-C. Effects of glucomannan noodle on diabetes risk factors in patients with metabolic syndrome: A double-blinded, randomized crossover controlled trial. J. Food Nutr. Res. 2017, 5, 622–628. [Google Scholar] [CrossRef] [Green Version]
- Ellis, P.R.; Dawoud, F.M.; Morris, E.R. Blood glucose, plasma insulin and sensory responses to guar-containing wheat breads: Effects of molecular weight and particle size of guar gum. Br. J. Nutr. 1991, 66, 363–379. [Google Scholar] [CrossRef] [Green Version]
- Wolf, B.W.; Wolever, T.M.S.; Lai, C.S.; Bolognesi, C.; Radmard, R.; Maharry, K.S.; Garleb, K.A.; Hertzler, S.R.; Firkins, J.L. Effects of a beverage containing an enzymatically induced-viscosity dietary fiber, with or without fructose, on the postprandial glycemic response to a high glycemic index food in humans. Eur. J. Clin. Nutr. 2003, 57, 1120–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.A.; Lai, C.-S.; Corwin, H.; Ma, Y.; Maki, K.C.; Garleb, K.A.; Wolf, B.W. Inclusion of guar gum and alginate into a crispy bar improves postprandial glycemia in humans. J. Nutr. 2004, 134, 886–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayakawa, A.; Suzuki, T.; Ikami, T.; Saito, M.; Yabe, D.; Seino, Y. Improvement of fasting plasma glucose level after ingesting moderate amount of dietary fiber in japanese men with mild hyperglycemia and visceral fat obesity. J. Diet. Suppl. 2013, 10, 129–141. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Jenkins, D.J.A.; Nineham, R.; Alberti, K.G.M.M. Guar gum and reduction of post-prandial glycaemia: Effect of incorporation into solid food, liquid food, and both. Br. J. Nutr. 1979, 41, 505–510. [Google Scholar] [CrossRef]
- Paquet, É.; Bédard, A.; Lemieux, S.; Turgeon, S.L. Effects of Apple Juice-Based Beverages Enriched with Dietary Fibres and Xanthan Gum on the Glycemic Response and Appetite Sensations in Healthy Men. Bioact. Carbohydr. Diet. Fibre 2014, 4, 39–47. [Google Scholar] [CrossRef]
- O’Connor, L.E.; Campbell, W.W. A Novel Fiber Composite Ingredient Incorporated into a Beverage and Bar Blunts Postprandial Serum Glucose and Insulin Responses: A Randomized Controlled Trial. Nutr. Res. 2016, 36, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Jovanovski, E.; Khayyat, R.; Zurbau, A.; Komishon, A.; Mazhar, N.; Sievenpiper, J.L.; Blanco Mejia, S.; Ho, H.V.T.; Li, D.; Jenkins, A.L.; et al. Should viscous fiber supplements be considered in diabetes control? Results from a systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2019, 42, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Watson, L.E.; Phillips, L.K.; Wu, T.; Bound, M.J.; Checklin, H.; Grivell, J.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Title: Differentiating the effects of whey protein and guar gum preloads on postprandial glycemia in type 2 diabetes. Clin. Nutr. 2019, 38, 2827–2832. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.; Holen, I.S.; Phillips, L.K.; Hatzinikolas, S.; Huynh, L.Q.; Wu, T.; Hausken, T.; Rayner, C.K.; Horowitz, M.; Jones, K.L. The effects of a whey protein and guar gum-containing preload on gastric emptying, glycaemia, small intestinal absorption and blood pressure in healthy older subjects. Nutrients 2019, 11, 2666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Zhou, Y.; Yuan, Y.; Fan, Z.; Wu, Y.; Liu, A.; Lu, X. Potato preload mitigated postprandial glycemic excursion in healthy subjects: An acute randomized trial. Nutrients 2020, 12, 2759. [Google Scholar] [CrossRef]
- Mirzababaei, A.; Zandkarimi, R.; Moradi, S.; Rasaei, N.; Amini, M.R.; Pourreza, S.; Abaj, F.; Clark, C.C.T.; Daneshzad, E.; Mirzaei, K. The effect of glucomannan on fasting and postprandial blood glucose in adults: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Metab. Disord. 2022, 21, 1055–1063. [Google Scholar] [CrossRef]
- Lee, S.-W.; Ro, H.-K.; Choi, I.-S.; Oh, S.-H. Effects of cellulose and pectin on postprandial blood glucose and plasma lipid concentration. Korean J. Nutr. 2006, 39, 244–251. [Google Scholar]
- Yamina, M.; Zahia, B.; Oumelkeir, S. Classification of some date cultivars algerian soft consistency according to their glycemic index. Appl. Biol. Sahar. Areas 2021, 3, 42–53. [Google Scholar]
- Spears, J.K.; Karr-Lilienthal, L.K.; Grieshop, C.M.; Flickinger, E.A.; Wolf, B.W.; Fahey, G.C. Glycemic, insulinemic, and breath hydrogen responses to pullulan in healthy humans. Nutr. Res. 2005, 25, 1029–1041. [Google Scholar] [CrossRef]
- Peters, H.P.F.; Ravestein, P.; van der Hijden, H.T.W.M.; Boers, H.M.; Mela, D.J. Effect of carbohydrate digestibility on appetite and its relationship to postprandial blood glucose and insulin levels. Eur. J. Clin. Nutr. 2011, 65, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livesey, G.; Tagami, H. Interventions to lower the glycemic response to carbohydrate foods with a low-viscosity fiber (resistant maltodextrin): Meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2009, 89, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Farhangi, M.A.; Dehghan, P.; Namazi, N. Prebiotic supplementation modulates advanced glycation end-products (AGEs), soluble receptor for AGEs (SRAGE), and cardiometabolic risk factors through improving metabolic endotoxemia: A randomized-controlled clinical trial. Eur. J. Nutr. 2020, 59, 3009–3021. [Google Scholar] [CrossRef] [PubMed]
- Lefranc-Millot, C.; Rodriguez, B.; Guerin-Deremaux, L.; Thabuis, C. NUTRIOSE(R), a resistant dextrin, displays low glycemic and insulinemic reponses: Combining technological, organoleptical and nutritional attributes for obesity and diabetes prevention. Obes. Facts 2015, 8 (Suppl. 1), 95. [Google Scholar]
- Astina, J.; Saphyakhajorn, W.; Borompichaichartkul, C.; Sapwarobol, S. Tapioca resistant maltodextrin as a carbohydrate source of oral nutrition supplement (ONS) on metabolic indicators: A clinical trial. Nutrients 2022, 14, 916. [Google Scholar] [CrossRef]
- Astina, J.; Sapwarobol, S. Attenuation of glycaemic and insulin responses following tapioca resistant maltodextrin consumption in healthy subjects: A randomised cross-over controlled trial. J. Nutr. Sci. 2020, 9, e29. [Google Scholar] [CrossRef]
- Tan, W.S.K.; Chia, P.F.W.; Ponnalagu, S.; Karnik, K.; Henry, C.J. The role of soluble corn fiber on glycemic and insulin response. Nutrients 2020, 12, 961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, A.L.; Jenkins, D.J.A.; Wolever, T.M.S.; Rogovik, A.L.; Jovanovski, E.; Bozikov, V.; Rahelić, D.; Vuksan, V. Comparable postprandial glucose reductions with viscous fiber blend enriched biscuits in healthy subjects and patients with diabetes mellitus: Acute randomized controlled clinical trial. Croat. Med. J. 2008, 49, 772–782. [Google Scholar] [CrossRef]
- Alshammari, N.A.; Taylor, M.A.; Stevenson, R.; Gouseti, O.; Alyami, J.; Muttakin, S.; Bakalis, S.; Lovegrove, A.; Aithal, G.P.; Marciani, L. Effect of intake of food hydrocolloids of bacterial origin on the glycemic response in humans: Systematic review and narrative synthesis. Nutrients 2021, 13, 2407. [Google Scholar] [CrossRef]
- Thompson, S.V.; Hannon, B.A.; An, R.; Holscher, H.D. Effects of isolated soluble fiber supplementation on body weight, glycemia, and insulinemia in adults with overweight and obesity: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 106, 1514–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sa’adah, S.; Candra, O.M.; Nugrahani, G.; Pramono, A.; Afifah, D.N. Nutritional composition, glycemic index, glycemic load, and organoleptical quality of glucomannan-enriched soy milk ice cream. IOP Conf. Ser. Earth Environ. Sci. 2018, 102, 012014. [Google Scholar] [CrossRef]
- Scazzina, F.; Siebenhandl-Ehn, S.; Pellegrini, N. The effect of dietary fibre on reducing the glycaemic index of bread. Br. J. Nutr. 2013, 109, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Culetu, A.; Duta, D.E.; Papageorgiou, M.; Varzakas, T. The role of hydrocolloids in gluten-free bread and pasta; Rheology, characteristics, staling and glycemic index. Foods 2021, 10, 3121. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Augustin, L.S.A.; Martini, M.C.; Axelsen, M.; Faulkner, D.; Vidgen, E.; Parker, T.; Lau, H.; Connelly, P.W.; et al. Effect of wheat bran on glycemic control and risk factors for cardiovascular disease in type 2 diabetes. Diabetes Care 2002, 25, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Tengblad, S.; Karlström, B.; Kamal-Eldin, A.; Landberg, R.; Basu, S.; Aman, P.; Vessby, B. Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. J. Nutr. 2007, 137, 1401–1407. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to article 13(1) of regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2207. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zhao, L.; Wang, L.; Liu, H. Microstructure-modified products from stone-milled wheat bran powder improve glycemic response and sustain colonic fermentation. Int. J. Biol. Macromol. 2020, 153, 1193–1201. [Google Scholar] [CrossRef]
- Tangthanantorn, J.; Wichienchot, S.; Sirivongpaisal, P. Development of fresh and dried noodle products with high resistant starch content from banana flour. Food Sci. Technol. 2021, 42. [Google Scholar] [CrossRef]
- Alhindi, Y.; Avery, A. The efficacy and safety of oral semaglutide for glycaemic management in adults with type 2 diabetes compared to subcutaneous semaglutide, placebo, and other GLP-1 RA comparators: A systematic review and network meta-analysis. Contemp. Clin. Trials Commun. 2022, 28, 100944. [Google Scholar] [CrossRef]
- Miehle, E.; Haas, M.; Bader-Mittermaier, S.; Eisner, P. The role of hydration properties of soluble dietary fibers on glucose diffusion. Food Hydrocoll. 2022, 131, 107822. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 432/2012 Establishing a List of Permitted Health Claims Made on Foods, Other Than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. 2012. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:136:0001:0040:en:PDF (accessed on 19 November 2022).
- Varallo, C. Glycaemic Index Labeling and Related Claims. 2019. Available online: https://foodlawlatest.com/2019/02/05/glycaemic-index-labeling-and-related-claims/ (accessed on 21 November 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuntini, E.B.; Sardá, F.A.H.; de Menezes, E.W. The Effects of Soluble Dietary Fibers on Glycemic Response: An Overview and Futures Perspectives. Foods 2022, 11, 3934. https://doi.org/10.3390/foods11233934
Giuntini EB, Sardá FAH, de Menezes EW. The Effects of Soluble Dietary Fibers on Glycemic Response: An Overview and Futures Perspectives. Foods. 2022; 11(23):3934. https://doi.org/10.3390/foods11233934
Chicago/Turabian StyleGiuntini, Eliana Bistriche, Fabiana Andrea Hoffmann Sardá, and Elizabete Wenzel de Menezes. 2022. "The Effects of Soluble Dietary Fibers on Glycemic Response: An Overview and Futures Perspectives" Foods 11, no. 23: 3934. https://doi.org/10.3390/foods11233934
APA StyleGiuntini, E. B., Sardá, F. A. H., & de Menezes, E. W. (2022). The Effects of Soluble Dietary Fibers on Glycemic Response: An Overview and Futures Perspectives. Foods, 11(23), 3934. https://doi.org/10.3390/foods11233934