Melatonin Treatment Affects Wax Composition and Maintains Storage Quality in ‘Kongxin’ Plum (Prunus salicina L. cv) during Postharvest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Observation of Peel Appearance and Microstructure
2.3. Extraction of Cuticular Wax
2.4. Chemical Analysis of Cuticular Wax
2.5. Determination of Fruit Firmness, Weight Loss, TSS, and Titratable Acid (TA) Content
2.6. Determination of Soluble Sugar and Soluble Protein Content
2.7. Determination of Pectin Substance and Cellulose Content
2.8. Determination of Polygalacturonase (PG), Cellulase (Cx), and β-Glucosidase (β-GC) Activity Assay
2.9. Statistical Analysis
3. Results
3.1. Fruit Appearance and Peel Microstructure during Storage
3.2. Cuticular Wax
3.2.1. Multivariate Analysis of Cuticular Wax during Storage
3.2.2. Cuticular Wax Composition Content
3.3. Fruit Quality during Storage as Affected by Melatonin Treatment
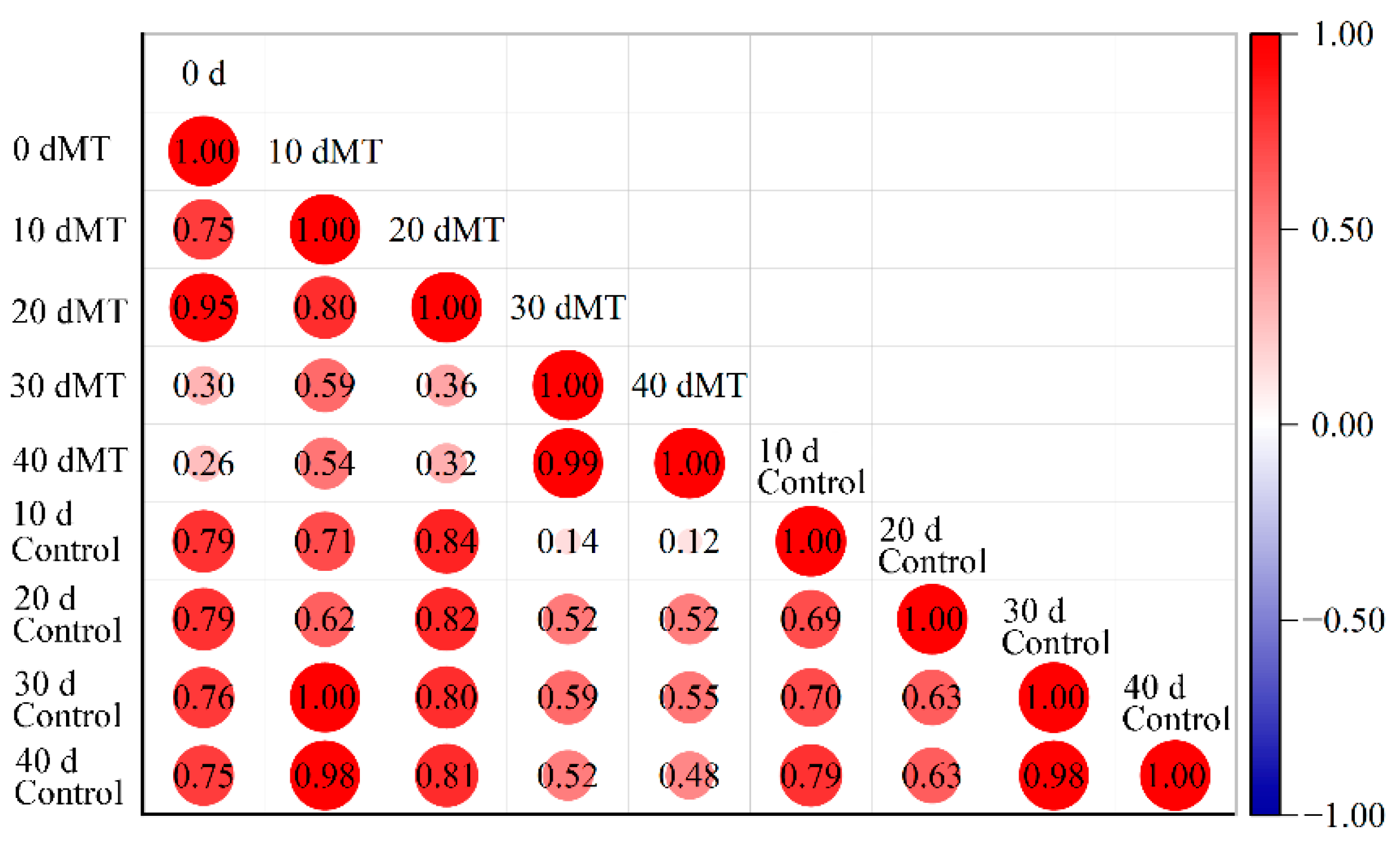
3.4. Correlation Analysis of Cuticular Wax Composition and Storage Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, P.; Sethi, S.; Sharma, R.R.; Srivastav, M.; Singh, D.; Varghese, E. Edible coatings influence the cold-storage life and quality of ‘Santa Rosa’ plum (Prunus salicina Lindell). J. Food Sci. Technol. 2018, 55, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- Minas, I.S.; Crisosto, G.M.; Holcroft, D.; Vasilakakis, M.; Crisosto, C.H. Postharvest handling of plums (Prunus salicina Lindl.) at 10 °C to save energy and preserve fruit quality using an innovative application system of 1-MCP. Postharvest Biol. Technol. 2013, 76, 1–9. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, C.H. Combined effects of aqueous chlorine dioxide and ultrasonic treatments on postharvest storage quality of plum fruit (Prunus salicina L.). Postharvest Biol. Technol. 2011, 61, 117–123. [Google Scholar] [CrossRef]
- Steffens, C.A.; Amarante, C.V.; Alves, E.O.; Brackmann, A. Fruit quality preservation of ‘Laetitia’ plums under controlled atmosphere storage. An. Acad. Bras. Cienc. 2014, 86, 485–494. [Google Scholar] [CrossRef]
- Lin, Y.F.; Lin, Y.X.; Lin, H.T.; Lin, M.S.; Li, H.; Yuan, F.; Chen, Y.H.; Xiao, J.B. Effects of paper containing 1-MCP postharvest treatment on the disassembly of cell wall polysaccharides and softening in Younai plum fruit during storage. Food Chem. 2018, 264, 1–8. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.H.; Lin, H.T.; Lin, M.S.; Chen, Y.H.; Lin, Y.F. 1-Methylcyclopropene containing-papers suppress the disassembly of cell wall polysaccharides in Anxi persimmon fruit during storage. Int. J. Biol. Macromol. 2020, 151, 723–729. [Google Scholar] [CrossRef]
- Wang, L.M.; Hong, K.; Xu, R.R.; Zhao, Z.L.; Cao, J.K. The alleviation of cold-stimulated flesh reddening in ‘Friar’ plum fruit by the elevated CO2 with polyvinyl chloride (PVC) packaging. Sci. Hortic. 2021, 281, 109997. [Google Scholar] [CrossRef]
- Kucuker, E.; Ozturk, B.; Celik, S.M.; Aksit, H. Pre-harvest spray application of methyl jasmonate plays an important role in fruit ripening, fruit quality and bioactive compounds of japanese plums. Sci. Hortic. 2014, 176, 162–169. [Google Scholar] [CrossRef]
- Alejandra, M.E.; María, S.; Daniel, V.; Domingo, M.R.; Salvador, C.; Pedro, Z. Enhancement of antioxidant systems and storability of two plum cultivars by preharvest treatments with salicylates. Int. J. Mol. Sci. 2017, 18, 1911. [Google Scholar] [CrossRef]
- Du, H.Y.; Liu, G.T.; Hua, C.M.; Liu, D.X.; He, Y.Y.; Liu, H.P.; Kurtenbach, R.; Ren, D.T. Exogenous melatonin alleviated chilling injury in harvested plum fruit via affecting the levels of polyamines conjugated to plasma membrane. Postharvest Biol. Technol. 2021, 179, 111585. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bal, E. Physicochemical changes in ‘Santa Rosa’plum fruit treated with melatonin during cold storage. J. Food Meas. Charact. 2019, 13, 1713–1720. [Google Scholar] [CrossRef]
- Wang, T.; Hu, M.J.; Yuan, D.B.; Yun, Z.; Gao, Z.Y.; Su, Z.H.; Zhang, Z.K. Melatonin alleviates pericarp browning in litchi fruit by regulating membrane lipid and energy metabolisms. Postharvest Biol. Technol. 2020, 160, 111066. [Google Scholar] [CrossRef]
- Tang, Q.; Li, C.Y.; Ge, Y.H.; Li, X.; Cheng, Y.; Hou, J.B.; Li, J.R. Exogenous application of melatonin maintains storage quality of jujubes by enhancing anti-oxidative ability and suppressing the activity of cell wall-degrading enzymes. LWT-Food Sci. Technol. 2020, 127, 109431. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Q.Y.; Chen, W.W.; Guo, Q.G.; Xia, Y.; Jing, D.L.; Liang, G.L. Melatonin treatment maintains quality and delays lignification in loquat fruit during cold storage. Sci. Hortic. 2021, 284, 110126. [Google Scholar] [CrossRef]
- Sharafi, Y.; Aghdam, M.S.; Luo, Z.S.; Jannatizadeh, A.; Razavi, F.; Fard, J.R.; Farmani, B. Melatonin treatment promotes endogenous melatonin accumulation and triggers gaba shunt pathway activity in tomato fruits during cold storage. Sci. Hortic. 2019, 254, 222–227. [Google Scholar] [CrossRef]
- Miranda, S.; Vilches, P.; Suazo, M.; Pavez, L.; García, K.; Méndez, M.A.; González, M.; Meisel, L.A.; Defilippi, B.G.; Del Pozo, T. Melatonin triggers metabolic and gene expression changes leading to improved quality traits of two sweet cherry cultivars during cold storage. Food Chem. 2020, 319, 126360. [Google Scholar] [CrossRef]
- Rastegar, S.; Khankahdani, H.H.; Rahimzadeh, M. Effects of melatonin treatment on the biochemical changes and antioxidant enzyme activity of mango fruit during storage. Sci. Hortic. 2020, 259, 108835. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Pu, H.L.; Shan, S.S.; Zhang, P.; Li, J.K.; Song, H.M.; Xu, X.B. Melatonin enhanced chilling tolerance and alleviated peel browning of banana fruit under low temperature storage. Postharvest Biol. Technol. 2021, 179, 111571. [Google Scholar] [CrossRef]
- Cao, Y.R.; Zang, Y.X.; Wu, S.C.; Li, T.; Li, J.; Xu, K.; Hong, S.B.; Wu, B.P.; Zhang, W.S.; Zheng, W.W. Melatonin affects cuticular wax profile in rabbiteye blueberry (Vaccinium ashei) during fruit development. Food Chem. 2022, 384, 132381. [Google Scholar] [CrossRef]
- Curry, E. Effects of 1-MCP applied postharvest on epicuticular wax of apples (Malus domestica borkh.) during storage. J. Sci. Food Agric. 2008, 88, 996–1006. [Google Scholar] [CrossRef]
- Wu, X.; Yin, H.; Chen, Y.Y.; Li, L.; Wang, Y.Z.; Hao, P.P.; Cao, P.; Qi, K.J.; Zhang, S.L. Chemical composition, crystal morphology and key gene expression of cuticular waxes of Asian pears at harvest and after storage. Postharvest Biol. Technol. 2017, 132, 71–80. [Google Scholar] [CrossRef]
- Yang, M.Y.; Luo, Z.S.; Gao, S.N.; Belwal, T.; Wang, L.; Qi, M.; Ban, Z.J.; Wu, B.; Wang, F.Z.; Li, L. The chemical composition and potential role of epicuticular and intracuticular wax in four cultivars of table grapes. Postharvest Biol. Technol. 2021, 173, 111430. [Google Scholar] [CrossRef]
- Costa, F. Mechanical investigation to assess the peel contribution in apple fruit. Postharvest Biol. Technol. 2016, 111, 41–47. [Google Scholar] [CrossRef]
- Verardo, G.; Pagani, E.; Geatti, P.; Martinuzzi, P. A thorough study of the surface wax of apple fruits. Anal. Bioanal. Chem. 2003, 376, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Q.; Rao, J.P.; Huber, D.J.; Chang, X.X.; Xin, F.C. Wax composition of ‘Red fuji’ apple fruit during development and during storage after 1-methylcyclopropene treatment. Hortic. Environ. Biotechnol. 2012, 53, 288–297. [Google Scholar] [CrossRef]
- Klein, B.; Thewes, F.R.; Oliveira, A.R.; Brackmann, A.; Barin, J.S.; Cichoski, A.J.; Wagner, R. Development of dispersive solvent extraction method to determine the chemical composition of apple peel wax. Food Res. Int. 2019, 116, 611–619. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, H.J.; Lv, Y.H.; Chen, G.G.; Jiang, Y. Comparative analysis of total wax content, chemical composition and crystal morphology of cuticular wax in Korla pear under different relative humidity of storage. Food Chem. 2020, 339, 128097. [Google Scholar] [CrossRef]
- Wang, Y.; Su, S.N.; Chen, G.G.; Mao, H.J.; Jiang, Y. Relationship between cuticular waxes and storage quality parameters of Korla pear under different storage methods. J. Plant Growth Regul. 2021, 40, 1152–1165. [Google Scholar] [CrossRef]
- Ding, S.H.; Zhang, J.; Wang, R.R.; Ou, S.Y.; Shan, Y. Changes in cuticle compositions and crystal structure of ‘Bingtang’ sweet orange fruits (Citrus sinensis) during storage. Int. J. Food Prop. 2018, 21, 2411–2427. [Google Scholar] [CrossRef]
- Romero, P.; Lafuente, M.T. Abscisic acid deficiency alters epicuticular wax metabolism and morphology that leads to increased cuticle permeability during sweet orange (Citrus sinensis) fruit ripening. Front. Plant Sci. 2020, 11, 594184. [Google Scholar] [CrossRef] [PubMed]
- Moggia, C.; Graell, J.; Lara, I.; Schmeda-Hirschmann, G.; Thomas-Valdés, S.; Lobos, G.A. Fruit characteristics and cuticle triterpenes as related to postharvest quality of highbush blueberries. Sci. Hortic. 2016, 211, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Chu, W.J.; Gao, H.Y.; Cao, S.F.; Fang, X.J.; Chen, H.J.; Xiao, S.Y. Composition and morphology of cuticular wax in blueberry (Vaccinium spp.) fruits. Food Chem. 2017, 219, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Liu, R.L.; Fang, X.J.; Tong, C.; Chen, H.J.; Gao, H.Y. Effects of salicylic acid treatment on fruit quality and wax composition of blueberry (Vaccinium virgatum Ait). Food Chem. 2021, 368, 130757. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Huang, S.A.; Zhang, Q.; Zhu, S.L.; Dong, X.Q. Changes in the Primary Metabolites of ‘Fengtang’ Plums during Storage Detected by Widely Targeted Metabolomics. Foods 2022, 11, 2830. [Google Scholar] [CrossRef]
- Cao, J.K.; Zhao, Y.M.; Jiang, W.B. Postharvest Physiological and Bioch Emical Experiment Technology of Fruits and Vegetables; China Light Industry Press: Beijing, China, 2007. [Google Scholar]
- He, Y.H.; Li, J.Y.; Ban, Q.Y.; Han, S.K.; Rao, J.P. Role of brassinosteroids in persimmon (Diospyros kaki L.) fruit ripening. J. Sci. Food Agric. 2018, 66, 2637–2644. [Google Scholar] [CrossRef]
- Bu, J.W.; Yu, Y.C.; Aisikaer, G.; Yin, T.J. Postharvest UV-C irradiation inhibits the production of ethylene and the activity of cell wall-degrading enzymes during softening of tomato (Lycopersicon esculentum L.) fruit. Postharvest Biol. Technol. 2013, 86, 337–345. [Google Scholar] [CrossRef]
- Wu, Y.X.; Xu, L.S.; Yin, Z.Y.; Dai, Q.Q.; Gao, X.N.; Feng, H.; Voegele, R.T.; Huang, L.L. Two members of the velvet family, vmvea and vmvelb, affect conidiation, virulence and pectinase expression in valsa mali. Mol. Plant Pathol. 2017, 19, 1639–1651. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.Q.; Du, G.C.; Qi, H.T.; Zhang, Y.N.; Yue, T.Q.; Wang, J.C.; Li, R.G. A nematicidal tannin from Punica granatum L. rind and its physiological effect on pine wood nematode (Bursaphelenchus xylophilus). Pestic. Biochem. Physiol. 2017, 135, 64–68. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Shi, F.F.; Yao, J.L.; Zhang, N. Effects of ultrasound irradiation on the properties of apricot kernels during accelerated debitterizing. RSC Adv. 2020, 10, 10624–10633. [Google Scholar] [CrossRef] [Green Version]
- Chai, Y.F.; Li, A.; Wai, S.C.; Song, C.C.; Zhao, Y.Y.; Duan, Y.Q.; Zhang, B.Q.; Lin, Q. Cuticular wax composition changes of 10 apple cultivars during postharvest storage. Food Chem. 2020, 324, 126903. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Ren, X.L.; Gong, H.S.; Huang, H.; Sun, S.Y.; Wang, P.; Zhao, J.; Fan, X.G.; Zhang, A. Skin greasiness in apple is caused by accumulations of liquid waxes: Evidence from chemical and thermodynamic analyses. LWT-Food Sci. Technol. 2021, 147, 111639. [Google Scholar] [CrossRef]
- Lin, Y.X.; Lin, H.T.; Wang, H.; Lin, M.S.; Chen, Y.H.; Fan, Z.Q.; Hung, Y.C.; Lin, Y.F. Effects of hydrogen peroxide treatment on pulp breakdown, softening, and cell wall polysaccharide metabolism in fresh longan fruit. Carbohydr. Polym. 2020, 242, 116427. [Google Scholar] [CrossRef] [PubMed]
- Uluisik, S.; Seymour, G.B. Pectate lyases: Their role in plants and importance in fruit ripening. Food Chem. 2020, 309, 125559. [Google Scholar] [CrossRef]
- He, M.Y.; Wu, Y.F.; Wang, Y.; Hong, M.; Li, T.T.; Deng, T.J.; Jiang, Y.M. Valeric acid suppresses cell wall polysaccharides disassembly to maintain fruit firmness of harvested ‘Waizuili’ plum (Prunus salicina Lindl). Sci. Hortic. 2021, 291, 110608. [Google Scholar] [CrossRef]
- Vorwerk, S.; Somerville, S.; Somerville, C. The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci. 2004, 9, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.J.; Gao, H.Y.; Chen, H.J.; Wu, W.J.; Fang, X.J. Changes in cuticular wax composition of two blueberry cultivars during fruit ripening and postharvest cold storage. J. Agric. Food Chem. 2018, 11, 2870–2876. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Shen, C.L.; Farnham, M.K.; Ku, K.M. Three-dimensional epicuticular wax on plant surface reduces attachment and survival rate of salmonella during storage. Postharvest Biol. Technol. 2020, 166, 111197. [Google Scholar] [CrossRef]
- Domínguez, E.; Heredia-Guerrero, J.A.; Heredia, A. The biophysical design of plant cuticles: An overview. New Phytol. 2011, 189, 938–949. [Google Scholar] [CrossRef]
- Furlan, C.M.; Santos, D.; Salatino, A.; Domingos, M. N-alkane distribution of leaves of psidium guajava exposed to industrial air pollutants. Environ. Exp. Bot. 2006, 58, 100–105. [Google Scholar] [CrossRef]
- Huang, H.; Burghardt, M.; Schuster, A.C.; Leide, J.; Lara, I.; Riederer, M. Chemical composition and water permeability of fruit and leaf cuticles of Olea europaea L. J. Agric. Food Chem. 2017, 65, 8790–8797. [Google Scholar] [CrossRef] [PubMed]
- Macel, M.; Visschers, I.G.S.; Peters, J.L.; Dam, N.M.; Graaf, R.M. High concentrations of very long chain leaf wax alkanes of thrips susceptible pepper accessions (Capsicum spp.). J. Chem. Ecol. 2020, 46, 1082–1089. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Huang, S.; Huber, D.J.; Zhang, Q.; Wan, X.; Peng, J.; Luo, D.; Dong, X.; Zhu, S. Melatonin Treatment Affects Wax Composition and Maintains Storage Quality in ‘Kongxin’ Plum (Prunus salicina L. cv) during Postharvest. Foods 2022, 11, 3972. https://doi.org/10.3390/foods11243972
Lin X, Huang S, Huber DJ, Zhang Q, Wan X, Peng J, Luo D, Dong X, Zhu S. Melatonin Treatment Affects Wax Composition and Maintains Storage Quality in ‘Kongxin’ Plum (Prunus salicina L. cv) during Postharvest. Foods. 2022; 11(24):3972. https://doi.org/10.3390/foods11243972
Chicago/Turabian StyleLin, Xin, Shian Huang, Donald J. Huber, Qin Zhang, Xuan Wan, Junsen Peng, Dengcan Luo, Xiaoqing Dong, and Shouliang Zhu. 2022. "Melatonin Treatment Affects Wax Composition and Maintains Storage Quality in ‘Kongxin’ Plum (Prunus salicina L. cv) during Postharvest" Foods 11, no. 24: 3972. https://doi.org/10.3390/foods11243972





