Ancient Wheat Varieties and Sourdough Fermentation as a Tool to Increase Bioaccessibility of Phenolics and Antioxidant Capacity of Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
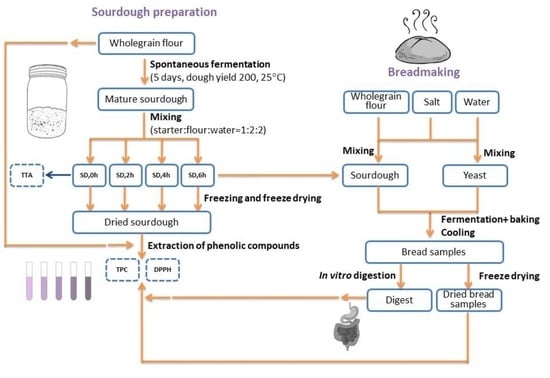
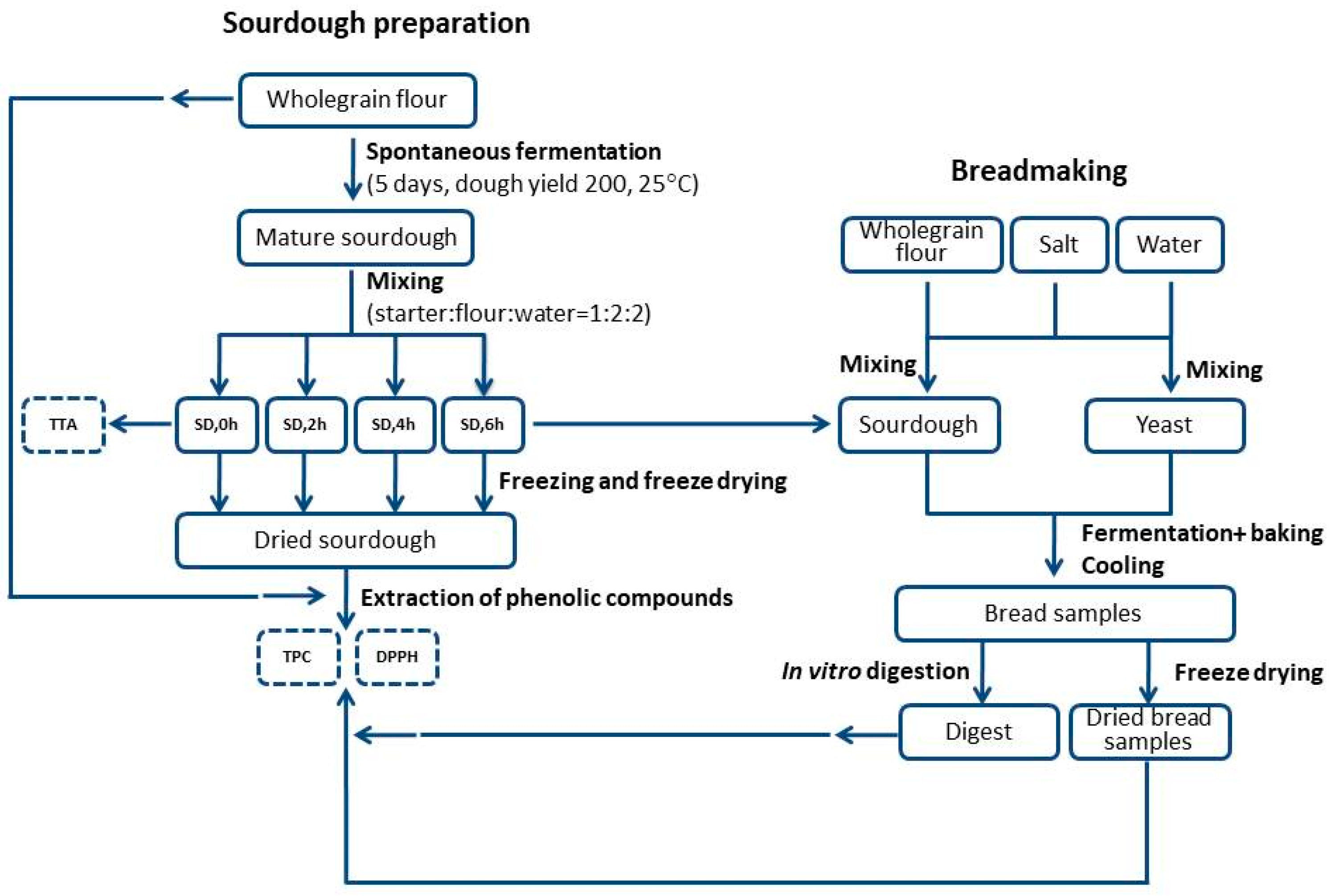
2.2. Sourdough Preparation and Total Titrable Acidity (TTA) Measurement
2.3. Bread Making Procedure
2.4. In Vitro Digestion of Bread Samples
2.5. Freeze Drying
2.6. Determination of Total Phenolic Content and Antioxidant Capacity
2.6.1. Extraction of Free Phenolic Compounds
2.6.2. Determination of Total Phenolic Content (TPC) in Flour, Dough, Bread and Bread after In Vitro Gastrointestinal Digestion
2.6.3. DPPH Antioxidant Capacity of Flour, Dough, Bread and Bread after In Vitro Gastrointestinal Digestion
2.7. Kinetic Analysis
2.8. Bioaccessibility Determination
2.9. Statistical Analysis
3. Results and Discussion
3.1. Changes in Total Titrable Acidity (TTA) during Sourdough Fermentation
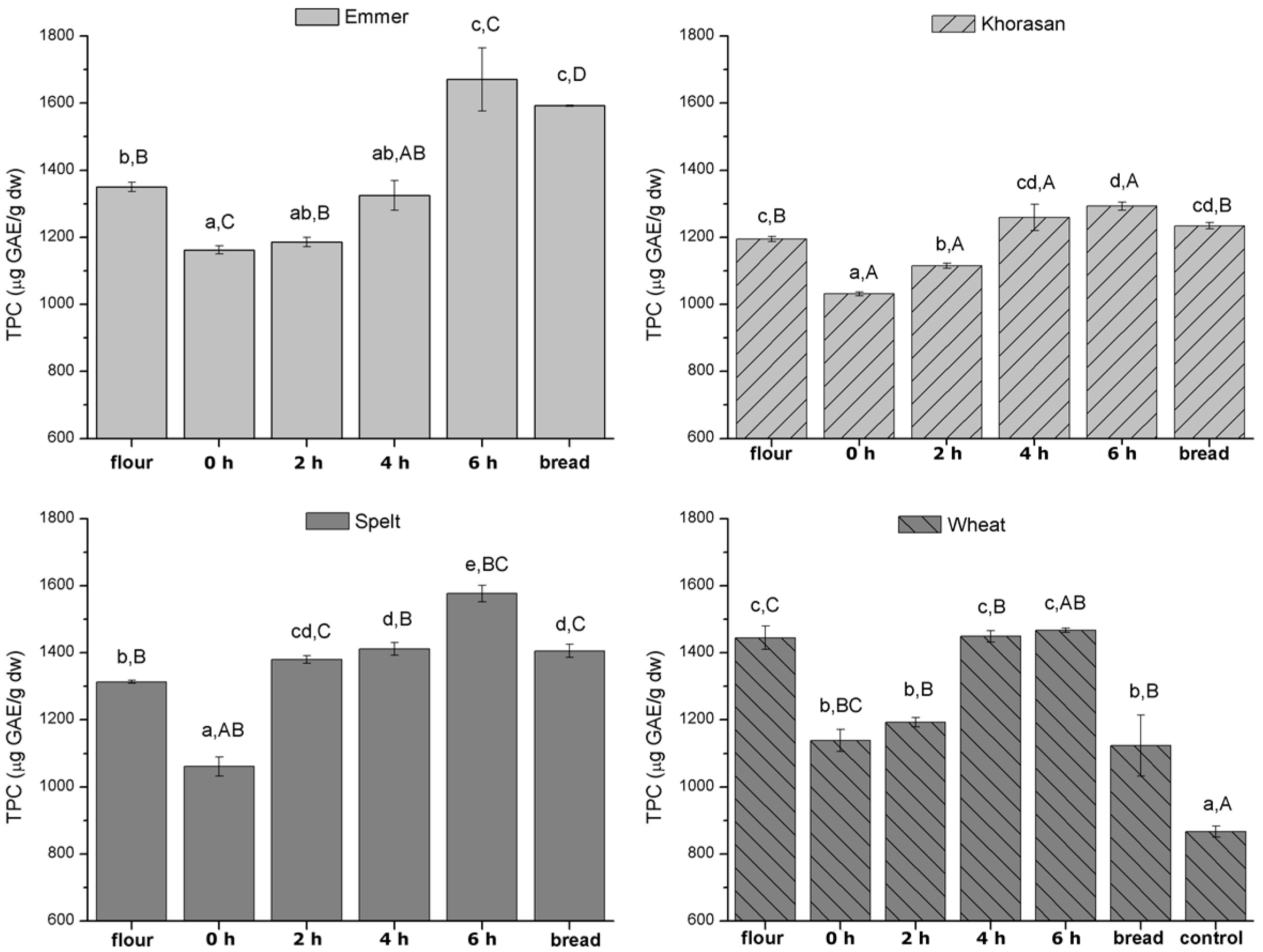
3.2. Total Phenolic Content (TPC) Evolution during Baking
3.3. DPPH Antioxidant Capacity Evolution during Breadmaking
3.4. Bioaccessibility Assay of TPC and Antioxidant Capacity after Simulated In Vitro Digestibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organisation of the United Nations (FAOSTAT). Available online: http://www.fao.org/faostat/en/#data (accessed on 5 April 2022).
- NDNS 2014. National Diet and Nutrition Survey Results from Years 1, 2, 3 and 4 (Combined) of the Rolling Programme (2008/2009–2011/2012) Public Health England. 160p. Available online: https://www.gov.uk/government/statistics/national-diet-and-nutrition-survey-results-from-years-1-to-4-combined-of-the-rolling-programme-for-2008-and-2009-to-2011-and-2012 (accessed on 7 April 2022).
- Shewry, P.R.; Hey, S. Do “Ancient” Wheat Species Differ from Modern Bread Wheat in Their Contents of Bioactive Components? J. Cereal Sci. 2015, 65, 236–243. [Google Scholar] [CrossRef]
- Arzani, A.; Ashraf, M. Cultivated Ancient Wheats (Triticum spp.): A Potential Source of Health-Beneficial Food Products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Angioloni, A.; Collar, C. Nutritional and Functional Added Value of Oat, Kamut ®, Spelt, Rye and Buckwheat versus Common Wheat in Breadmaking. J. Sci. Food Agric. 2011, 91, 1283–1292. [Google Scholar] [CrossRef]
- Geisslitz, S.; Longin, C.F.H.; Scherf, K.A.; Koehler, P. Comparative Study on Gluten Protein Composition of Ancient (Einkorn, Emmer and Spelt) and Modern Wheat Species (Durum and Common Wheat). Foods 2019, 8, 409. [Google Scholar] [CrossRef]
- Suchowilska, E.; Wiwart, M.; Kandler, W.; Krska, R. A Comparison of Macro- and Microelement Concentrations in the Whole Grain of Four Triticum Species. Plant Soil Environ. 2012, 58, 141–147. [Google Scholar] [CrossRef]
- Shewry, P.R. Do Ancient Types of Wheat Have Health Benefits Compared with Modern Bread Wheat? J. Cereal Sci. 2018, 79, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Dinelli, G.; Segura-Carretero, A.; Di Silvestro, R.; Marotti, I.; Arráez-Román, D.; Benedettelli, S.; Ghiselli, L.; Fernadez-Gutierrez, A. Profiles of Phenolic Compounds in Modern and Old Common Wheat Varieties Determined by Liquid Chromatography Coupled with Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2011, 1218, 7670–7681. [Google Scholar] [CrossRef]
- Carter, J.W.; Madl, R.; Padula, F. Wheat Antioxidants Suppress Intestinal Tumor Activity in Min Mice. Nutr. Res. 2006, 26, 33–38. [Google Scholar] [CrossRef]
- Fardet, A. New Hypotheses for the Health-Protective Mechanisms of Whole-Grain Cereals: What Is beyond Fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Lorusso, A.; Russo, V.; Pinto, D.; Marzani, B.; Gobbetti, M. Improving the Antioxidant Properties of Quinoa Flour through Fermentation with Selected Autochthonous Lactic Acid Bacteria. Int. J. Food Microbiol. 2017, 241, 252–261. [Google Scholar] [CrossRef]
- Mateo Anson, N.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Bioavailability of Ferulic Acid Is Determined by Its Bioaccessibility. J. Cereal Sci. 2009, 49, 296–300. [Google Scholar] [CrossRef]
- Zhao, Z.; Egashira, Y.; Sanada, H. Digestion and Absorption of Ferulic Acid Sugar Esters in Rat Gastrointestinal Tract. J. Agric. Food Chem. 2003, 51, 5534–5539. [Google Scholar] [CrossRef] [PubMed]
- Pejcz, E.; Lachowicz-Wiśniewska, S.; Nowicka, P.; Wojciechowicz-Budzisz, A.; Spychaj, R.; Gil, Z. Effect of Inoculated Lactic Acid Fermentation on the Fermentable Saccharides and Polyols, Polyphenols and Antioxidant Activity Changes in Wheat Sourdough. Molecules 2021, 26, 4193. [Google Scholar] [CrossRef] [PubMed]
- Banu, I.; Vasilean, I.; Aprodu, I. Effect of Lactic Fermentation on Antioxidant Capacity of Rye Sourdough and Bread. Food Sci. Technol. Res. 2010, 16, 571–576. [Google Scholar] [CrossRef]
- Moroni, A.V.; Zannini, E.; Sensidoni, G.; Arendt, E.K. Exploitation of Buckwheat Sourdough for the Production of Wheat Bread. Eur. Food Res. Technol. 2012, 235, 659–668. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Rabalski, I. Effect of Baking on Free and Bound Phenolic Acids in Wholegrain Bakery Products. J. Cereal Sci. 2013, 57, 312–318. [Google Scholar] [CrossRef]
- Han, H.-M.; Koh, B.-K. Antioxidant Activity of Hard Wheat Flour, Dough and Bread Prepared Using Various Processes with the Addition of Different Phenolic Acids. J. Sci. Food Agric. 2011, 91, 604–608. [Google Scholar] [CrossRef]
- Gelinas, P.; McKinnon, C.M. Effect of Wheat Variety, Farming Site, and Bread-Baking on Total Phenolics. Int. J. Food Sci. Technol. 2006, 41, 329–332. [Google Scholar] [CrossRef]
- Cheng, Z.; Su, L.; Moore, J.; Zhou, K.; Luther, M.; Yin, J.-J.; Yu, L. (Lucy) Effects of Postharvest Treatment and Heat Stress on Availability of Wheat Antioxidants. J. Agric. Food Chem. 2006, 54, 5623–5629. [Google Scholar] [CrossRef]
- Holtekjølen, A.K.; Bævre, A.B.; Rødbotten, M.; Berg, H.; Knutsen, S.H. Antioxidant Properties and Sensory Profiles of Breads Containing Barley Flour. Food Chem. 2008, 110, 414–421. [Google Scholar] [CrossRef]
- Menga, V.; Fares, C.; Troccoli, A.; Cattivelli, L.; Baiano, A. Effects of Genotype, Location and Baking on the Phenolic Content and Some Antioxidant Properties of Cereal Species. Int. J. Food Sci. Technol. 2009, 45, 7–16. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Milićević, N.; Kojić, P.; Sakač, M.; Mišan, A.; Kojić, J.; Perussello, C.; Banjac, V.; Pojić, M.; Tiwari, B. Kinetic Modelling of Ultrasound-Assisted Extraction of Phenolics from Cereal Brans. Ultrason. Sonochem. 2021, 79, 105761. [Google Scholar] [CrossRef] [PubMed]
- Saharan, P.; Sadh, P.K.; Singh Duhan, J. Comparative Assessment of Effect of Fermentation on Phenolics, Flavanoids and Free Radical Scavenging Activity of Commonly Used Cereals. Biocatal. Agric. Biotechnol. 2017, 12, 236–240. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of Ultrasound-Assisted Extraction of Phenolic Compounds from Wheat Bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Santana Andrade, J.K.; Chagas Barros, R.G.; Pereira, U.C.; Nogueira, J.P.; Gualberto, N.C.; Santos de Oliveira, C.; Shanmugam, S.; Narain, N. Bioaccessibility of Bioactive Compounds after in Vitro Gastrointestinal Digestion and Probiotics Fermentation of Brazilian Fruits Residues with Antioxidant and Antidiabetic Potential. LWT 2022, 153, 112469. [Google Scholar] [CrossRef]
- Katina, K.; Arendt, E.; Liukkonen, K.-H.; Autio, K.; Flander, L.; Poutanen, K. Potential of Sourdough for Healthier Cereal Products. Trends Food Sci. Technol. 2005, 16, 104–112. [Google Scholar] [CrossRef]
- Coda, R.; Nionelli, L.; Rizzello, C.G.; De Angelis, M.; Tossut, P.; Gobbetti, M. Spelt and Emmer Flours: Characterization of the Lactic Acid Bacteria Microbiota and Selection of Mixed Starters for Bread Making. J. Appl. Microbiol. 2010, 108, 925–935. [Google Scholar] [CrossRef]
- Van der Meulen, R.; Scheirlinck, I.; Van Schoor, A.; Huys, G.; Vancanneyt, M.; Vandamme, P.; De Vuyst, L. Population Dynamics and Metabolite Target Analysis of Lactic Acid Bacteria during Laboratory Fermentations of Wheat and Spelt Sourdoughs. Appl. Environ. Microbiol. 2007, 73, 4741–4750. [Google Scholar] [CrossRef]
- Venturi, M.; Galli, V.; Pini, N.; Guerrini, S.; Sodi, C.; Granchi, L. Influence of Different Leavening Agents on Technological and Nutritional Characteristics of Whole Grain Breads Obtained from Ancient and Modern Flour Varieties. Eur. Food Res. Technol. 2021, 247, 1701–1710. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Gerhardt, K.; Wendin, K. Biochemical Characteristics and Potential Applications of Ancient Cereals—An Underexploited Opportunity for Sustainable Production and Consumption. Trends Food Sci. Technol. 2021, 107, 114–123. [Google Scholar] [CrossRef]
- Yu, L.; Nanguet, A.-L.; Beta, T. Comparison of Antioxidant Properties of Refined and Whole Wheat Flour and Bread. Antioxidants 2013, 2, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Drakula, S.; Novotni, D.; Čukelj Mustač, N.; Voučko, B.; Krpan, M.; Hruškar, M.; Ćurić, D. Alteration of Phenolics and Antioxidant Capacity of Gluten-Free Bread by Yellow Pea Flour Addition and Sourdough Fermentation. Food Biosci. 2021, 44, 101424. [Google Scholar] [CrossRef]
- Nionelli, L.; Curri, N.; Curiel, J.A.; Di Cagno, R.; Pontonio, E.; Cavoski, I.; Gobbetti, M.; Rizzello, C.G. Exploitation of Albanian Wheat Cultivars: Characterization of the Flours and Lactic Acid Bacteria Microbiota, and Selection of Starters for Sourdough Fermentation. Food Microbiol. 2014, 44, 96–107. [Google Scholar] [CrossRef]
- Benincasa, P.; Galieni, A.; Manetta, A.C.; Pace, R.; Guiducci, M.; Pisante, M.; Stagnari, F. Phenolic Compounds in Grains, Sprouts and Wheatgrass of Hulled and Non-Hulled Wheat Species. J. Sci. Food Agric. 2015, 95, 1795–1803. [Google Scholar] [CrossRef]
- Li, L.; Shewry, P.R.; Ward, J.L. Phenolic Acids in Wheat Varieties in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef]
- Van Boxstael, F.; Aerts, H.; Linssen, S.; Latré, J.; Christiaens, A.; Haesaert, G.; Dierickx, I.; Brusselle, J.; De Keyzer, W. A Comparison of the Nutritional Value of Einkorn, Emmer, Khorasan and Modern Wheat: Whole Grains, Processed in Bread, and Population-level Intake Implications. J. Sci. Food Agric. 2020, 100, 4108–4118. [Google Scholar] [CrossRef]
- Olojede, A.O.; Sanni, A.I.; Banwo, K.; Adesulu-Dahunsi, A.T. Sensory and Antioxidant Properties and In-Vitro Digestibility of Gluten-Free Sourdough Made with Selected Starter Cultures. LWT 2020, 129, 109576. [Google Scholar] [CrossRef]
- Li, Y.; Ma, D.; Sun, D.; Wang, C.; Zhang, J.; Xie, Y.; Guo, T. Total Phenolic, Flavonoid Content, and Antioxidant Activity of Flour, Noodles, and Steamed Bread Made from Different Colored Wheat Grains by Three Milling Methods. Crop J. 2015, 3, 328–334. [Google Scholar] [CrossRef]
- Lu, Y.; Luthria, D.; Fuerst, E.P.; Kiszonas, A.M.; Yu, L.; Morris, C.F. Effect of Processing on Phenolic Composition of Dough and Bread Fractions Made from Refined and Whole Wheat Flour of Three Wheat Varieties. J. Agric. Food Chem. 2014, 62, 10431–10436. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Velázquez, O.A.; Mulero, M.; Cuevas-Rodríguez, E.O.; Mondor, M.; Arcand, Y.; Hernández-Álvarez, A.J. In Vitro Gastrointestinal Digestion Impact on Stability, Bioaccessibility and Antioxidant Activity of Polyphenols from Wild and Commercial Blackberries (Rubus spp.). Food Funct. 2021, 12, 7358–7378. [Google Scholar] [CrossRef] [PubMed]

| Sample | Wheat | Spelt | Emmer | Khorasan |
|---|---|---|---|---|
| Moisture | 11.20 ± 0.09 b | 10.80 ± 0.07 a | 11.31 ± 0.11 b | 10.70 ± 0.06 a |
| Protein (d.b.) | 11.30 ± 0.32 a | 15.87 ± 0.37 b | 15.99 ± 0.30 b | 11.49 ± 0.43 a |
| Total dietary fiber (d.b.) | 10.45 ± 0.38 b | 10.10 ± 0.42 b | 9.58 ± 0.28 a | 9.85 ± 0.31 ab |
| Ash (d.b.) | 1.48 ± 0.08 a | 1.98 ± 0.06 b | 1.87 ± 0.04 b | 1.50 ± 0.07 a |
| TTA | 0 h | 6 h |
|---|---|---|
| Emmer | 4.85 ± 0.085 d | 11.60 ± 0.042 h |
| Khorasan | 3.61 ± 0.007 a | 9.21 ± 0.041 e |
| Spelt | 4.46 ± 0.011 c | 10.61 ± 0.014 f |
| Wheat | 4.08 ± 0.042 b | 11.12 ± 0.056 g |
| Sample | TPC | DPPH | ||||
|---|---|---|---|---|---|---|
| A0 | k | r2 | A0 | k | r2 | |
| Emmer | 980 ± 12.3 a | 0.127 ± 0.0055 c | 0.864 ± 0.0086 | 3.01 ± 0.028 a | 0.069 ± 0.0050 c | 0.907 ± 0.0356 |
| Khorasan | 958 ± 4.0 a | 0.080 ± 0.0036 a | 0.950 ± 0.0393 | 3.17 ± 0.010 bc | 0.040 ± 0.0008 a | 0.947 ± 0.0116 |
| Spelt | 993 ± 22.6 a | 0.114 ± 0.008b c | 0.868 ± 0.0193 | 3.21 ± 0.013 c | 0.056 ± 0.0047 bc | 0.957 ± 0.0236 |
| Wheat | 1026 ± 33.7 a | 0.096 ± 0.0074 ab | 0.887 ± 0.0268 | 3.12 ± 0.026 b | 0.045 ± 0.0001 ab | 0.868 ± 0.0080 |
| Sample | TPC | DPPH | ||
|---|---|---|---|---|
| Digestion Product (μg GAE/g dw) | BI (%) | Digestion Product (μmol TE/g dw) | BI (%) | |
| Emmer | 5302.1 ± 110.30 d | 333.0 ± 7.21 a | 14.26 ± 0.002 a | 371.2 ± 0.92 a |
| Khorasan | 4088.8 ± 35.80 b | 331.2 ± 0.42 a | 14.35 ± 0.032 a | 393.4 ± 0.35 c |
| Spelt | 4843.9 ± 15.59 c | 344.6 ± 3.60 a | 14.73 ± 0.046 b | 398.7 ± 5.36 c |
| Wheat | 4054.1 ± 8.66 b | 361.9 ± 28.49 a | 15.17 ± 0.009 c | 393.8 ± 1.39 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dapčević-Hadnađev, T.; Stupar, A.; Stevanović, D.; Škrobot, D.; Maravić, N.; Tomić, J.; Hadnađev, M. Ancient Wheat Varieties and Sourdough Fermentation as a Tool to Increase Bioaccessibility of Phenolics and Antioxidant Capacity of Bread. Foods 2022, 11, 3985. https://doi.org/10.3390/foods11243985
Dapčević-Hadnađev T, Stupar A, Stevanović D, Škrobot D, Maravić N, Tomić J, Hadnađev M. Ancient Wheat Varieties and Sourdough Fermentation as a Tool to Increase Bioaccessibility of Phenolics and Antioxidant Capacity of Bread. Foods. 2022; 11(24):3985. https://doi.org/10.3390/foods11243985
Chicago/Turabian StyleDapčević-Hadnađev, Tamara, Alena Stupar, Dušan Stevanović, Dubravka Škrobot, Nikola Maravić, Jelena Tomić, and Miroslav Hadnađev. 2022. "Ancient Wheat Varieties and Sourdough Fermentation as a Tool to Increase Bioaccessibility of Phenolics and Antioxidant Capacity of Bread" Foods 11, no. 24: 3985. https://doi.org/10.3390/foods11243985
APA StyleDapčević-Hadnađev, T., Stupar, A., Stevanović, D., Škrobot, D., Maravić, N., Tomić, J., & Hadnađev, M. (2022). Ancient Wheat Varieties and Sourdough Fermentation as a Tool to Increase Bioaccessibility of Phenolics and Antioxidant Capacity of Bread. Foods, 11(24), 3985. https://doi.org/10.3390/foods11243985