Novel Peptide Sequences with ACE-Inhibitory and Antioxidant Activities Derived from the Heads and Bones of Hybrid Groupers (Epinephelus lanceolatus × Epinephelus fuscoguttatus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
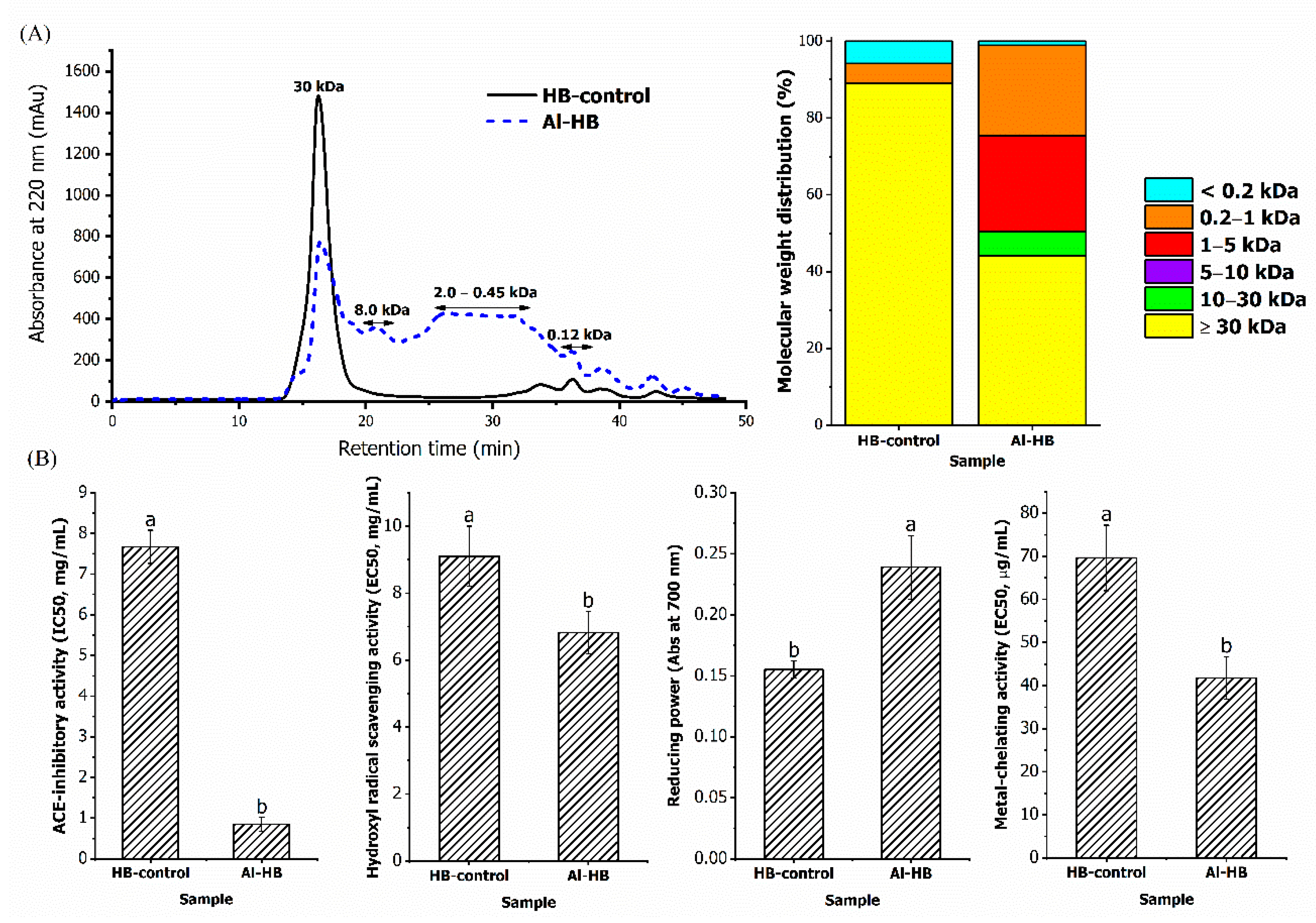
2.2. Production of Alcalase-Treated Bone Hydrolysate
2.3. Determination of Protein Content and Yield of Fractions
2.4. Determination of Molecular Weight Distribution of Hydrolysates Using Gel Filtration Chromatography
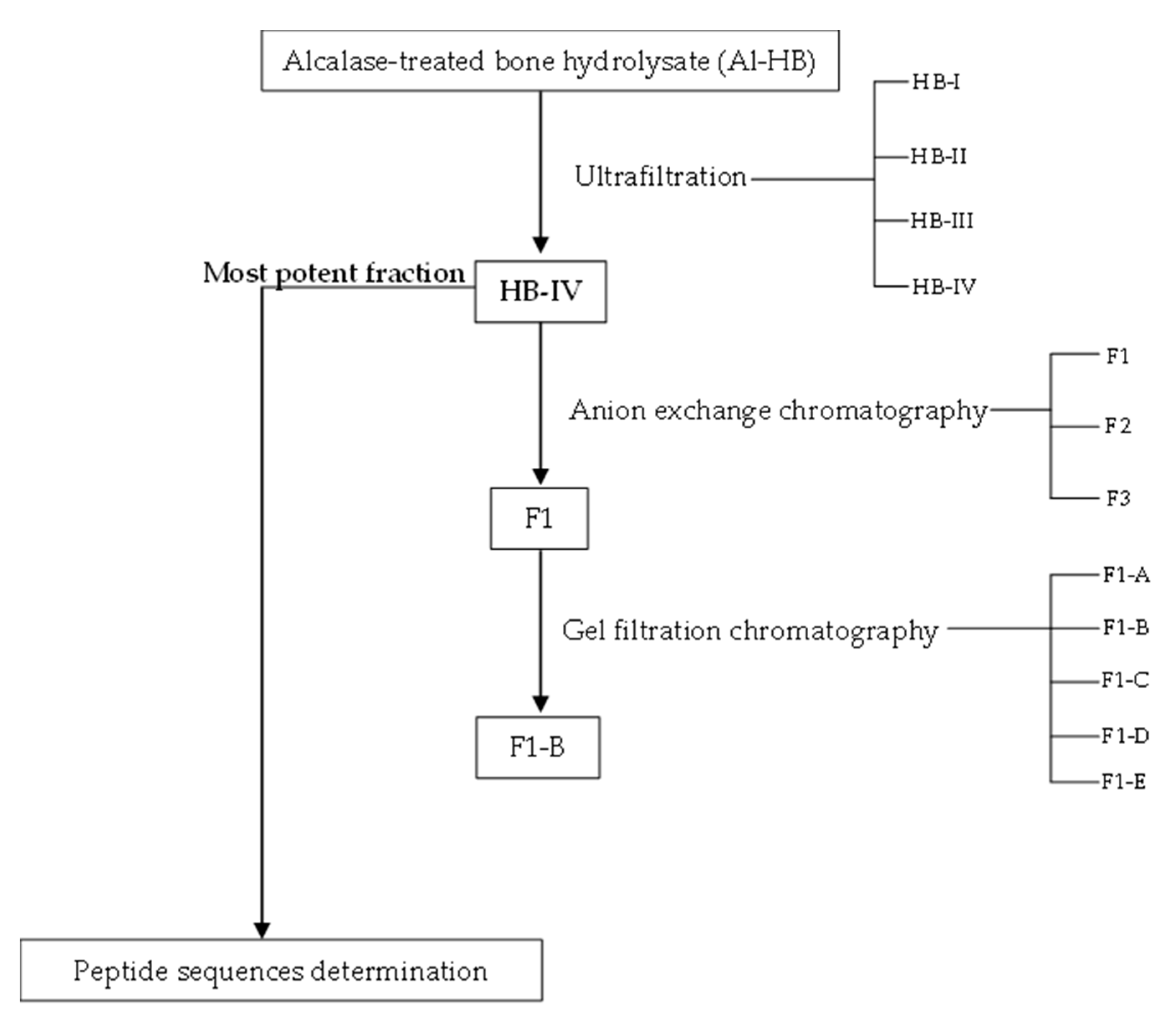
2.5. Fractionation of Alcalase-Treated Bone Hydrolysate
2.5.1. Ultrafiltration
2.5.2. Anion Exchange Chromatography
2.5.3. Gel Filtration Chromatography
2.6. Determination of Amino Acids Composition of Fractions
2.7. Determination of ACE-Inhibitory Activity of Fractions
2.8. Determination of In Vitro Antioxidant Activities of Peptide Fractions
2.8.1. Hydroxyl Radical Scavenging Activity
2.8.2. Reducing Power
2.8.3. Metal-Chelating Activity
2.9. Identification of Peptide Sequences
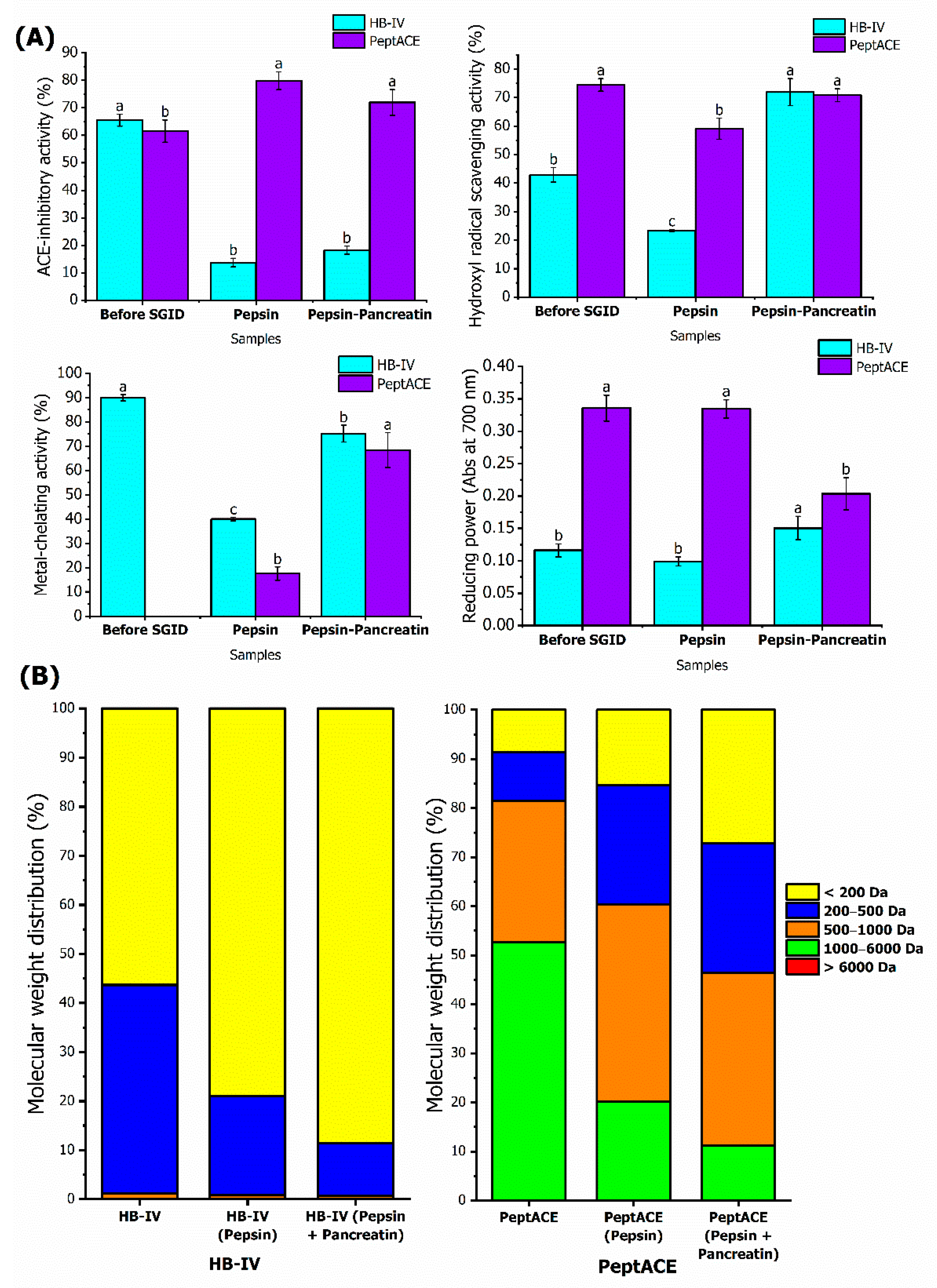
2.10. Stability of Peptide Fraction against In Vitro Stimulated Gastrointestinal Digestion
2.11. Stability of Peptide Fraction against ACE Using Pre-Incubation Experiment
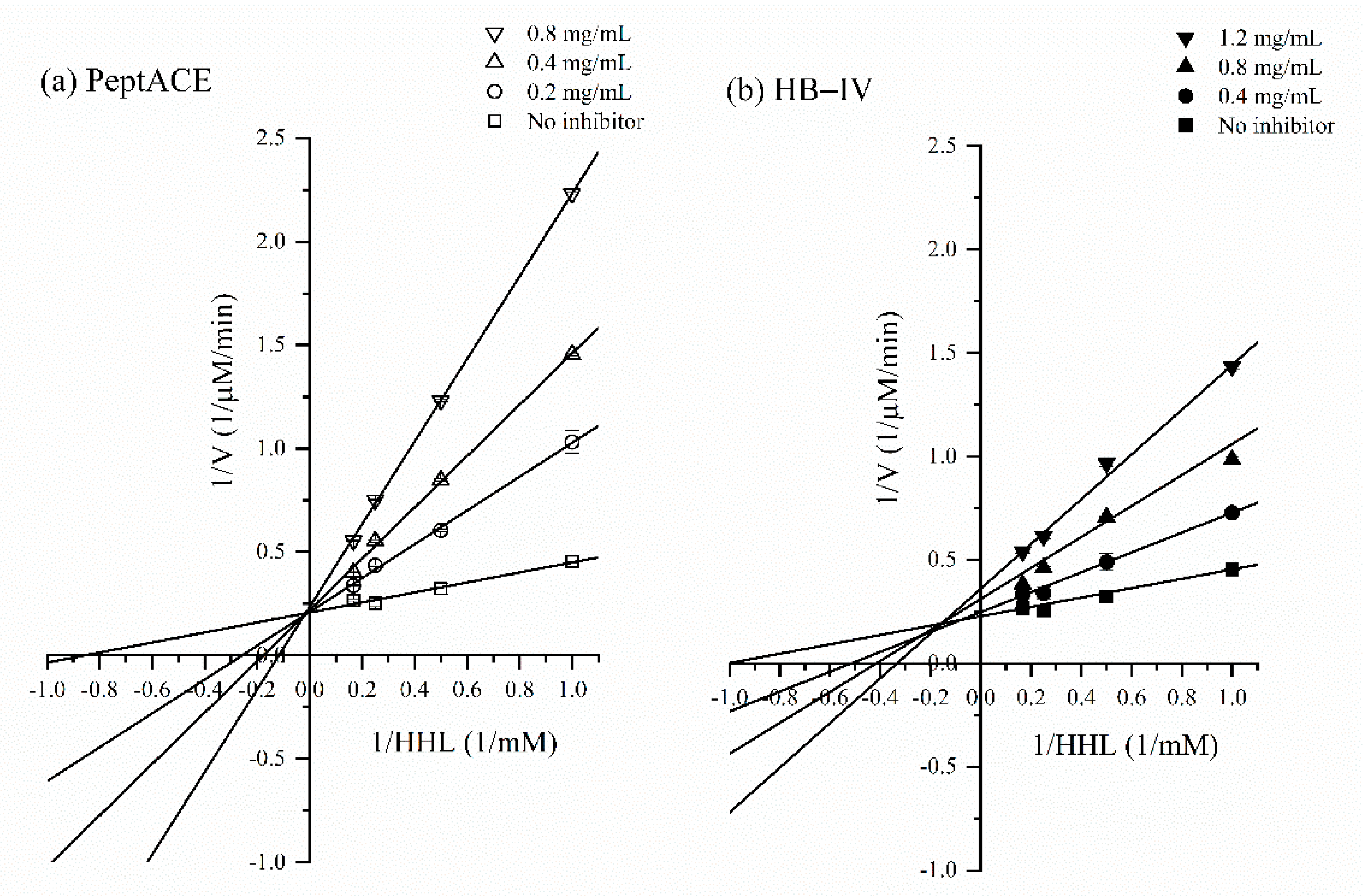
2.12. Determination of ACE-Inhibition Kinetics
2.13. Statistical Analysis
3. Results and Discussion
3.1. ACE-Inhibitory and Antioxidant Activities of Alcalase Hydrolysate
3.2. Purification of ACE-Inhibitory and Antioxidant Peptides
3.2.1. Ultrafiltration
3.2.2. Anion Exchange Chromatography
3.2.3. Gel Filtration Chromatography
3.3. Identification of the Peptide Sequences in HB-IV Fraction
3.4. Stability of HB-IV Fraction against In Vitro Stimulated Gastrointestinal Digestion
3.5. Stability of HB-IV Fraction against ACE
3.6. ACE-Inhibitory Kinetics of HB-IV Fraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Ryu, B.; Zhang, Y.; Liang, P.; Li, C.; Zhou, C.; Yang, P.; Hong, P.; Qian, Z. Comparison of an Angiotensin-I-Converting Enzyme Inhibitory Peptide from Tilapia (Oreachromix niloticus) with Captopril: Inhibition Kinetics, in Vivo Effect, Stimulated Gastrointestinal Digestion and a Molecular Docking Study. J. Sci. Food Agric. 2020, 100, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Ishak, N.H.; Shaik, M.I.; Yellapu, N.K.; Howell, N.K.; Sarbon, N.M. Purification, Characterization and Molecular Docking Study of Angiotensin-I Converting Enzyme (ACE) Inhibitory Peptide from Shortfin Scad (Decapterus macrosoma) Protein Hydrolysate. J. Food Sci. Technol. 2021, 58, 4567–4577. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.M.I.; Sohn, J.H.; Kim, J.-S.; Choi, J.-S. Identification and Characterization of Novel Antioxidant Peptides from Mackerel (Scomber japonicus) Muscle Protein Hydrolysates. Food Chem. 2020, 323, 126809. [Google Scholar] [CrossRef] [PubMed]
- Najafian, L.; Babji, A.S. Production of Bioactive Peptides Using Enzymatic Hydrolysis and Identification Antioxidative Peptides from Patin (Pangasius sutchi) Sarcoplasmic Protein Hydolysate. J. Funct. Foods 2014, 9, 280–289. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Production and Identification of Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides from Discarded Sardine pilchardus Protein. Food Chem. 2020, 328, 127096. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, R.; Gautam, H.; Jadon, G.; Dahiya, S. Bioactive Oxazole-Based Cyclopolypeptides from Marine Resources and Their Health Potential. In Bioactive Peptides: Production, Bioavailability, Health Potential, and Regulatory Issues; Onuh, J.O., Selvamuthukumaran, M., Pathak, Y.V., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 133–168. [Google Scholar]
- Charoensiddhi, S.; Conlon, M.A.; Franco, C.M.M.; Zhang, W. The Development of Seaweed-Derived Bioactive Compounds for Use as Prebiotics and Nutraceuticals Using Enzyme Technologies. Trends Food Sci. Technol. 2017, 70, 20–33. [Google Scholar] [CrossRef] [Green Version]
- Chalamaiah, M.; Dinesh Kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish Protein Hydrolysates: Proximate Composition, Amino Acid Composition, Antioxidant Activities and Applications: A Review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Kumar, J. Epidemiology of Hypertension. Clin. Queries Nephrol. 2013, 2, 56–61. [Google Scholar] [CrossRef]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global Burden of Hypertension: Analysis of Worldwide Data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- World Health Organization (WHO). A Global Brief on Hypertension; World Health Organization: Geneva, Switzerland, 2013; pp. 1–40. [Google Scholar]
- Ministry of Health (MOH). National Health and Morbility Survey 2019; Institute for Public Health: Shah Alam, Malaysia, 2019; Volume 1. [Google Scholar]
- Wijesekara, I.; Kim, S.-K. Angiotensin-I-Converting Enzyme (ACE) Inhibitors from Marine Resources: Prospects in the Pharmaceutical Industry. Mar. Drugs 2010, 8, 1080–1093. [Google Scholar] [CrossRef]
- Havelka, J.; Boerlin, H.J.; Studer, A.; Greminger, P.; Tenschert, W.; Luescher, T.; Siegenthaler, W.; Vetter, W.; Walger, P.; Vetter, H. Long-Term Experience with Captopril in Severe Hypertension. Br. J. Clin. Pharmacol. 1982, 14, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, T.; Seki, E.; Osajima, K.; Yoshida, M.; Asada, K.; Matsui, T.; Osajima, Y. Antihypertensive Effect of Valyl-Tyrosine, a Short Chain Peptide Derived from Sardine Muscle Hydrolyzate, on Mild Hypertensive Subjects. J. Hum. Hypertens. 2000, 14, 519–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tkaczewska, J.; Borczak, B.; Piątkowska, E.; Kapusta-Duch, J.; Morawska, M.; Czech, T. Effect of Protein Hydrolysates from Carp (Cyprinus carpio) Skin Gelatine on Oxidative Stress Biomarkers and Other Blood Parameters in Healthy Rats. J. Funct. Foods 2019, 60, 103411. [Google Scholar] [CrossRef]
- Ch’ng, C.L.; Senoo, S. Egg and Larval Development of a New Hybrid Grouper, Tiger Grouper Epinephelus fuscoguttatus x Giant Grouper, E. lanceolatus. Aquac. Sci. 2008, 56, 505–512. [Google Scholar]
- Ching, F.F.; Othman, N.; Anuar, A.; Shapawi, R.; Senoo, S. Natural Spawning, Embryonic and Larval Development of F2 Hybrid Grouper, Tiger Grouper Epinephelus fuscoguttatus × Giant Grouper, E. lanceolatus. Int. Aquat. Res. 2018, 10, 391–402. [Google Scholar] [CrossRef] [Green Version]
- Department of Fisheries Malaysia. Annual Fisheries Statistics 2017. Available online: https://www.dof.gov.my/en/resources/fisheries-statistics-i/ (accessed on 7 September 2021).
- Department of Fisheries Malaysia. Annual Fisheries Statistics. 2018. Available online: https://www.dof.gov.my/en/resources/fisheries-statistics-i/ (accessed on 7 September 2021).
- Yang, X.R.; Zhao, Y.Q.; Qiu, Y.T.; Chi, C.F.; Wang, B. Preparation and Characterization of Gelatin and Antioxidant Peptides from Gelatin Hydrolysate of Skipjack Tuna (Katsuwonus pelamis) Bone Stimulated by in Vitro Gastrointestinal Digestion. Mar. Drugs 2019, 17, 78. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.; De Gobba, C.; Skibsted, L.H.; Otte, J. Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Activity of Bioactive Peptides Produced by Enzymatic Hydrolysis of Skin from Grass Carp (Ctenopharyngodon idella). Int. J. Food Prop. 2017, 20, 1129–1144. [Google Scholar] [CrossRef] [Green Version]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; Alashi, M.A.; Aluko, R.E.; FitzGerald, R.J. Peptide Identification in a Salmon Gelatin Hydrolysate with Antihypertensive, Dipeptidyl Peptidase IV Inhibitory and Antioxidant Activities. Food Res. Int. 2017, 100, 112–120. [Google Scholar] [CrossRef]
- Gómez, L.J.; Gómez, N.A.; Zapata, J.E.; López-García, G.; Cilla, A.; Alegría, A. In-Vitro Antioxidant Capacity and Cytoprotective/Cytotoxic Effects upon Caco-2 Cells of Red Tilapia (Oreochromis spp.) Viscera Hydrolysates. Food Res. Int. 2019, 120, 52–61. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Regenstein, J.; Su, X. In Vitro and in Vivo Anti-Oxidation and Anti-Fatigue Effect of Monkfish Liver Hydrolysate. Food Biosci. 2017, 18, 9–14. [Google Scholar] [CrossRef]
- Chan, P.-T.; Matanjun, P.; Budiman, C.; Shapawi, R.; Lee, J.-S. ACE-Inhibitory and Antioxidant Activities of Hydrolysates from the By-Products of Hybrid Grouper (Epinephelus lanceolatus × Epinephelus fuscoguttatus). Sains Malays. 2020, 49, 261–270. [Google Scholar] [CrossRef]
- Girgih, A.T.; Udenigwe, C.C.; Li, H.; Adebiyi, A.P.; Aluko, R.E. Kinetics of Enzyme Inhibition and Antihypertensive Effects of Hemp Seed (Cannabis sativa L.) Protein Hydrolysates. J. Am. Oil Chem. Soc. 2012, 88, 1767–1774. [Google Scholar] [CrossRef]
- Saidi, S.; Deratani, A.; Belleville, M.P.; Amar, R. Ben Antioxidant Properties of Peptide Fractions from Tuna Dark Muscle Protein By-Product Hydrolysate Produced by Membrane Fractionation Process. Food Res. Int. 2014, 65, 329–336. [Google Scholar] [CrossRef]
- Raghavan, S.; Kristinsson, H.G. ACE-Inhibitory Activity of Tilapia Protein Hydrolysates. Food Chem. 2009, 117, 582–588. [Google Scholar] [CrossRef]
- Toopcham, T.; Roytrakul, S.; Yongsawatdigul, J. Characterization and Identification of Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides Derived from Tilapia Using Virgibacillus Halodenitrificans SK1-3-7 Proteinases. J. Funct. Foods 2015, 14, 435–444. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Gilani, G.S. Amino Acid Analysis. In Current Protocols in Protein Science; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 11.9.1–11.9.37. ISBN 0471140864. [Google Scholar]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric Assay and Properties Of The Angiotensin Converting Enzyme Of The Rabbit Lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Lee, J.K.; Jeon, J.K.; Byun, H.G. Effect of Angiotensin I Converting Enzyme Inhibitory Peptide Purified from Skate Skin Hydrolysate. Food Chem. 2011, 125, 495–499. [Google Scholar] [CrossRef]
- Je, J.Y.; Lee, K.H.; Lee, M.H.; Ahn, C.B. Antioxidant and Antihypertensive Protein Hydrolysates Produced from Tuna Liver by Enzymatic Hydrolysis. Food Res. Int. 2009, 42, 1266–1272. [Google Scholar] [CrossRef]
- Kim, S.S.; Ahn, C.B.; Moon, S.W.; Je, J.Y. Purification and Antioxidant Activities of Peptides from Sea Squirt (Halocynthia roretzi) Protein Hydrolysates Using Pepsin Hydrolysis. Food Biosci. 2018, 25, 128–133. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [Green Version]
- Khositanon, P.; Panya, N.; Roytrakul, S.; Krobthong, S.; Chanroj, S.; Choksawangkarn, W. Effects of Fermentation Periods on Antioxidant and Angiotensin I-Converting Enzyme Inhibitory Activities of Peptides from Fish Sauce by-Products. LWT Food Sci. Technol. 2021, 135, 110122. [Google Scholar] [CrossRef]
- Cinq-Mars, C.D.; Hu, C.; Kitts, D.D.; Li-Chan, E.C.Y. Investigations into Inhibitor Type and Mode, Simulated Gastrointestinal Digestion, and Cell Transport of the Angiotensin I-Converting Enzyme-Inhibitory Peptides in Pacific Hake (Merluccius productus) Fillet Hydrolysate. J. Agric. Food Chem. 2008, 56, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Ghassem, M.; Arihara, K.; Babji, A.S. Isolation, Purification and Characterisation of Angiotensin I-Converting Enzyme-Inhibitory Peptides Derived from Catfish (Clarias batrachus) Muscle Protein Thermolysin Hydrolysates. Int. J. Food Sci. Technol. 2012, 47, 2444–2451. [Google Scholar] [CrossRef]
- Lee, J.K.; Jeon, J.-K.; Byun, H.-G. Antihypertensive Effect of Novel Angiotensin I Converting Enzyme Inhibitory Peptide from Chum Salmon (Oncorhynchus keta) Skin in Spontaneously Hypertensive Rats. J. Funct. Foods 2014, 7, 381–389. [Google Scholar] [CrossRef]
- Ghassem, M.; Babji, A.S.; Said, M.; Mahmoodani, F.; Arihara, K. Angiotensin I-Converting Enzyme Inhibitory Peptides from Snakehead Fish Sarcoplasmic Protein Hydrolysate. J. Food Biochem. 2014, 38, 140–149. [Google Scholar] [CrossRef]
- Zhou, D.-Y.; Tang, Y.; Zhu, B.-W.; Qin, L.; Li, D.-M.; Yang, J.-F.; Lei, K. Antioxidant Activity of Hydrolysates Obtained from Scallop (Patinopecten yessoensis) and Abalone (Haliotis discus hannai Ino) Muscle. Food Chem. 2012, 132, 815–822. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Hu, F.Y.; Wang, Y.M.; Zhang, B.; Deng, S.G.; Wu, C.W. Purification and Identification of Three Novel Antioxidant Peptides from Protein Hydrolysate of Bluefin Leatherjacket (Navodon septentrionalis) Skin. Food Res. Int. 2014, 73, 124–129. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, P.-J.; Kwon, J.Y.; Kim, S.-K. A Novel Angiotensin I Converting Enzyme Inhibitory Peptide from Alaska Pollack (Theragra chalcogramma) Frame Protein Hydrolysate. J. Agric. Food Chem. 2004, 52, 7842–7845. [Google Scholar] [CrossRef]
- Onuh, J.O.; Girgih, A.T.; Malomo, S.A.; Aluko, R.E.; Aliani, M. Kinetics of in Vitro Renin and Angiotensin Converting Enzyme Inhibition by Chicken Skin Protein Hydrolysates and Their Blood Pressure Lowering Effects in Spontaneously Hypertensive Rats. J. Funct. Foods 2015, 14, 133–143. [Google Scholar] [CrossRef]
- Norris, R.; FitzGerald, R.J. Antihypertensive Peptides from Food Proteins. In Bioactive Food Peptides in Health and Disease; Hernandez-Ledesma, B., Hsieh, C.-C., Eds.; InTechOpen Ltd.: London, UK, 2013; pp. 45–72. [Google Scholar]
- Zhang, P.; Chang, C.; Liu, H.; Li, B.; Yan, Q.; Jiang, Z. Identification of Novel Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides from Wheat Gluten Hydrolysate by the Protease of Pseudomonas aeruginosa. J. Funct. Foods 2020, 65, 103751. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Deng, Y.Y.; Wang, Y.M.; Deng, S.G.; Ma, J.Y. Isolation and Characterization of Three Antioxidant Pentapeptides from Protein Hydrolysate of Monkfish (Lophius litulon) Muscle. Food Res. Int. 2014, 55, 222–228. [Google Scholar] [CrossRef]
- Kimatu, B.M.; Zhao, L.; Biao, Y.; Ma, G.; Yang, W.; Pei, F.; Hu, Q. Antioxidant Potential of Edible Mushroom (Agaricus bisporus) Protein Hydrolysates and Their Ultrafiltration Fractions. Food Chem. 2017, 230, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino Acid Composition and Antioxidant Properties of Pea Seed (Pisum sativum L.) Enzymatic Protein Hydrolysate Fractions. J. Agric. Food Chem. 2010, 58, 4712–4718. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Chemometric Analysis of the Amino Acid Requirements of Antioxidant Food Protein Hydrolysates. Int. J. Mol. Sci. 2011, 12, 3148–3161. [Google Scholar] [CrossRef] [Green Version]
- Girgih, A.T.; Udenigwe, C.C.; Aluko, R.E. In Vitro Antioxidant Properties of Hemp Seed (Cannabis sativa L.) Protein Hydrolysate Fractions. J. Am. Oil Chem. Soc. 2011, 88, 381–389. [Google Scholar] [CrossRef]
- Je, J.Y.; Kim, S.Y.; Kim, S.K. Preparation and Antioxidative Activity of Hoki Frame Protein Hydrolysate Using Ultrafiltration Membranes. Eur. Food Res. Technol. 2005, 221, 157–162. [Google Scholar] [CrossRef]
- Gianfranceschi, G.L.; Gianfranceschi, G.; Quassinti, L.; Bramucci, M. Biochemical Requirements of Bioactive Peptides for Nutraceutical Efficacy. J. Funct. Foods 2018, 47, 252–263. [Google Scholar] [CrossRef]
- Meisel, H.; Walsh, D.J.; Murray, B.; FitzGerald, R.J. ACE-Inhibitory Peptides. In Nutraceutical Proteins and Peptides in Health and Disease; Mine, Y., Shahidi, F., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 269–315. [Google Scholar]
- Quirós, A.; del Contreras, M.M.; Ramos, M.; Amigo, L.; Recio, I. Stability to Gastrointestinal Enzymes and Structure-Activity Relationship of β-Casein-Peptides with Antihypertensive Properties. Peptides 2009, 30, 1848–1853. [Google Scholar] [CrossRef]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef]
- Byun, H.; Kim, S. Structure and Activity of Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Alaskan Pollack Skin. Biochem. Mol. Biol. 2002, 35, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory Peptides: Quantitative Structure-Activity Relationship Study of Di- and Tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, I.D.; Aluko, R.E. Structural and Functional Properties of Food Protein-Derived Antioxidant Peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarmadi, B.H.; Ismail, A. Antioxidative Peptides from Food Proteins: A Review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-M.; Deng, S.-G.; Huang, S.-B. Antioxidant Activities of Ferrous-Chelating Peptides Isolated from Five Types of Low-Value Fish Protein Hydrolysates. J. Food Biochem. 2014, 38, 627–633. [Google Scholar] [CrossRef]
- Qian, Z.J.; Je, J.Y.; Kim, S.K. Antihypertensive Effect of Angiotensin I Converting Enzyme-Inhibitory Peptide from Hydrolysates of Bigeye Tuna Dark Muscle, Thunnus Obesus. J. Agric. Food Chem. 2007, 55, 8398–8403. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Y.; Li, L.; Chi, C. Four Antioxidant Peptides from Protein Hydrolysate of Red Stingray (Dasyatis Akajei) Cartilages: Isolation, Identification, and In Vitro Activity Evaluation. Mar. Drugs 2019, 17, 263. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Montoro, M.; Olalla-herrera, M.; Rufian-Henares, J.Á.; Martínez, R.G.; Miralles, B.; Bergillos, T.; Navarro-Alarcón, M.; Jauregi, P. Antioxidant, ACE-Inhibitory and Antimicrobial Activity of Fermented Goat Milk: Activity and Physicochemical Property Relationship of the Peptide Components. Food Funct. 2017, 8, 2783–2791. [Google Scholar] [CrossRef] [Green Version]
- Ao, J.; Li, B. Stability and Antioxidative Activities of Casein Peptide Fractions during Simulated Gastrointestinal Digestion in Vitro: Charge Properties of Peptides Affect Digestive Stability. Food Res. Int. 2013, 52, 334–341. [Google Scholar] [CrossRef]
- Drauz, K.; Grayson, I.; Kleemann, A.; Krimmer, H.-P.; Leuchtenberger, W.; Weckbecker, C. Amino Acids. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; Volume 3, pp. 1–51. [Google Scholar]
- Zhu, L.; Jie, C.; Tang, X.; Xiong, Y.L. Reducing, Radical Scavenging, and Chelation Properties of in Vitro Digests of Alcalase-Treated Zein Hydrolysate. J. Agric. Food Chem. 2008, 56, 2714–2721. [Google Scholar] [CrossRef]
- Weng, W.; Tang, L.; Wang, B.; Chen, J.; Su, W.; Osako, K.; Tanaka, M. Antioxidant Properties of Fractions Isolated from Blue Shark (Prionace glauca) Skin Gelatin Hydrolysates. J. Funct. Foods 2014, 11, 342–351. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Zhong, Q.; Wu, Y.; Xia, W. Peptides Purification and Characterization of a Novel Angiotensin-I Converting Enzyme (ACE) Inhibitory Peptide Derived from Enzymatic Hydrolysate of Grass Carp Protein. Peptides 2012, 33, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; He, H.L.; Chen, X.L.; Sun, C.Y.; Zhang, Y.Z.; Zhou, B.C. Purification and Identification of Novel Angiotensin-I-Converting Enzyme Inhibitory Peptides from Shark Meat Hydrolysate. Process Biochem. 2008, 43, 457–461. [Google Scholar] [CrossRef]
- Tuz, M.A.O.; Campos, M.R.S. Purification of Mucuna pruriens (L) Peptide Fractions and Evaluation of Their ACE Inhibitory Effect. Biocatal. Agric. Biotechnol. 2017, 10, 390–395. [Google Scholar] [CrossRef]
- Balti, R.; Bougatef, A.; Sila, A.; Guillochon, D.; Dhulster, P.; Nedjar-arroume, N. Nine Novel Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides from Cuttlefish (Sepia officinalis) Muscle Protein Hydrolysates and Antihypertensive Effect of the Potent Active Peptide in Spontaneously Hypertensive Rats. Food Chem. 2015, 170, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.; Jeon, Y.; Kim, Y.; Je, J. Angiotensin I Converting Enzyme (ACE) Inhibitory Peptides from Salmon Byproduct Protein Hydrolysate by Alcalase Hydrolysis. Process Biochem. 2012, 47, 2240–2245. [Google Scholar] [CrossRef]
- Chai, T.-T.; Law, Y.-C.; Wong, F.-C.; Kim, S.-K. Enzyme-Assisted Discovery of Antioxidant Peptides from Edible Marine Invertebrates: A Review. Mar. Drugs 2017, 15, 42. [Google Scholar] [CrossRef]
- Zhang, P.; Roytrakul, S.; Sutheerawattananonda, M. Production and Purification of Glucosamine and Angiotensin-I Converting Enzyme (ACE) Inhibitory Peptides from Mushroom Hydrolysates. J. Funct. Foods 2017, 36, 72–83. [Google Scholar] [CrossRef]
- Gallego, M.; Mauri, L.; Aristoy, M.C.; Toldrá, F.; Mora, L. Antioxidant Peptides Profile in Dry-Cured Ham as Affected by Gastrointestinal Digestion. J. Funct. Foods 2020, 69, 103956. [Google Scholar] [CrossRef]
- Yokoyama, K.; Chiba, H.; Yoshikawa, M. Peptide Inhibitors for Angiotensin I-Converting Enzyme from Thermolysin Digest of Dried Bonitot. Biosci. Biotechnol. Biochem. 1992, 56, 1541–1545. [Google Scholar] [CrossRef]
- Thuanthong, M.; De Gobba, C.; Sirinupong, N.; Youravong, W.; Otte, J. Purification and Characterization of Angiotensin-Converting Enzyme- Inhibitory Peptides from Nile Tilapia (Oreochromis niloticus) Skin Gelatine Produced by an Enzymatic Membrane Reactor. J. Funct. Foods 2017, 36, 243–254. [Google Scholar] [CrossRef]
- Ketnawa, S.; Benjakul, S.; Martínez-Alvarez, O.; Rawdkuen, S. Fish Skin Gelatin Hydrolysates Produced by Visceral Peptidase and Bovine Trypsin: Bioactivity and Stability. Food Chem. 2017, 215, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; He, T.; Li, H.; Tang, H.; Xia, E. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Lima, K.O.; da Costa de Quadros, C.; da Rocha, M.; de Lacerda, J.T.J.G.; Juliano, M.A.; Dias, M.; Mendes, M.A.; Prentice, C. Bioactivity and Bioaccessibility of Protein Hydrolyzates from Industrial Byproducts of Stripped Weakfish (Cynoscion Guatucupa). LWT Food Sci. Technol. 2019, 111, 408–413. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Gelatin Hydrolysate from Blacktip Shark Skin Prepared Using Papaya Latex Enzyme: Antioxidant Activity and Its Potential in Model Systems. Food Chem. 2012, 135, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Khantaphant, S.; Benjakul, S.; Kishimura, H. Antioxidative and ACE Inhibitory Activities of Protein Hydrolysates from the Muscle of Brownstripe Red Snapper Prepared Using Pyloric Caeca and Commercial Proteases. Process Biochem. 2011, 46, 318–327. [Google Scholar] [CrossRef]
- Fujita, H.; Yokoyama, K.; Yoshikawa, M. Classification and Antihypertensive Activity of Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Food Proteins. J. Food Sci. 2000, 65, 564–569. [Google Scholar] [CrossRef]
- Fujita, H.; Yamagami, T.; Ohshima, K. Effects of an Ace-Inhibitory Agent, Katsuobushi Oligopeptide, in the Spontaneously Hypertensive Rat and in Borderline and Mildly Hypertensive Subjects. Nutr. Res. 2001, 21, 1149–1158. [Google Scholar] [CrossRef]
- Forghani, B.; Zarei, M.; Ebrahimpour, A.; Philip, R.; Bakar, J.; Abdul Hamid, A.; Saari, N. Purification and Characterization of Angiotensin Converting Enzyme-Inhibitory Peptides Derived from Stichopus Horrens: Stability Study against the ACE and Inhibition Kinetics. J. Funct. Foods 2016, 20, 276–290. [Google Scholar] [CrossRef]
- Ono, S.; Hosokawa, M.; Miyashita, K.; Takahashi, K. Inhibition Properties of Dipeptides from Salmon Muscle Hydrolysate on Angiotensin I-Converting Enzyme. Int. J. Food Sci. Technol. 2006, 41, 383–386. [Google Scholar] [CrossRef]


| Fraction | MW (kDa) | ACE-Inhibitory Activity (IC50, mg/mL) | Hydroxyl Radical Scavenging Activity (EC50, mg/mL) | Reducing Power * (Abs at 700 nm) | Metal-chelating Activity (EC50, ug/mL) | Protein Content (mg/g) | Yield |
|---|---|---|---|---|---|---|---|
| HB-I | >5 | 1.19 ± 0.17 a | 6.97 ± 0.70 a | 0.352 ± 0.039 a | 156.23 ± 18.00 a | 104.34 ± 2.37 a | 25.10 ± 0.78 a |
| HB-II | 3–5 | 0.39 ± 0.06 b | 6.74 ± 0.71 a | 0.264 ± 0.010 b | 83.24 ± 9.99 b | 83.25 ± 2.08 b | 19.87 ± 0.21 b |
| HB-III | 1–3 | 0.44 ± 0.10 b | 5.90 ± 0.60 a | 0.203 ± 0.024 b | 34.34 ± 3.20 c | 77.15 ± 4.01 b | 19.27 ± 0.32 b |
| HB-IV | <1 | 0.28 ± 0.03 b | 3.48 ± 0.14 b | 0.116 ± 0.007 c | 34.07 ± 2.24 c | 37.41 ± 0.30 c | 14.37 ± 0.57 c |
| PeptACE ^ | 0.22 ± 0.03 b | 0.44 ± 0.02 c | 0.336 ± 0.024 a | >1000 | ND | ND | |
| Ascorbic acid | ND | 1.57 ± 0.20 c | 0.928 ± 0.027 # | ND | ND | ND | |
| EDTA | ND | ND | ND | 4.34 ± 0.43 d | ND | ND |
| Amino Acids | Amino Acid Composition (% Total Amino Acids) | |||||
|---|---|---|---|---|---|---|
| Al-HB | HB-I | HB-II | HB-III | HB-IV | PeptACE | |
| Asp | 8.00 ± 0.15 d | 7.87 ± 0.29 d | 9.49 ± 0.12 b | 8.75 ± 0.15 c | 7.79 ± 0.30 d | 10.75 ± 0.06 a |
| Glu | 13.89 ± 0.27 cd | 13.08 ± 0.48 d | 15.10 ± 0.96 bc | 15.93 ± 0.36 ab | 15.55 ± 0.41 ab | 16.82 ± 0.12 a |
| Ser | 4.04 ± 0.13 c | 3.86 ± 0.19 c | 3.82 ± 0.28 c | 4.64 ± 0.17 b | 5.31 ± 0.25 a | 4.19 ± 0.02 bc |
| His | 1.98 ± 0.24 cd | 1.68 ± 0.23 d | 2.26 ± 0.17 bcd | 2.61 ± 0.43 bc | 2.90 ± 0.33 b | 4.97 ± 0.20 a |
| Gly | 11.83 ± 0.38 b | 15.46 ± 0.80 a | 7.78 ± 0.16 c | 6.20 ± 0.24 d | 8.12 ± 1.03 c | 4.37 ± 0.04 e |
| Thr | 4.04 ± 0.11 cd | 3.48 ± 0.24 d | 4.20 ± 0.25 bc | 4.94 ± 0.14 a | 4.51 ± 0.41 abc | 4.72 ± 0.07 ab |
| Arg | 7.88 ± 0.19 ab | 8.81 ± 0.33 a | 7.76 ± 0.86 ab | 7.13 ± 0.21 bc | 5.66 ± 0.21 d | 6.15 ± 0.09 cd |
| Ala | 8.17 ± 0.15 b | 8.09 ± 0.28 b | 6.83 ± 0.26 c | 8.17 ± 0.18 b | 13.41 ± 0.78 a | 6.84 ± 0.05 c |
| Tyr | 2.46 ± 0.06 c | 1.68 ± 0.04 d | 2.74 ± 0.15 b | 3.78 ± 0.04 a | 3.57 ± 0.11 a | 2.72 ± 0.04 b |
| Val | 4.54 ± 0.10 bc | 3.97 ± 0.10 c | 5.16 ± 0.37 ab | 5.78 ± 0.12 a | 4.48 ± 0.50 bc | 4.63 ± 0.09 bc |
| Met | 2.47 ± 0.32 c | 1.98 ± 0.10 d | 2.41 ± 0.01 c | 3.44 ± 0.05 a | 3.80 ± 0.14 a | 2.96 ± 0.09 b |
| Trp | 0.73 ± 0.15 b | 0.93 ± 0.12 b | 1.83 ± 0.12 a | 0.95 ± 0.27 b | 1.01 ± 0.17 b | 0.71 ± 0.02 b |
| Phe | 4.07 ± 0.05 bc | 3.64 ± 0.19 c | 4.29 ± 0.12 abc | 5.04 ± 0.07 a | 4.66 ± 0.63 ab | 3.76 ± 0.06 c |
| Ile | 3.90 ± 0.12 c | 3.14 ± 0.09 d | 4.82 ± 0.12 b | 5.27 ± 0.06 a | 3.68 ± 0.30 c | 4.58 ± 0.04 b |
| Leu | 6.88 ± 0.12 c | 5.36 ± 0.16 d | 7.91 ± 0.24 b | 9.44 ± 0.15 a | 9.42 ± 0.09 a | 8.14 ± 0.05 b |
| Lys | 6.31 ± 1.02 bc | 3.52 ± 0.78 d | 7.37 ± 0.90 ab | 7.13 ± 1.27 bc | 4.66 ± 0.81 cd | 10.04 ± 1.03 a |
| Hyp | 2.36 ± 0.13 b | 3.39 ± 0.42 a | 1.36 ± 0.14 c | 0.84 ± 0.16 c | 1.41 ± 0.28 c | ND |
| Pro | 6.47 ± 0.31 b | 10.07 ± 1.98 a | 4.84 ± 0.40 bc | 2.90 ± 0.30 cd | 1.53 ± 0.14 d | 3.63 ± 0.39 cd |
| HAA | 33.21 ± 0.46 d | 28.79 ± 0.65 e | 36.01 ± 0.44 c | 41.85 ± 0.48 b | 44.28 ± 1.04 a | 34.34 ± 0.21 d |
| PCAA | 16.18 ± 1.08 bcd | 14.00 ± 0.50 cd | 17.39 ± 1.49 b | 16.86 ± 1.29 bc | 13.29 ± 1.02 d | 21.17 ± 0.74 a |
| NCAA | 21.89 ± 0.42 cd | 20.94 ± 0.77 d | 24.59 ± 0.85 b | 24.68 ± 0.50 b | 23.46 ± 0.49 bc | 27.57 ± 0.18 a |
| AroAA | 7.25 ± 0.09 c | 6.25 ± 0.13 d | 8.87 ± 0.06 b | 9.76 ± 0.23 a | 9.28 ± 0.48 ab | 7.18 ± 0.06 c |
| BCAA | 15.31 ± 0.32 c | 12.47 ± 0.33 d | 17.89 ± 0.36 b | 20.49 ± 0.31 a | 17.68 ± 0.46 b | 17.36 ± 0.11 b |
| Amino Acids | Amino Acid Composition (% Total Amino Acids) | ||
|---|---|---|---|
| F1 | F2 | F3 | |
| Asp | 2.49 ± 0.20 c | 9.10 ± 0.29 a | 7.89 ± 0.45 b |
| Glu | 7.33 ± 0.63 c | 27.19 ± 1.14 a | 29.30 ± 1.50 a |
| Ser | 5.85 ± 0.42 b | 7.92 ± 0.34 a | 4.68 ± 0.32 c |
| His | 5.90 ± 0.57 a | ND | ND |
| Gly | 17.61 ± 0.39 a | 12.22 ± 0.83 b | 13.02 ± 2.30 b |
| Thr | 2.63 ± 0.27 a | 1.54 ± 0.23 b | 0.91 ± 0.16 c |
| Arg | 13.25 ± 1.20 ab | 10.29 ± 1.15 b | 15.33 ± 1.74 a |
| Ala | 25.60 ± 3.13 a | 2.56 ± 0.35 b | 1.29 ± 0.18 b |
| Tyr | 3.68 ± 0.55 c | 6.70 ± 0.70 b | 8.24 ± 0.44 a |
| Val | 2.31 ± 0.42 a | 2.17 ± 0.31 a | 1.33 ± 0.24 b |
| Met | 1.39 ± 0.26 a | 0.94 ± 0.21 a | 0.42 ± 0.04 b |
| Phe | 2.27 ± 0.26 a | 2.71 ± 0.50 a | 2.75 ± 0.21 a |
| Ile | 1.28 ± 0.07 b | 1.82 ± 0.20 a | 0.98 ± 0.19 b |
| Leu | 6.21 ± 0.61 ab | 7.36 ± 0.34 a | 4.77 ± 0.79 b |
| Lys | 2.21 ± 0.10 c | 7.47 ± 0.04 b | 9.08 ± 1.08 a |
| HAA | 42.74 ± 2.44 a | 24.27 ± 1.31 b | 19.78 ± 1.16 c |
| PCAA | 21.36 ± 1.37 b | 17.75 ± 1.16 c | 24.41 ± 0.92 a |
| NCAA | 9.82 ± 0.83 b | 36.29 ± 1.69 a | 37.19 ± 1.92 a |
| AroAA | 5.95 ± 0.35 b | 9.42 ± 1.08 a | 10.99 ± 0.57 a |
| BCAA | 8.88 ± 0.82 a | 10.12 ± 0.45 a | 6.16 ± 0.93 b |
| Fractions | Step | Bioactivities | |||
|---|---|---|---|---|---|
| ACE-Inhibitory Activity (IC50, mg/mL) | Hydroxyl Radical Scavenging Activity (EC50, mg/mL) | Reducing Power * (Abs at 700 nm) | Metal-chelating Activity (EC50, mg/mL) | ||
| Al-HB | 0.84 ± 0.17 | 6.82 ± 0.63 | 0.239 ± 0.026 | 0.042 ± 0.005 | |
| HB-IV | Ultrafiltration | 0.28 ± 0.03 | 3.48 ± 0.14 | 0.116 ± 0.007 | 0.034 ± 0.002 |
| F1 | Anion exchange chromatography | 3.18 ± 0.53 | 4.45 ± 0.72 | 0.136 ± 0.008 | 5.15 ± 0.98 |
| F1-B | Gel filtration chromatography | 5.76 ± 0.78 | 9.01 ± 1.98 | 0.039 ± 0.004 | 5.89 ± 0.60 |
| Identified Peptide Sequences * | Observed Mass (Da) | Identified Peptide Sequences * | Observed Mass (Da) |
|---|---|---|---|
| RL | 287.19 | FDLK | 521.28 |
| LL | 244.18 | FCVH | 504.22 |
| ALLL | 428.29 | LPML | 472.27 |
| LRL | 400.28 | LKLF | 519.34 |
| LGGL | 358.22 | MLCM | 496.18 |
| AGPL | 356.21 | LGFL | 448.27 |
| PCLN | 445.20 | MMVF | 542.22 |
| LLGL | 414.28 | MPFE | 522.21 |
| LAFL | 463.28 | LGSF | 422.22 |
| LSFL | 478.28 | FAGL | 406.22 |
| LGLL | 414.28 | MLLP | 472.27 |
| FLGM | 466.23 | MPDLR | 630.32 |
| TLPF | 476.26 | FADTF | 599.26 |
| LPAL | 412.27 | MGGVF | 509.23 |
| AFLK | 477.30 | MPLLM | 619.31 |
| DPLL | 456.26 | LDAGF | 521.25 |
| LGPL | 398.25 | GPAGPL | 510.28 |
| LNFL | 505.29 | YPGLWMR | 921.45 |
| VALF | 448.27 | YPGLADMR | 921.44 |
| ALFL | 462.28 | PPACLTAL | 784.42 |
| LYLF | 554.31 | LLMLLL | 714.47 |
| Samples | ACE-Inhibitory Activity (IC50, mg/mL) | Inhibitor Category | |
|---|---|---|---|
| Without Pre-Incubation | With Pre-Incubation | ||
| HB-IV | 0.28 ± 0.03 b | 1.52 ± 0.11 a | Substrate |
| HB-IV (SGID) | 1.76 ± 0.28 a | 1.96 ± 0.22 a | True inhibitor |
| PeptACE | 0.22 ± 0.03 a | 0.15 ± 0.01 b | Pro-drug |
| PeptACE (SGID) | 0.19 ± 0.04 a | 0.13 ± 0.02 b | Pro-drug |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, P.-T.; Matanjun, P.; Budiman, C.; Shapawi, R.; Lee, J.-S. Novel Peptide Sequences with ACE-Inhibitory and Antioxidant Activities Derived from the Heads and Bones of Hybrid Groupers (Epinephelus lanceolatus × Epinephelus fuscoguttatus). Foods 2022, 11, 3991. https://doi.org/10.3390/foods11243991
Chan P-T, Matanjun P, Budiman C, Shapawi R, Lee J-S. Novel Peptide Sequences with ACE-Inhibitory and Antioxidant Activities Derived from the Heads and Bones of Hybrid Groupers (Epinephelus lanceolatus × Epinephelus fuscoguttatus). Foods. 2022; 11(24):3991. https://doi.org/10.3390/foods11243991
Chicago/Turabian StyleChan, Pei-Teng, Patricia Matanjun, Cahyo Budiman, Rossita Shapawi, and Jau-Shya Lee. 2022. "Novel Peptide Sequences with ACE-Inhibitory and Antioxidant Activities Derived from the Heads and Bones of Hybrid Groupers (Epinephelus lanceolatus × Epinephelus fuscoguttatus)" Foods 11, no. 24: 3991. https://doi.org/10.3390/foods11243991
APA StyleChan, P.-T., Matanjun, P., Budiman, C., Shapawi, R., & Lee, J.-S. (2022). Novel Peptide Sequences with ACE-Inhibitory and Antioxidant Activities Derived from the Heads and Bones of Hybrid Groupers (Epinephelus lanceolatus × Epinephelus fuscoguttatus). Foods, 11(24), 3991. https://doi.org/10.3390/foods11243991







