Interaction between Kidney-Bean Polysaccharides and Duck Myofibrillar Protein as Affected by Ultrasonication: Effects on Gel Properties and Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction of Myofibrillar Proteins
2.3. Sample Treatment
2.4. Gel Properties
2.4.1. Gel Strength
2.4.2. Water Holding Capacity
2.4.3. Dynamic Rheological Testing
2.4.4. Low-Field Nuclear Magnetic Resonance (NMR)
2.4.5. Scanning Electron Microscopy
2.5. Physicochemical Properties
2.5.1. Solubility
2.5.2. Surface Hydrophobicity
2.5.3. Total SH
2.5.4. SDS-PAGE
2.5.5. Particle Size and Zeta Potential
2.5.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.7. Chemical Forces
2.6. Statistical Analysis
3. Results and Discussion
3.1. Gel Strength and WHC
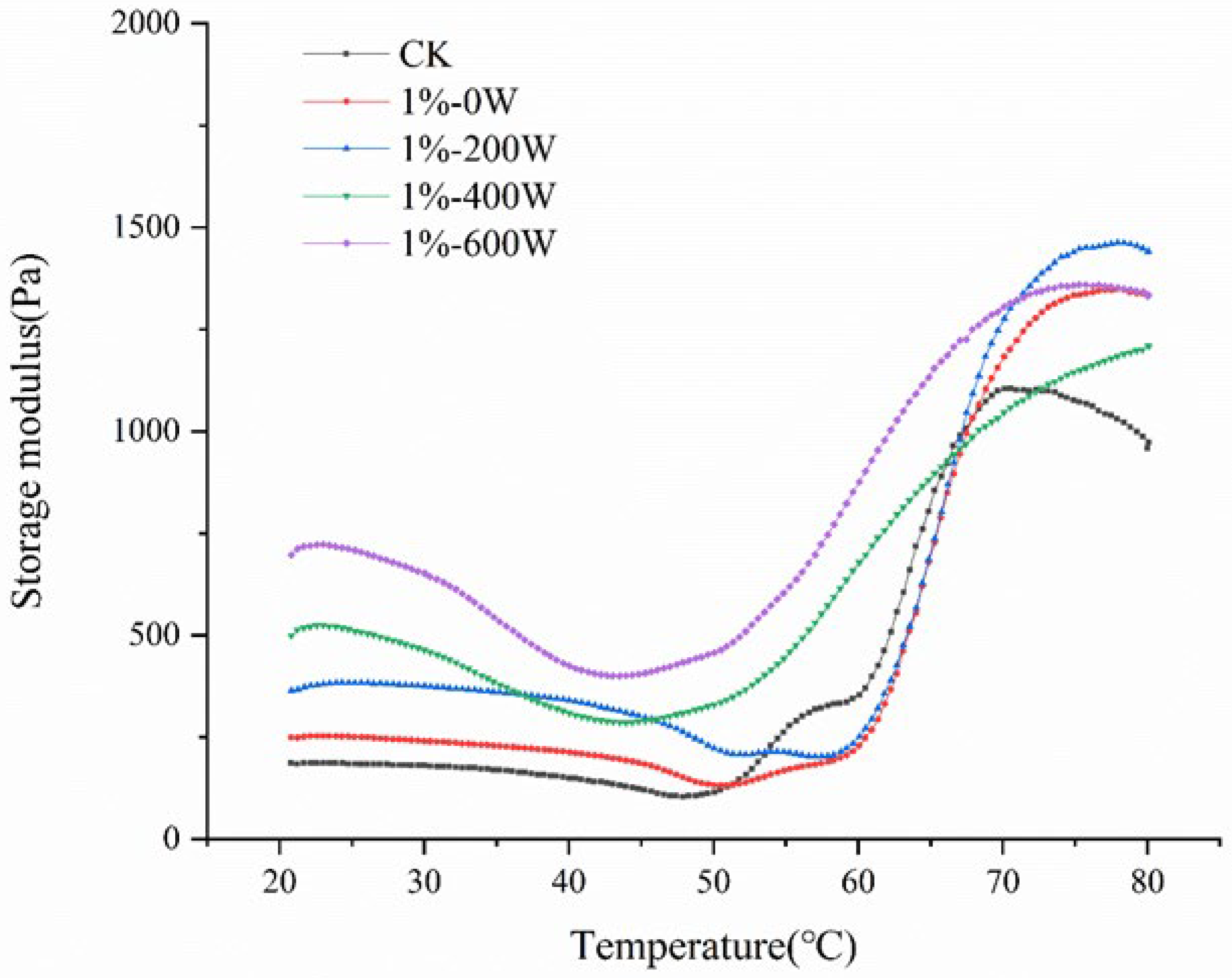
3.2. Dynamic Rheological Analysis
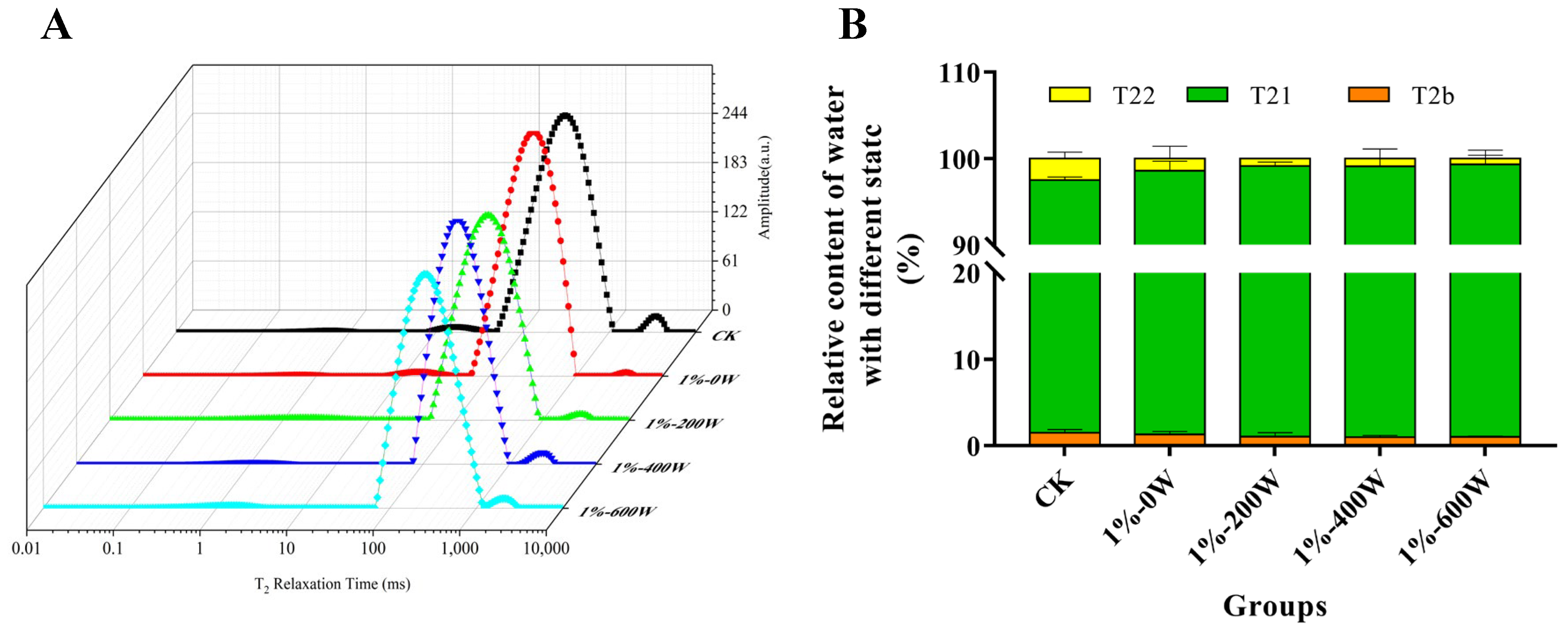
3.3. Water State and Distribution of MP Gel
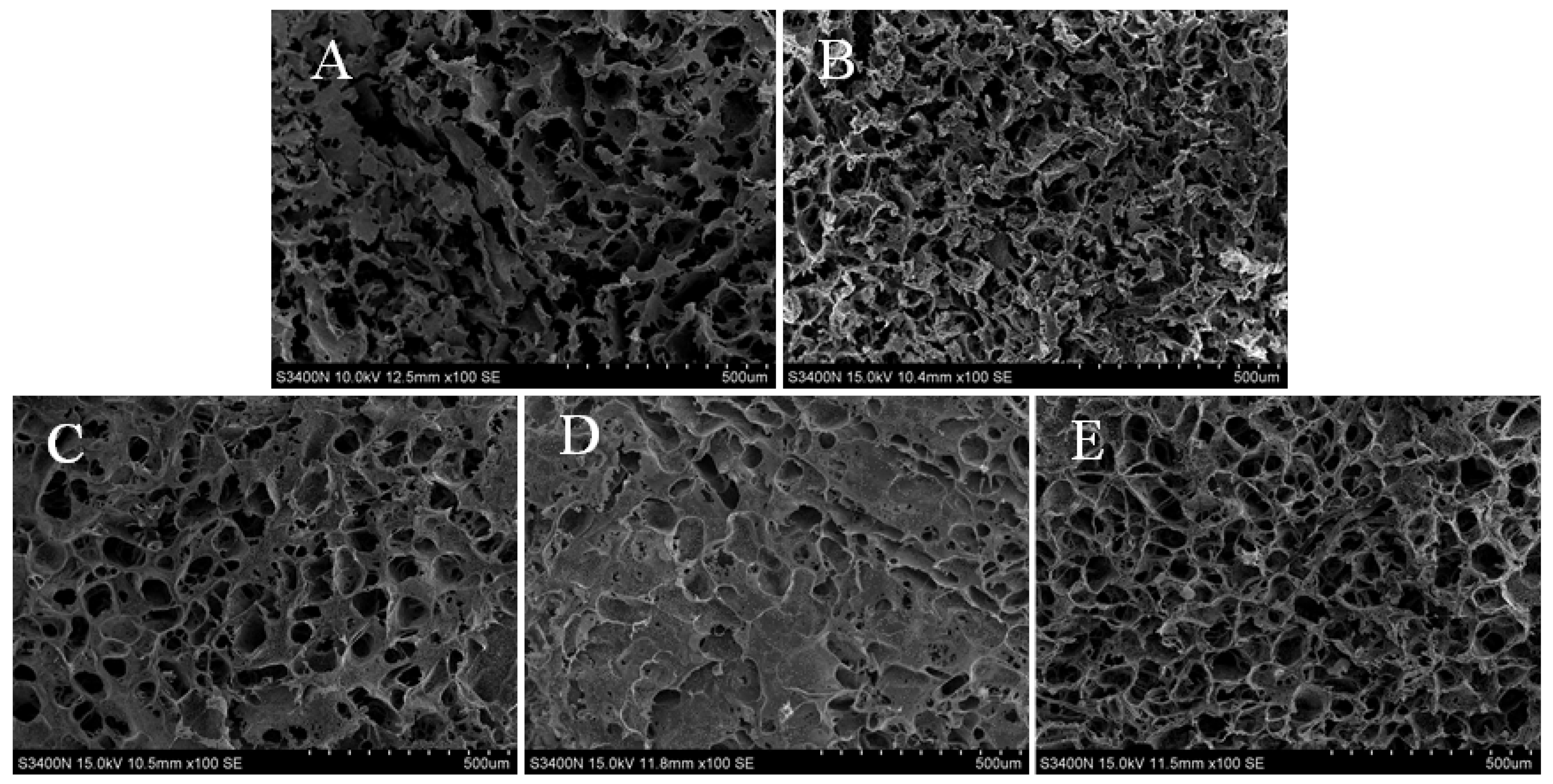
3.4. Microstructure Analysis
3.5. Changes the Solubility
3.6. Surface Hydrophobicity
3.7. Total SH
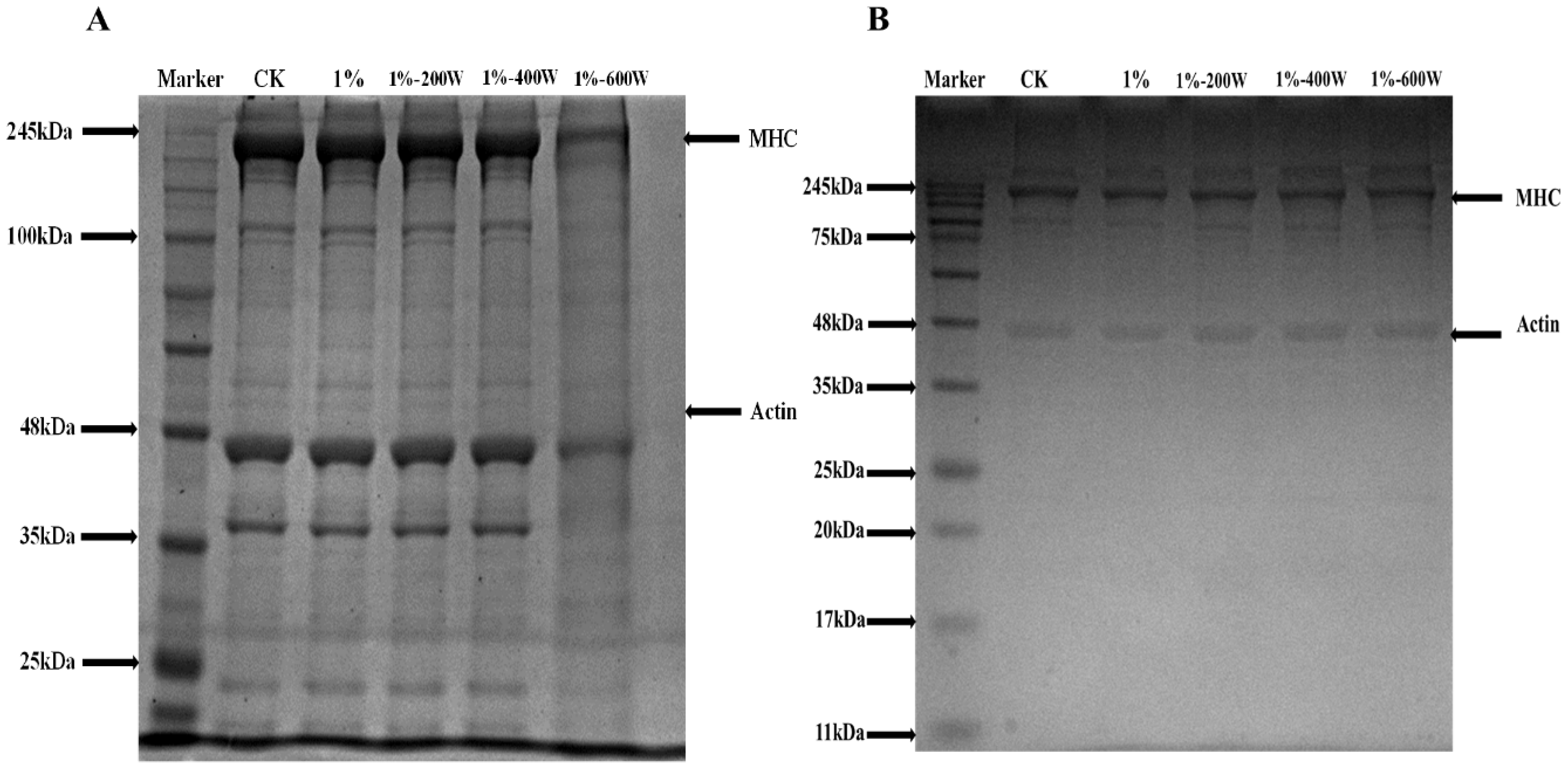
3.8. SDS-PAGE
3.9. Particle Size and Zeta Potential
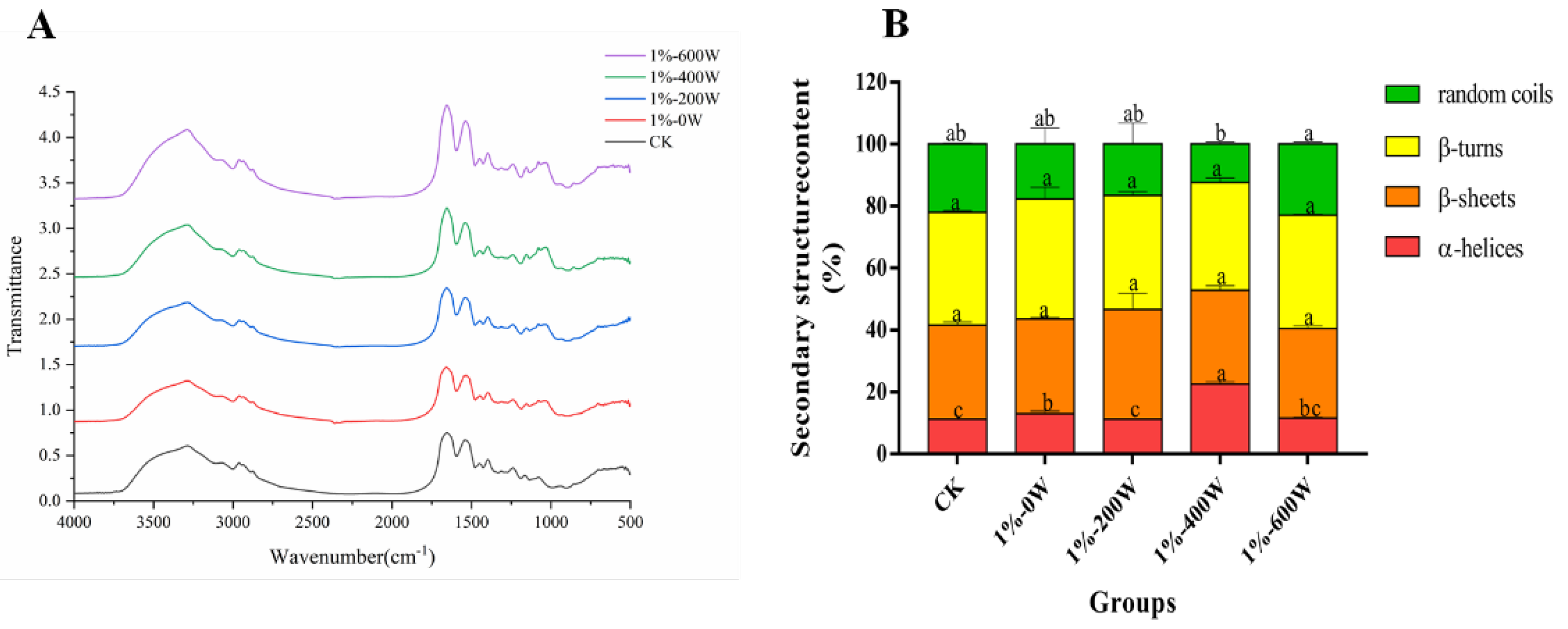
3.10. Changes in Secondary Structure
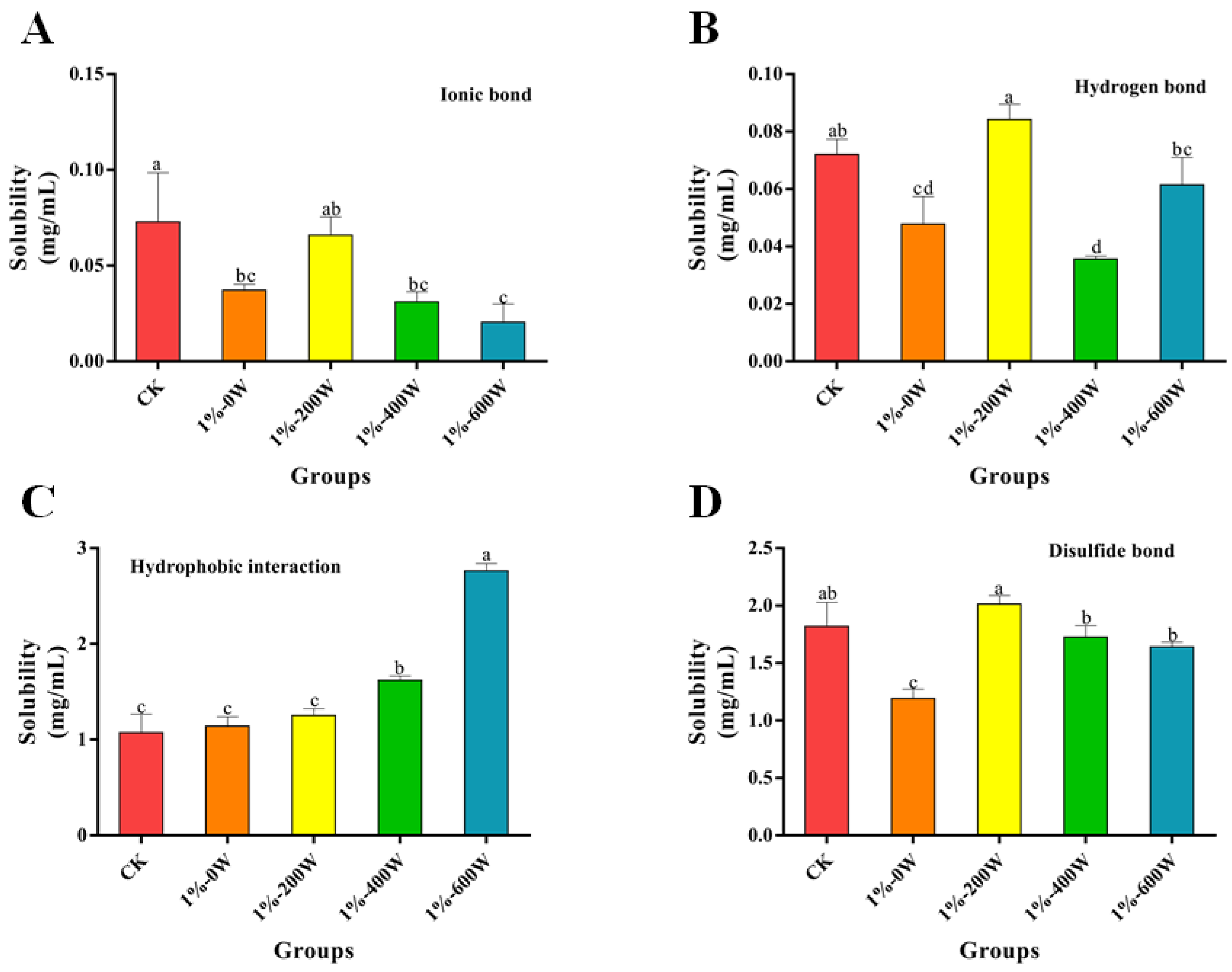
3.11. Chemical Forces Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biswas, S.; Banerjee, R.; Bhattacharyya, D.; Patra, G.; Das, A.K.; Das, S.K. Technological investigation into duck meat and its products—A potential alternative to chicken. World Poult. Sci. J. 2019, 75, 609–620. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef] [PubMed]
- Ramadhan, K.; Huda, N.; Ahmad, R. Effect of number and washing solutions on functional properties of surimi-like material from duck meat. J. Food Sci. Technol. 2014, 51, 256–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Bai, Z.; Wang, J.; Zhou, Y.; Jiang, J.; Zeng, Q.; Song, K. Comparative study on the chemical composition, anthocyanins, tocopherols and carotenoids of selected legumes. Food Chem. 2018, 260, 317–326. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Dang, Y.; Cao, J.; Pan, D.; Guo, Y.; He, J. Water-insoluble dietary fibers from oats enhance gel properties of duck myofibrillar proteins. Food Chem. 2021, 344, 128690. [Google Scholar] [CrossRef]
- Zhuang, X.; Han, M.; Jiang, X.; Bai, Y.; Zhou, H.; Li, C.; Xu, X.-L.; Zhou, G.-H. The effects of insoluble dietary fiber on myofibrillar protein gelation: Microstructure and molecular conformations. Food Chem. 2019, 275, 770–777. [Google Scholar] [CrossRef]
- Niu, Y.; Fang, H.; Huo, T.; Sun, X.; Gong, Q.; Yu, L. A novel fat replacer composed by gelatin and soluble dietary fibers from black bean coats with its application in meatballs. LWT 2020, 122, 109000. [Google Scholar] [CrossRef]
- Jiang, L.; Ge, H.; Liu, F.; Chen, D. Investigations on dynamics of interacting cavitation bubbles in strong acoustic fields. Ultrason Sonochem. 2017, 34, 90–97. [Google Scholar] [CrossRef]
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O.E.; Pilosof, A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.-A.; Wang, H.; Xia, X.; Kong, B. Ultrasound-assisted immersion freezing reduces the structure and gel property deterioration of myofibrillar protein from chicken breast. Ultrason Sonochem. 2020, 67, 105137. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Zhang, W.; Liu, R.; Liu, Y.; Xing, L.; Han, M.; Kang, Z.-L.; Xu, X.-L.; Zhou, G.-H. Improved gel functionality of myofibrillar proteins incorporation with sugarcane dietary fiber. Food Res. Int. 2017, 100, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, R.; Feng, X.; Fan, X.; Liu, Y.; Xu, X.; Zhou, G.; Zhu, B.; Ullah, N.; Chen, L. Effects of low-frequency and high-intensity ultrasonic treatment combined with curdlan gels on the thermal gelling properties and structural properties of soy protein isolate. Food Hydrocoll. 2022, 127, 107506. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, Y.; Wang, Y.; Wang, R.; Zeng, M. Effect of κ-carrageenan on the gelation properties of oyster protein. Food Chem. 2022, 382, 132329. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, J.; Lorenzo, J.M.; Zhang, W. Effects of ultrasound emulsification on the properties of pork myofibrillar protein-fat mixed gel. Food Chem. 2021, 345, 128751. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, Y.; Yang, K.; Yin, X.; Ma, J.; Li, Z.; Sun, W.; Han, M. Effect of low-frequency magnetic field on the gel properties of pork myofibrillar proteins. Food Chem. 2019, 274, 775–781. [Google Scholar] [CrossRef]
- Du, J.; Zhou, C.; Xia, Q.; Wang, Y.; Geng, F.; He, J.; Sun, Y.; Pan, D.; Cao, J. The effect of fibrin on rheological behavior, gelling properties and microstructure of myofibrillar proteins. LWT 2022, 153, 112457. [Google Scholar] [CrossRef]
- Pan, J.; Lian, H.; Jia, H.; Li, S.; Hao, R.; Wang, Y.; Zhang, X.; Dong, X. Ultrasound treatment modified the functional mode of gallic acid on properties of fish myofibrillar protein. Food Chem. 2020, 320, 126637. [Google Scholar] [CrossRef]
- Alavi, F.; Chen, L.; Emam-Djomeh, Z. Effect of ultrasound-assisted alkaline treatment on functional property modifications of faba bean protein. Food Chem. 2021, 354, 129494. [Google Scholar] [CrossRef]
- Liu, G.; Xiong, Y.L. Electrophoretic Pattern, Thermal Denaturation, and in Vitro Digestibility of Oxidized Myosin. J. Agric. Food Chem. 2000, 48, 624–630. [Google Scholar] [CrossRef]
- Qayum, A.; Hussain, M.; Li, M.; Li, J.; Shi, R.; Li, T.; Anwar, A.; Ahmed, Z.; Hou, J.; Jiang, Z. Gelling, microstructure and water-holding properties of alpha-lactalbumin emulsion gel: Impact of combined ultrasound pretreatment and laccase cross-linking. Food Hydrocoll. 2021, 110, 106122. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, M.; Xia, X.; Liu, Q.; Kong, B. Effect of porcine plasma protein hydrolysates on long-term retrogradation of corn starch. Food Chem. 2018, 239, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, T.; Wang, X.; Zou, Y.; Wang, D.; Xu, W. Effects of the structure and gel properties of myofibrillar protein on chicken breast quality treated with ultrasound-assisted potassium alginate. Food Chem. 2021, 358, 129873. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Katano, T.; Yoshimoto, Y.; Gao, Y.; Nakazawa, N.; Osako, K.; Okazaki, E. Effect of small granules in potato starch and wheat starch on quality changes of direct heated surimi gels after freezing. Food Hydrocoll. 2020, 104, 105732. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.; Feng, J.; Liu, Y.; Suo, R.; Jin, J.; Wang, W. Ultrasonic pretreatment improves the gelation properties of low-salt Penaeus vannamei (Litopenaeus vannamei) surimi. Ultrason Sonochem. 2022, 86, 106031. [Google Scholar] [CrossRef]
- Zheng, H.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Effect of κ-carrageenan on gelation and gel characteristics of Antarctic krill (Euphausia superba) protein isolated with isoelectric solubilization/precipitation. Food Chem. 2019, 278, 644–652. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, G.; Zhang, W. Effects of regenerated cellulose fiber on the characteristics of myofibrillar protein gels. Carbohyd Polym. 2019, 209, 276–281. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Bhandari, B.; Gao, Z. Effects of malondialdehyde-induced protein modification on water functionality and physicochemical state of fish myofibrillar protein gel. Food Res. Int. 2016, 86, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Cen, K.; Yu, X.; Gao, C.; Yang, Y.; Tang, X.; Feng, X. Effects of quinoa protein Pickering emulsion on the properties, structure and intermolecular interactions of myofibrillar protein gel. Food Chem. 2022, 394, 133456. [Google Scholar] [CrossRef]
- Yang, Z.; de Campo, L.; Gilbert, E.P.; Knott, R.; Cheng, L.; Storer, B.; Lin, X.; Luo, L.; Patole, S.; Hemar, Y. Effect of NaCl and CaCl2 concentration on the rheological and structural characteristics of thermally-induced quinoa protein gels. Food Hydrocoll. 2022, 124, 107350. [Google Scholar] [CrossRef]
- Song, Y.; Cheng, S.; Wang, H.; Zhu, B.-W.; Zhou, D.; Yang, P.; Tan, M. Variable Temperature Nuclear Magnetic Resonance and Magnetic Resonance Imaging System as a Novel Technique for In Situ Monitoring of Food Phase Transition. J. Agric. Food Chem. 2018, 66, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Ma, J.; Yang, K.; Feng, X.; You, X.; Wang, S.; Zhang, Y.; Xiong, G.; Wang, L.; et al. Effects of radio frequency heating on water distribution and structural properties of grass carp myofibrillar protein gel. Food Chem. 2021, 343, 128557. [Google Scholar] [CrossRef]
- Zhang, Z.; Regenstein, J.M.; Zhou, P.; Yang, Y. Effects of high intensity ultrasound modification on physicochemical property and water in myofibrillar protein gel. Ultrason Sonochem. 2017, 34, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-P.; Kang, Z.-L.; Sukmanov, V.; Ma, H.-J. Effects of soy protein isolate on gel properties and water holding capacity of low-salt pork myofibrillar protein under high pressure processing. Meat Sci. 2021, 176, 108471. [Google Scholar] [CrossRef]
- Wang, B.; Du, X.; Kong, B.; Liu, Q.; Li, F.; Pan, N.; Xia, X.; Zhang, D. Effect of ultrasound thawing, vacuum thawing, and microwave thawing on gelling properties of protein from porcine longissimus dorsi. Ultrason Sonochem. 2020, 64, 104860. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Tu, Y.; Zhang, G.; Xin, X.; Hu, H.; Qiu, W.; Ruan, D.; Zhao, Y. Mechanism of ultrasound and tea polyphenol assisted ultrasound modification of egg white protein gel. Ultrason Sonochem. 2021, 81, 105857. [Google Scholar] [CrossRef] [PubMed]
- Arzeni, C.; Pérez, O.E.; Pilosof, A.M.R. Functionality of egg white proteins as affected by high intensity ultrasound. Food Hydrocoll. 2012, 29, 308–316. [Google Scholar] [CrossRef]
- Mousakhani-Ganjeh, A.; Hamdami, N.; Soltanizadeh, N. Impact of high voltage electric field thawing on the quality of frozen tuna fish (Thunnus albacares). J. Food Eng. 2015, 156, 39–44. [Google Scholar] [CrossRef]
- Tang, C.-H.; Wang, X.-Y.; Yang, X.-Q.; Li, L. Formation of soluble aggregates from insoluble commercial soy protein isolate by means of ultrasonic treatment and their gelling properties. J. Food Eng. 2009, 92, 432–437. [Google Scholar] [CrossRef]
- Higuera-Barraza, O.A.; Torres-Arreola, W.; Ezquerra-Brauer, J.M.; Cinco-Moroyoqui, F.J.; Figueroa, J.C.R.; Marquez-Ríos, E. Effect of pulsed ultrasound on the physicochemical characteristics and emulsifying properties of squid (Dosidicus gigas) mantle proteins. Ultrason Sonochem. 2017, 38, 829–834. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, H.; Dong, K.; Yang, S.; Ye, X.; Chen, S. Analysis of the tenderisation of jumbo squid (Dosidicus gigas) meat by ultrasonic treatment using response surface methodology. Food Chem. 2014, 160, 219–225. [Google Scholar] [CrossRef]
- Geng, M.; Liu, J.; Hu, H.; Qin, L.; Taha, A.; Zhang, Z. A comprehensive study on structures and characterizations of 7S protein treated by high intensity ultrasound at different pH and ionic strengths. Food Chem. 2022, 373, 131378. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Chen, L.; Foegeding, E.A. Mechanical and Water-Holding Properties and Microstructures of Soy Protein Isolate Emulsion Gels Induced by CaCl2, Glucono-δ-lactone (GDL), and Transglutaminase: Influence of Thermal Treatments before and/or after Emulsification. J. Agric. Food Chem. 2011, 59, 4071–4077. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Mayer, S.G.; Park, J.W. FT-IR and Raman spectroscopies determine structural changes of tilapia fish protein isolate and surimi under different comminution conditions. Food Chem. 2017, 226, 156–164. [Google Scholar] [CrossRef]
- Yu, L.H.; Yao, K.L.; Liu, Z.L.; Zhang, Y.S. Electronic structure of half-Heusler semiconductor LiBeN. Phys. Lett. A. 2007, 367, 389–393. [Google Scholar] [CrossRef]


| Groups | Size (μm) | Zeta Potential (mV) |
|---|---|---|
| CK | 27.40 ± 0.37 b | −25.73 ± 1.60 c |
| 1%-0 W | 20.15 ± 0.10 c | −20.43 ± 0.59 b |
| 1%-200 W | 19.47 ± 0.25 c | −18.00 ± 0.78 a |
| 1%-400 W | 31.52 ± 0.44 a | −16.83 ± 1.10 a |
| 1%-600 W | 15.62 ± 0.76 d | −18.47 ± 0.42 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Du, Q.; Fan, X.; Zhou, C.; He, J.; Sun, Y.; Xia, Q.; Pan, D. Interaction between Kidney-Bean Polysaccharides and Duck Myofibrillar Protein as Affected by Ultrasonication: Effects on Gel Properties and Structure. Foods 2022, 11, 3998. https://doi.org/10.3390/foods11243998
Wu Y, Du Q, Fan X, Zhou C, He J, Sun Y, Xia Q, Pan D. Interaction between Kidney-Bean Polysaccharides and Duck Myofibrillar Protein as Affected by Ultrasonication: Effects on Gel Properties and Structure. Foods. 2022; 11(24):3998. https://doi.org/10.3390/foods11243998
Chicago/Turabian StyleWu, Yang, Qiwei Du, Xiankang Fan, Changyu Zhou, Jun He, Yangying Sun, Qiang Xia, and Daodong Pan. 2022. "Interaction between Kidney-Bean Polysaccharides and Duck Myofibrillar Protein as Affected by Ultrasonication: Effects on Gel Properties and Structure" Foods 11, no. 24: 3998. https://doi.org/10.3390/foods11243998
APA StyleWu, Y., Du, Q., Fan, X., Zhou, C., He, J., Sun, Y., Xia, Q., & Pan, D. (2022). Interaction between Kidney-Bean Polysaccharides and Duck Myofibrillar Protein as Affected by Ultrasonication: Effects on Gel Properties and Structure. Foods, 11(24), 3998. https://doi.org/10.3390/foods11243998







