Simulated Digestion and Fermentation In Vitro by Obese Human Gut Microbiota of Sulforaphane from Broccoli Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction and Purification of SFN
2.3. Simulated Saliva Digestion
2.4. Simulated Gastric Digestion
2.5. Simulated Small Intestinal Digestion
2.6. In Vitro Fermentation of SFN
2.7. Detection of SFN
2.8. Determination of Optical Density and SCFAs
2.9. Analysis of Gut Microbiota
2.10. Statistical Analysis
3. Results and Discussion
3.1. Extraction and Purification of SFN
3.2. Change of SFN in Digestion of Simulated Saliva
3.3. Change of SFN in Simulated Gastrointestinal Digestion
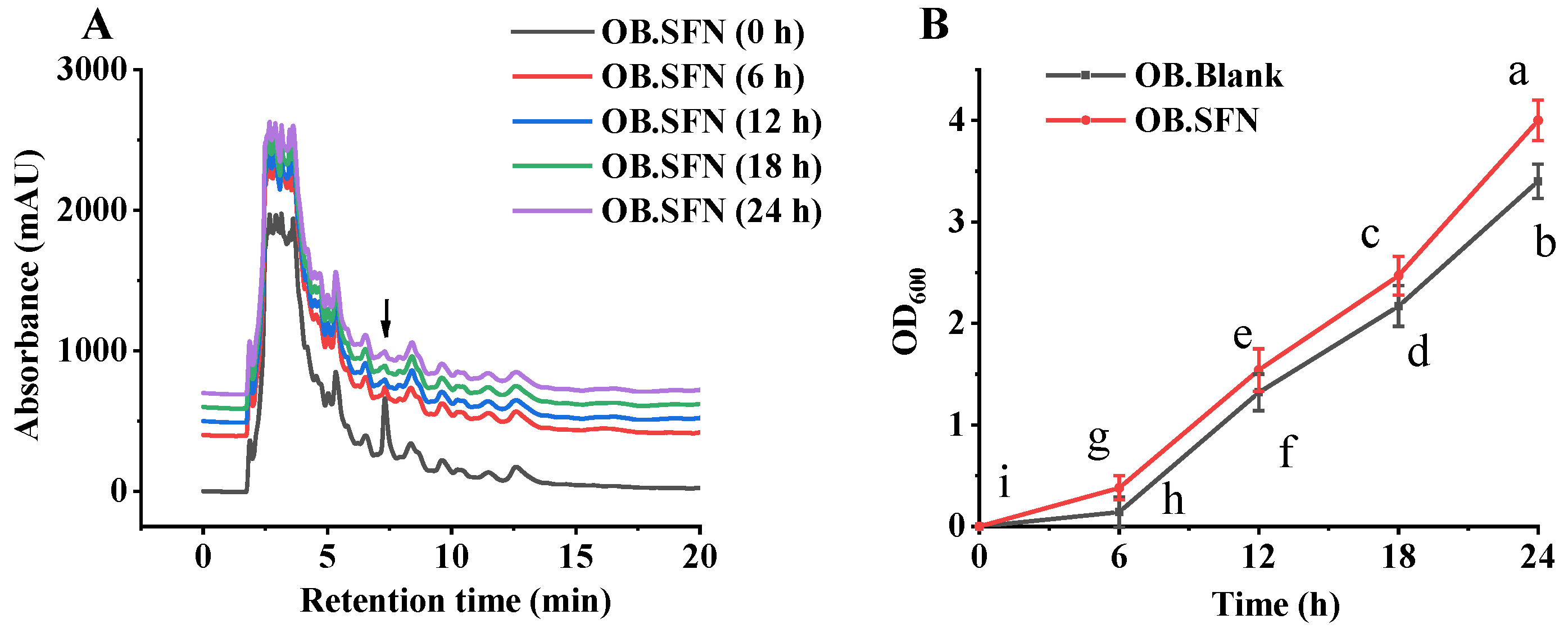
3.4. Fermentation In Vitro of SFN by Human Gut Microbiota
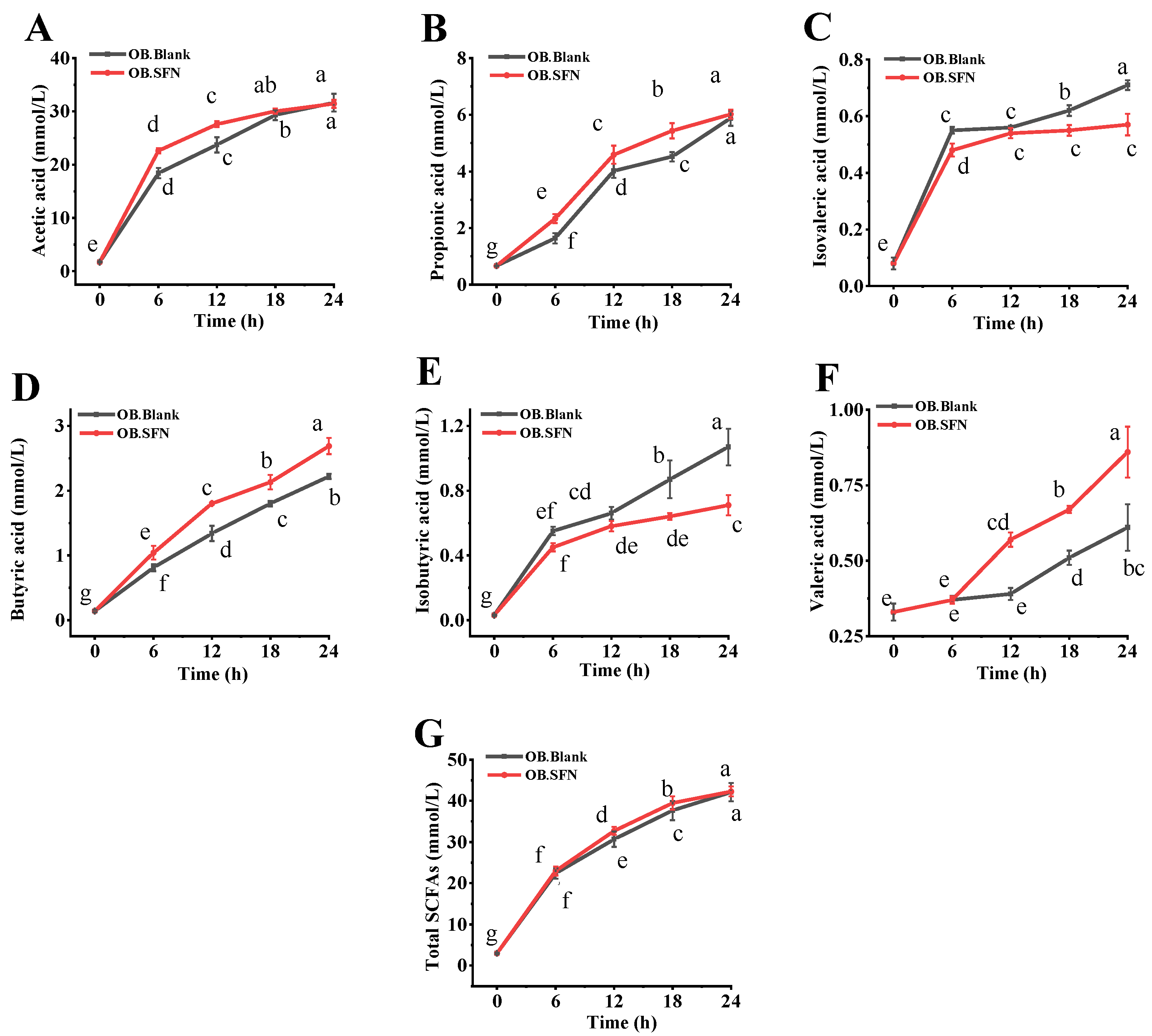
3.5. Effects of SFN Fermentation In Vitro on SCFAs Production
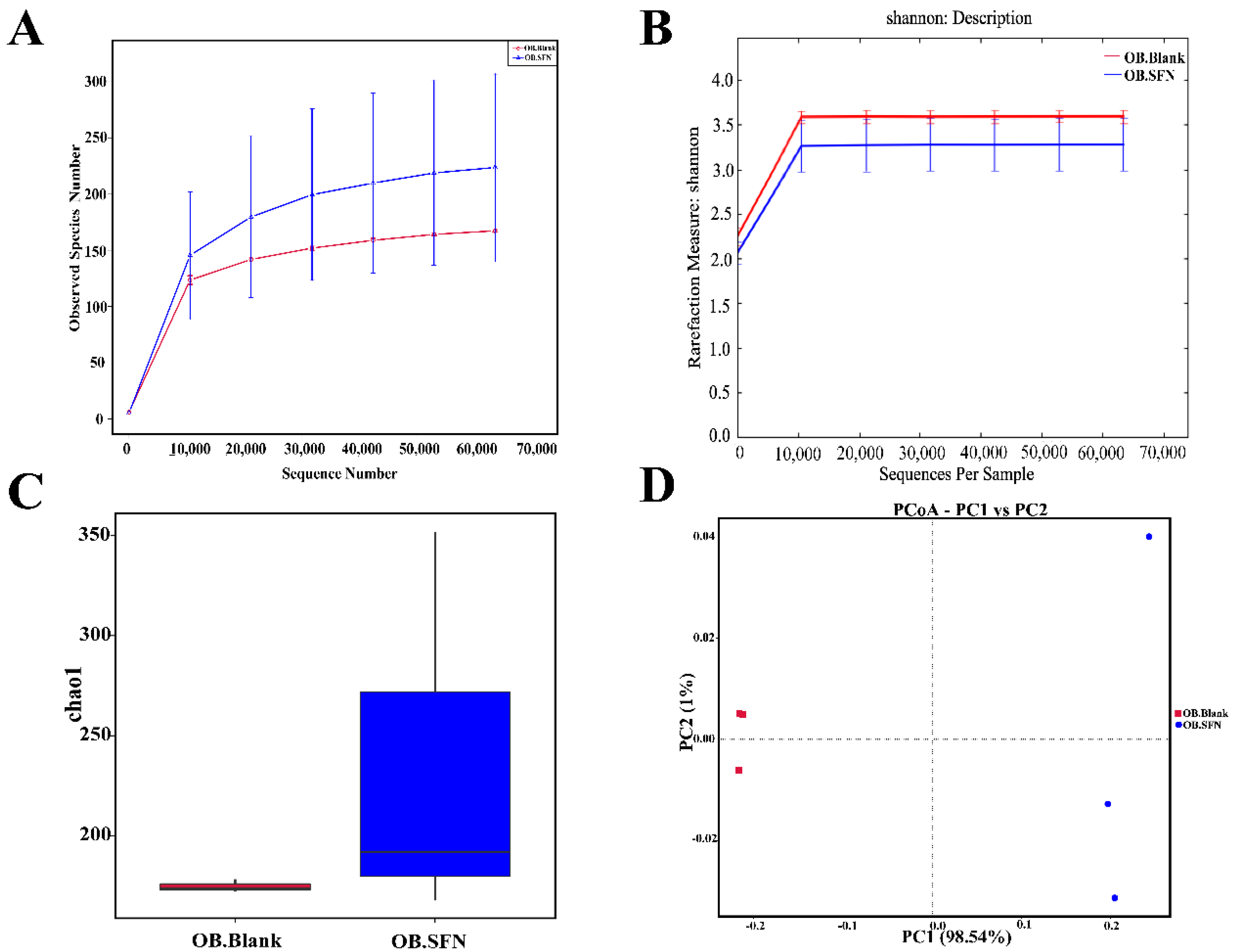
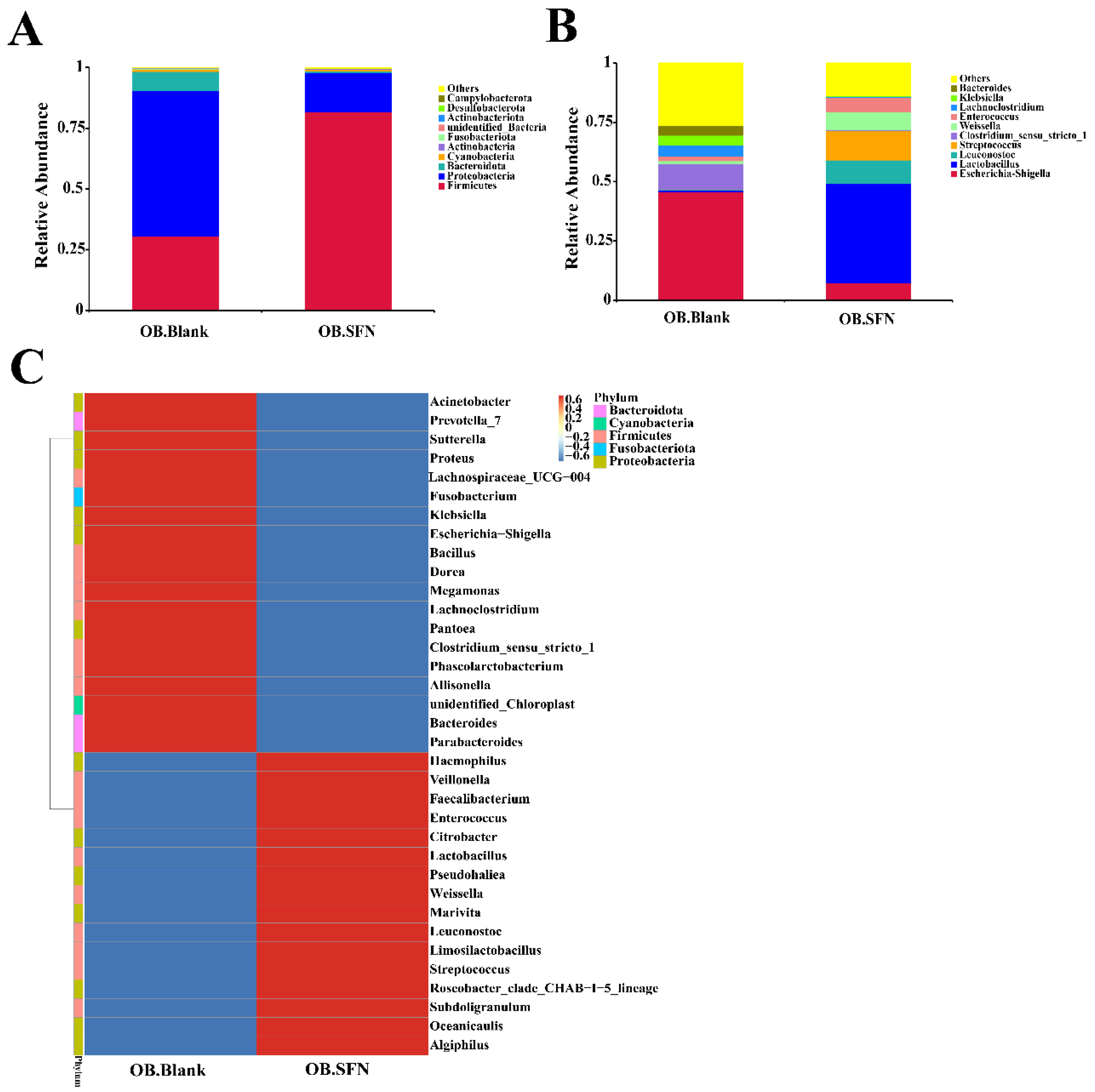
3.6. Effect of SFN on Gut Microbiota
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Wei, C.-X.; Min, L.; Zhu, L.-Y. Good or bad: Gut bacteria in human health and diseases. Biotechnol. Biotechnol. Equip. 2018, 32, 1075–1080. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Mining Lactobacillus and Bifidobacterium for organisms with long-term gut colonization potential. Clin. Nutr. 2020, 39, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.F.; Sakanaka, M.; von Burg, N.; Mörbe, U.; Andersen, D.; Moll, J.M.; Pekmez, C.T.; Rivollier, A.; Michaelsen, K.F.; Mølgaard, C.; et al. Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut. Nat. Microbiol. 2021, 6, 1367–1382. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Matsushima, Y.; Mizutani, K.; Yamanaka, K. The Role of Gut Microbiome in Psoriasis: Oral Administration of Staphylococcus aureus and Streptococcus danieliae Exacerbates Skin Inflammation of Imiquimod-Induced Psoriasis-Like Dermatitis. Int. J. Mol. Sci. 2020, 21, 3303. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, M.; Thaiss, C.A.; Elinav, E. Metabolites: Messengers between the microbiota and the immune system. Genes Dev. 2016, 30, 1589–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postler, T.S.; Ghosh, S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Li, Y.; Li, P.; Wang, M.; Wang, J.; Tang, Z.; Wang, T.; Luo, L.; Wang, C.; Zhao, B. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed. Pharm. 2019, 117, 109138. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef]
- Singh, K.; Connors, S.L.; Macklin, E.A.; Smith, K.D.; Fahey, J.W.; Talalay, P.; Zimmerman, A.W. Sulforaphane treatment of autism spectrum disorder (ASD). Proc. Natl. Acad. Sci. USA 2014, 111, 15550–15555. [Google Scholar] [CrossRef]
- Wu, Y.K.; Ren, Z.N.; Zhu, S.L.; Wu, Y.Z.; Wang, G.; Zhang, H.; Chen, W.; He, Z.; Ye, X.L.; Zhai, Q.X. Sulforaphane ameliorates non-alcoholic fatty liver disease in mice by promoting FGF21/FGFR1 signaling pathway. Acta. Pharm. Sin. 2022, 43, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-M.; Lee, Y.-S.; Kim, W.; Kim, S.J.; Shin, K.-O.; Yu, J.-Y.; Lee, M.K.; Lee, Y.-M.; Hong, J.T.; Yun, Y.-P.; et al. Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. J. Nutr. Biochem. 2014, 25, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Janczewski, Ł. Sulforaphane and Its Bifunctional Analogs: Synthesis and Biological Activity. Molecules 2022, 27, 1750. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, S.M.; Royce, S.G.; Licciardi, P.V.; Karagiannis, T.C. Dietary Sulforaphane in Cancer Chemoprevention: The Role of Epigenetic Regulation and HDAC Inhibition. Antioxid. Redox Signal. 2015, 22, 1382–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkashty, O.A.; Tran, S.D. Sulforaphane as a Promising Natural Molecule for Cancer Prevention and Treatment. Curr. Med. Sci. 2021, 41, 250–269. [Google Scholar] [CrossRef]
- He, C.; Huang, L.; Lei, P.; Liu, X.; Li, B.; Shan, Y. Sulforaphane Normalizes Intestinal Flora and Enhances Gut Barrier in Mice with BBN-Induced Bladder Cancer. Mol. Nutr. Food Res. 2018, 62, e1800427. [Google Scholar] [CrossRef]
- Tian, S.; Li, X.; Wang, Y.; Lu, Y. The protective effect of sulforaphane on type II diabetes induced by high-fat diet and low-dosage streptozotocin. Food Sci. Nutr. 2021, 9, 747–756. [Google Scholar] [CrossRef]
- He, C.; Gao, M.; Zhang, X.; Lei, P.; Yang, H.; Qing, Y.; Zhang, L. The Protective Effect of Sulforaphane on Dextran Sulfate Sodium-Induced Colitis Depends on Gut Microbial and Nrf2-Related Mechanism. Front. Nutr. 2022, 9, 893344. [Google Scholar] [CrossRef]
- Abukhabta, S.; Ghawi, S.K.; Karatzas, K.A.; Charalampopoulos, D.; McDougall, G.; Allwood, J.W.; Verrall, S.; Lavery, S.; Latimer, C.; Pourshahidi, L.K.; et al. Sulforaphane-enriched extracts from glucoraphanin-rich broccoli exert antimicrobial activity against gut pathogens in vitro and innovative cooking methods increase in vivo intestinal delivery of sulforaphane. Eur. J. Nutr. 2021, 60, 1263–1276. [Google Scholar] [CrossRef]
- Vale, A.P.; Santos, J.; Melia, N.; Peixoto, V.; Brito, N.V.; Oliveira, M.B.P.P. Phytochemical composition and antimicrobial properties of four varieties of Brassica oleracea sprouts. Food Control. 2015, 55, 248–256. [Google Scholar] [CrossRef]
- Fahey, J.W.; Stephenson, K.K.; Wade, K.L.; Talalay, P. Urease from Helicobacter pylori is inactivated by sulforaphane and other isothiocyanates. Biochem. Biophys. Res. Commun. 2013, 435, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Yan, Y.; Mi, J.; Zhang, H.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. Simulated Digestion and Fermentation in vitro by Human Gut Microbiota of Polysaccharides from Bee Collected Pollen of Chinese Wolfberry. J. Agric. Food Chem. 2018, 66, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-T.; Hu, G.-A.; Zhu, L.; Zhao, Z.-C.; Jiang, Y.; Gao, M.-J.; Zhan, X.-B. In vitro digestion and fecal fermentation of highly resistant starch rice and its effect on the gut microbiota. Food Chem. 2021, 361, 130095. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhu, J.; Pan, Y.; Huang, Q. Assessment of dynamic bioaccessibility of curcumin encapsulated in milled starch particle stabilized Pickering emulsions using TNO’s gastrointestinal model. Food Funct. 2019, 10, 2583–2594. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Singh, R.P. A Human Gastric Simulator (HGS) to Study Food Digestion in Human Stomach. J. Food Sci. 2010, 75, 627–635. [Google Scholar] [CrossRef]
- Mandalari, G.; Rigby, N.M.; Bisignano, C.; Curto, R.B.L.; Mulholland, F.; Su, M.; Venkatachalam, M.; Robotham, J.M.; Willison, L.N.; Lapsley, K.; et al. Effect of food matrix and processing on release of almond protein during simulated digestion. LWT Food. Sci. Technol. 2014, 59, 439–447. [Google Scholar] [CrossRef]
- Hopgood, M.; Reynolds, G.; Barker, R. Using Computational Fluid Dynamics to Compare Shear Rate and Turbulence in the TIM-Automated Gastric Compartment with USP Apparatus II. J. Pharm. Sci. 2018, 107, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-T.; Zhu, L.; Zhang, W.-L.; Zhan, X.-B.; Gao, M.-J. New dynamic digestion model reactor that mimics gastrointestinal function. Biochem. Eng. J. 2019, 154, 107431. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H. Natural Sulforaphane from Broccoli Seeds Against Influenza A Virus Replication in MDCK Cells. Nat. Prod. Commun. 2019, 14, 1934578X1985822. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Zhou, Q.; Gao, Z.; Lin, X.; Zhou, K.; Cheng, X.; Chitrakar, B.; Chen, H.; Zhao, W. In vitro digestion and fecal fermentation behaviors of polysaccharides from Ziziphus Jujuba cv. Pozao and its interaction with human gut microbiota. Food Res. Int. 2022, 162, 112022. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Li, W. Artificial simulated saliva, gastric and intestinal digestion and fermentation in vitro by human gut microbiota of intrapolysaccharide from Paecilomyces Cicadae Tjj1213. Food. Sci. Hum. Well. 2023, 12, 622–633. [Google Scholar]
- Ding, Y.; Yan, Y.; Peng, Y.; Chen, D.; Mi, J.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int. J. Biol. Macromol. 2019, 125, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, B.; Liu, C.; Hua, H.; Guo, Y.; Cheng, Y.; Yao, W.; Qian, H. Comprehensive analysis of Sparassis crispa polysaccharide characteristics during the in vitro digestion and fermentation model. Food Res. Int. 2022, 154, 111005. [Google Scholar] [CrossRef]
- Ares, A.M.; Valverde, S.; Bernal, J.L.; Nozal, M.J. Development and validation of a LC–MS/MS method to determine sulforaphane in honey. Food Chem. 2015, 181, 263–269. [Google Scholar] [CrossRef]
- Chen, D.; Chen, G.; Wan, P.; Hu, B.; Chen, L.; Ou, S.; Zeng, X.; Ye, H. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro induced by human gut microbiota of polysaccharides from the flowers of Camellia sinensis. Food. Funct. 2017, 8, 4619–4629. [Google Scholar] [CrossRef]
- Xu, J.; Sun, W.; Li, H.; Gao, Z.; Hu, G.; Wu, J.; Zhang, H.; Li, Z.; Gao, M.; Zhu, L.; et al. Xanthan gum oligosaccharides ameliorate glucose metabolism and related gut microbiota dysbiosis in type 2 diabetic mice. Food Biosci. 2022, 50, 102002. [Google Scholar] [CrossRef]
- Kokotou, M.G.; Revelou, P.-K.; Pappas, C.; Constantinou-Kokotou, V. High resolution mass spectrometry studies of sulforaphane and indole-3-carbinol in broccoli. Food Chem. 2017, 237, 566–573. [Google Scholar] [CrossRef]
- Gachons, C.P.D.; Breslin, P.A.S. Salivary Amylase: Digestion and Metabolic Syndrome. Curr. Diabetes Rep. 2016, 16, 102. [Google Scholar] [CrossRef]
- Johansson, N.L.; Pavia, C.S.; Chiao, J.W. Growth Inhibition of a Spectrum of Bacterial and Fungal Pathogens by Sulforaphane, an Isothiocyanate Product Found in Broccoli and Other Cruciferous Vegetables. Planta Med. 2008, 74, 747–750. [Google Scholar] [CrossRef]
- Wagner, A.E.; Ernst, I.; Iori, R.; Desel, C.; Rimbach, G. Sulforaphane but not ascorbigen, indole-3-carbinole and ascorbic acid activates the transcription factor Nrf2 and induces phase-2 and antioxidant enzymes in human keratinocytes in culture. Exp. Dermatol. 2010, 19, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauf, A.; Khalil, A.A.; Rahman, U.-U.; Khalid, A.; Naz, S.; Shariati, M.A.; Rebezov, M.; Urtecho, E.Z.; de Albuquerque, R.D.D.G.; Anwar, S.; et al. Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): An updated review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6034–6054. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, E.; Grootaert, C.; Verstraete, W.; Van De Wiele, T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 2011, 69, 245–258. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [Green Version]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Slattery, C.; Cotter, P.D.; O’Toole, P.W. Analysis of Health Benefits Conferred by Lactobacillus Species from Kefir. Nutrients 2019, 11, 1252. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; HuangFu, H.; Wang, X.; Zhao, S.; Liu, Y.; Lv, H.; Qin, G.; Tan, Z. Antibacterial Activity of Lactic Acid Producing Leuconostoc mesenteroides QZ1178 Against Pathogenic Gallibacterium anatis. Front. Veter. Sci. 2021, 8, 630294. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Zhu, H.; Zhu, L.; Chen, C.; Shen, C.; Zhang, R. Small intestinal microbiota composition and the prognosis of infants with ileostomy resulting from distinct primary diseases. BMC Gastroenterol. 2020, 20, 224. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Johnston, L.J.; Wu, C.; Ma, X. Gut microbiota and its metabolites: Bridge of dietary nutrients and obesity-related diseases. Crit. Rev. Food Sci. Nutr. 2021, 40, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Faden, H. The Role of Faecalibacterium, Roseburia and Butyrate in Inflammatory Bowel Disease. Dig. Dis. 2022, 40, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, K.; Saito, M.; Park, J.; Murakami, H.; Shibata, N.; Ando, M.; Nagatake, T.; Konishi, K.; Ohno, H.; Tanisawa, K.; et al. Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat. Commun. 2022, 13, 4477. [Google Scholar] [CrossRef]
- Zou, R.; Xu, F.; Wang, Y.; Duan, M.; Guo, M.; Zhang, Q.; Zhao, H.; Zheng, H. Changes in the Gut Microbiota of Children with Autism Spectrum Disorder. Autism Res. 2020, 13, 1614–1625. [Google Scholar] [CrossRef]
- Chiu, C.-M.; Huang, W.-C.; Weng, S.-L.; Tseng, H.-C.; Liang, C.; Wang, W.-C.; Yang, T.; Yang, T.-L.; Weng, C.-T.; Chang, T.-H.; et al. Systematic Analysis of the Association between Gut Flora and Obesity through High-Throughput Sequencing and Bioinformatics Approaches. BioMed. Res. Int. 2014, 2014, 906168. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, H.; Liu, H.; Zhang, H.; Bao, Y.; Di, J.; Hu, C. The genus Sutterella is a potential contributor to glucose metabolism improvement after Roux-en-Y gastric bypass surgery in T2D. Diabetes Res. Clin. Pract. 2020, 162, 108116. [Google Scholar] [CrossRef]
- Lu, H.; You, Y.; Zhou, X.; He, Q.; Wang, M.; Chen, L.; Zhou, L.; Sun, X.; Liu, Y.; Jiang, P.; et al. Citrus reticulatae pericarpium Extract Decreases the Susceptibility to HFD-Induced Glycolipid Metabolism Disorder in Mice Exposed to Azithromycin in Early Life. Front. Immunol. 2021, 12, 774433. [Google Scholar] [CrossRef]
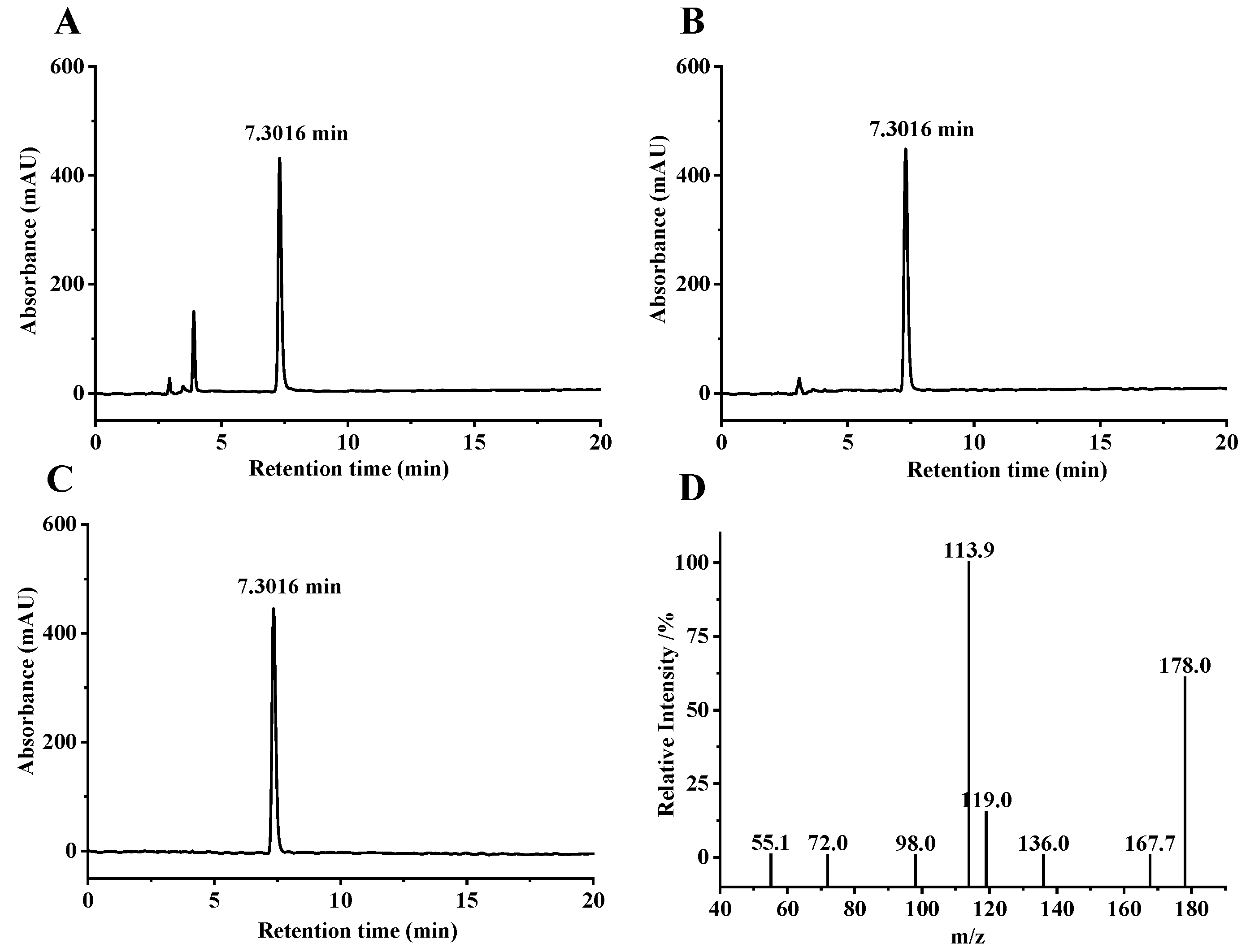
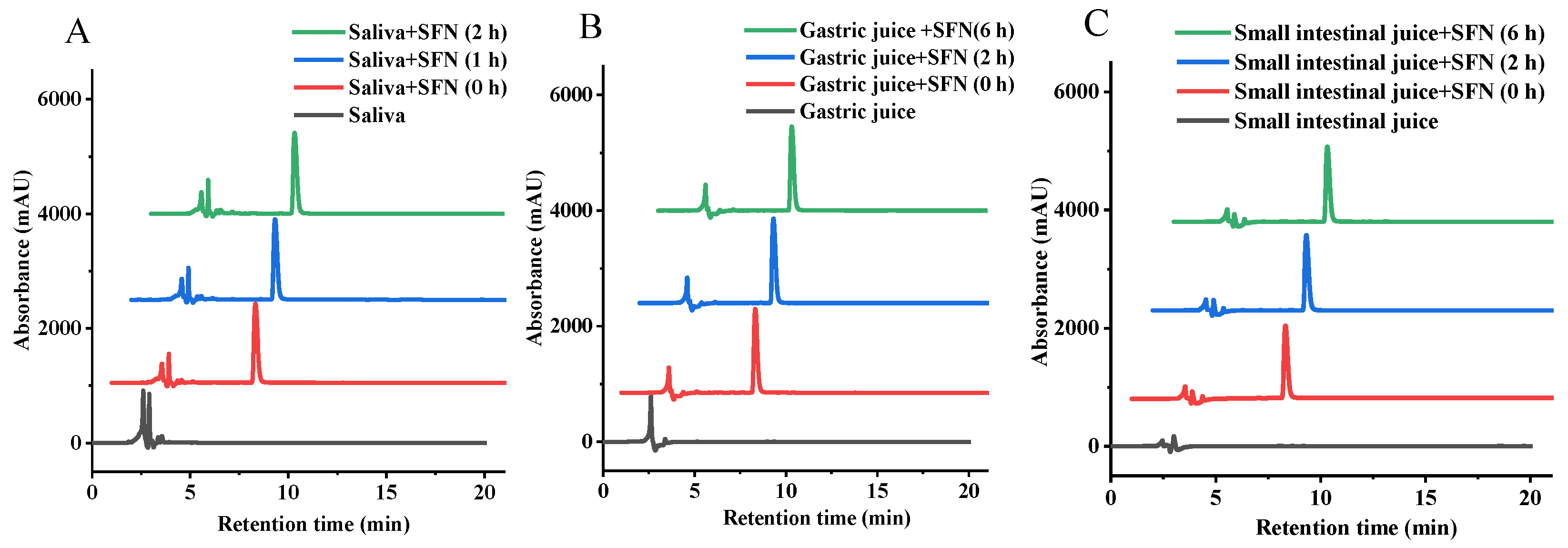
| Process | Digestion Time (h) | Content of SFN (mg/mL) |
|---|---|---|
| Saliva digestion | 0 | 1.5139 ± 0.0037 a |
| 1 | 1.5165 ± 0.0035 a | |
| 2 | 1.5133 ± 0.0028 a | |
| Gastric digestion | 0 | 1.6136 ± 0.0059 a |
| 2 | 1.6140 ± 0.0027 a | |
| 6 | 1.6133 ± 0.0020 a | |
| Small intestinal digestion | 0 | 1.2780 ± 0.0024 a |
| 2 | 1.2768 ± 0.0015 a | |
| 6 | 1.2751 ± 0.0030 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Tang, Z.; Hao, T.; Qiu, Z.; Zhang, B. Simulated Digestion and Fermentation In Vitro by Obese Human Gut Microbiota of Sulforaphane from Broccoli Seeds. Foods 2022, 11, 4016. https://doi.org/10.3390/foods11244016
Sun Y, Tang Z, Hao T, Qiu Z, Zhang B. Simulated Digestion and Fermentation In Vitro by Obese Human Gut Microbiota of Sulforaphane from Broccoli Seeds. Foods. 2022; 11(24):4016. https://doi.org/10.3390/foods11244016
Chicago/Turabian StyleSun, Yifei, Zhaocheng Tang, Tingting Hao, Zeyu Qiu, and Baolong Zhang. 2022. "Simulated Digestion and Fermentation In Vitro by Obese Human Gut Microbiota of Sulforaphane from Broccoli Seeds" Foods 11, no. 24: 4016. https://doi.org/10.3390/foods11244016






