Effects of Sheep Sires on Muscle Fiber Characteristics, Fatty Acid Composition and Volatile Flavor Compounds in F1 Crossbred Lambs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Histological Analysis of the Muscle
2.3. Fluorescence Quantitative PCR
2.4. Fatty Acid Composition Analysis
2.5. Identification of Volatile Flavor Compounds
2.6. Statistical Analysis
3. Results
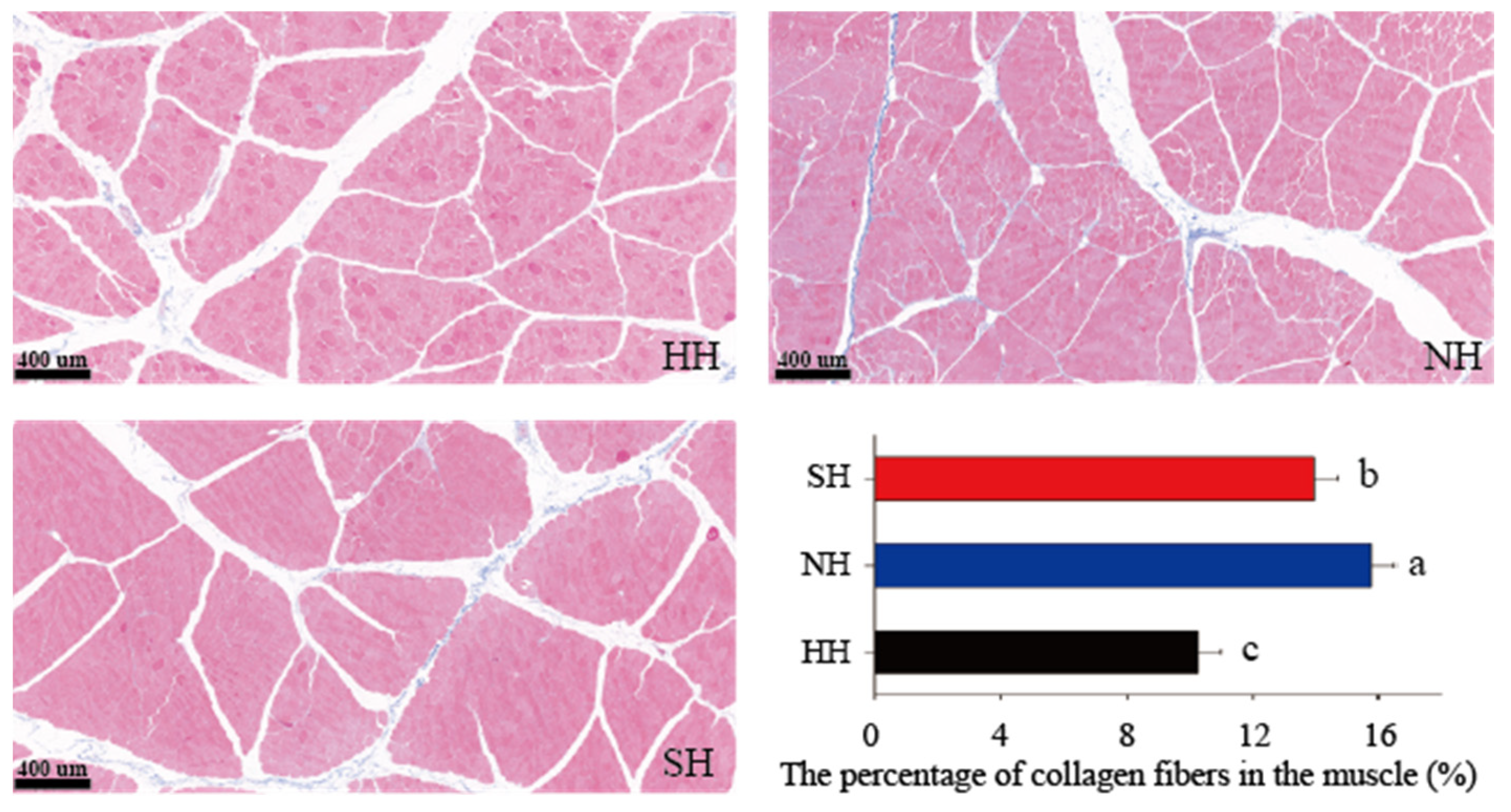
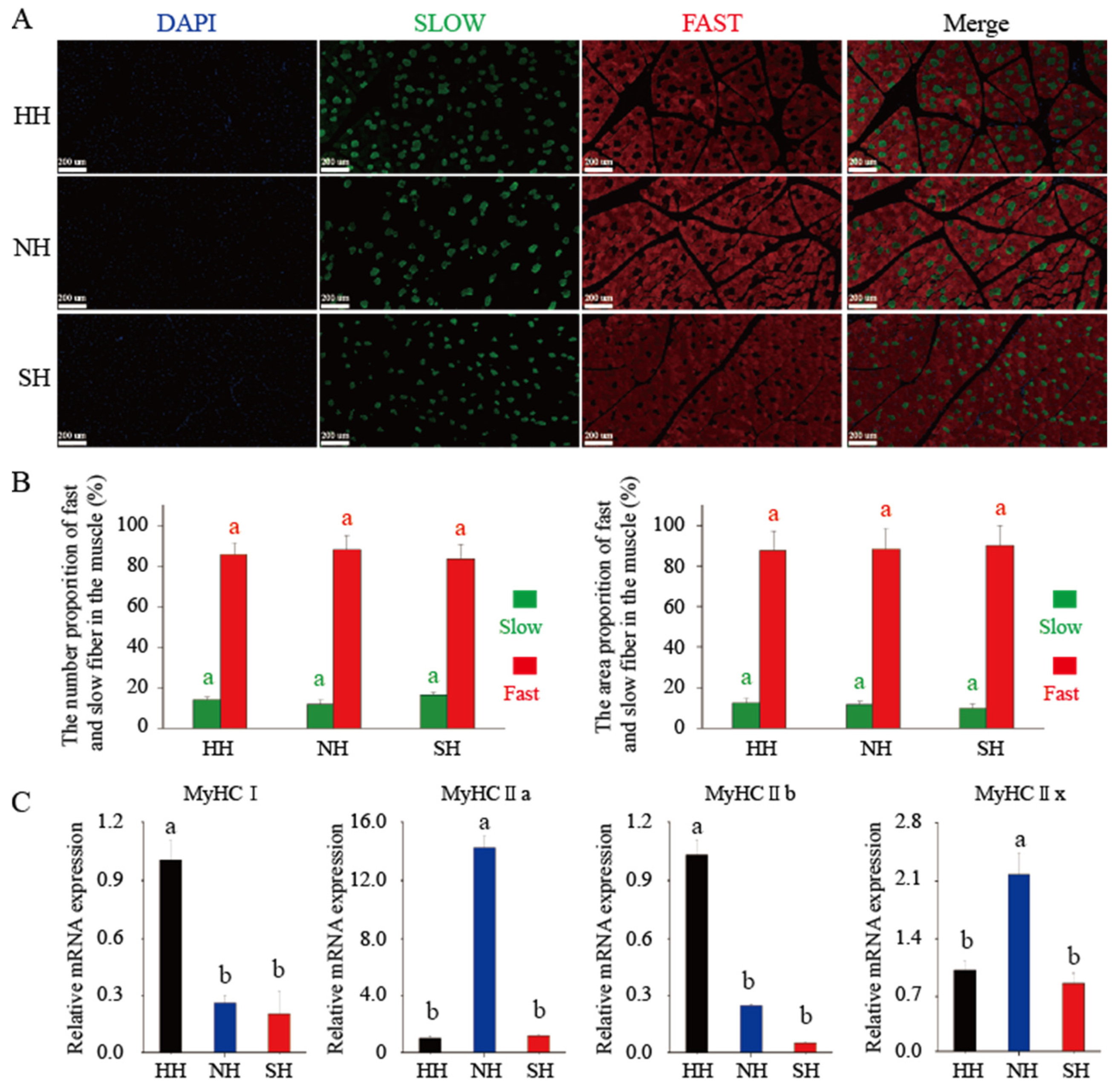
3.1. Muscle Fiber Characteristics of the Three Lamb Hybrids
3.2. Fatty Acid Composition Analysis and Contents in the F1 Crossbred Lambs
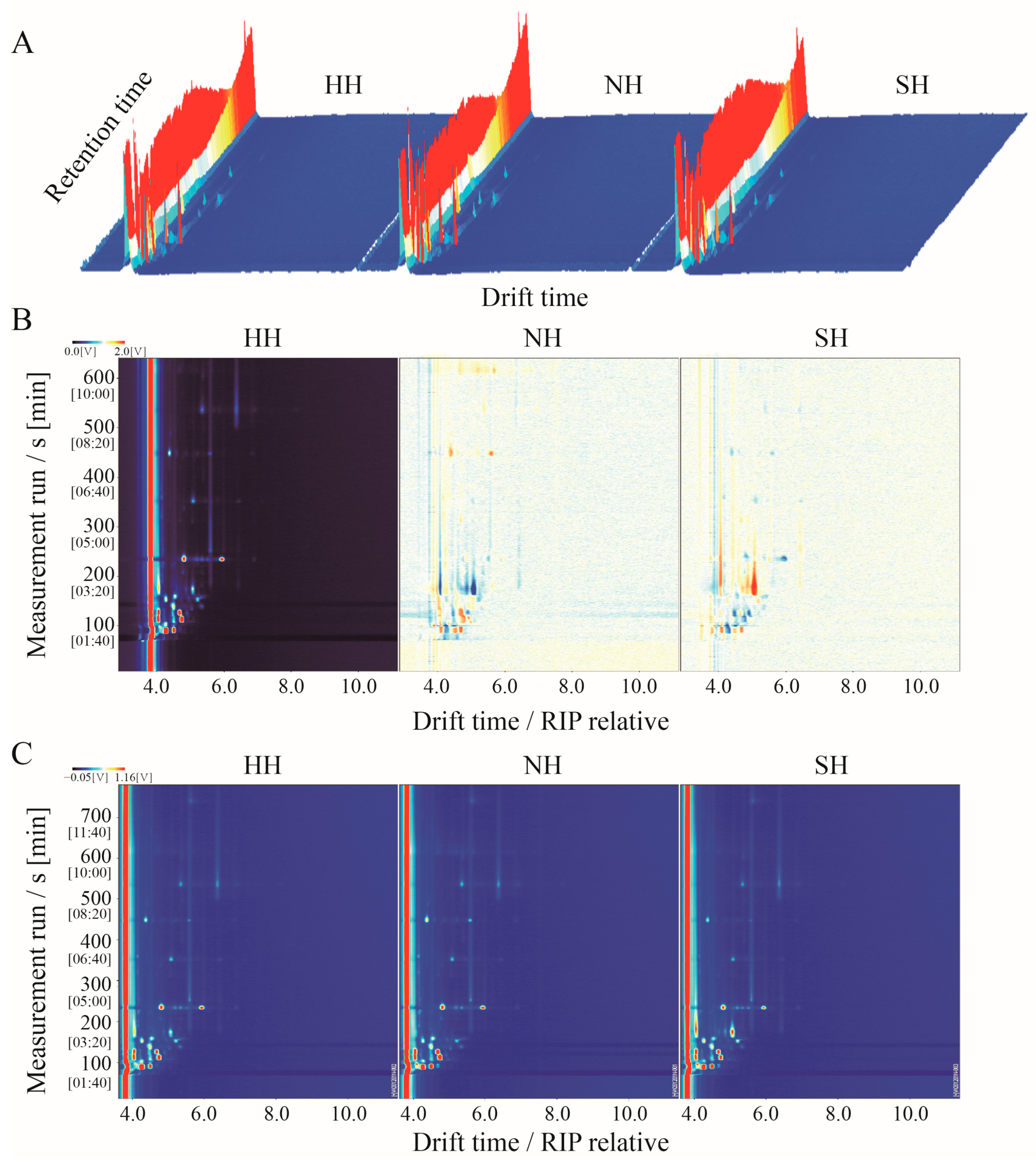
3.3. GC-IMS Topographic Maps of the F1 Crossbred Lambs
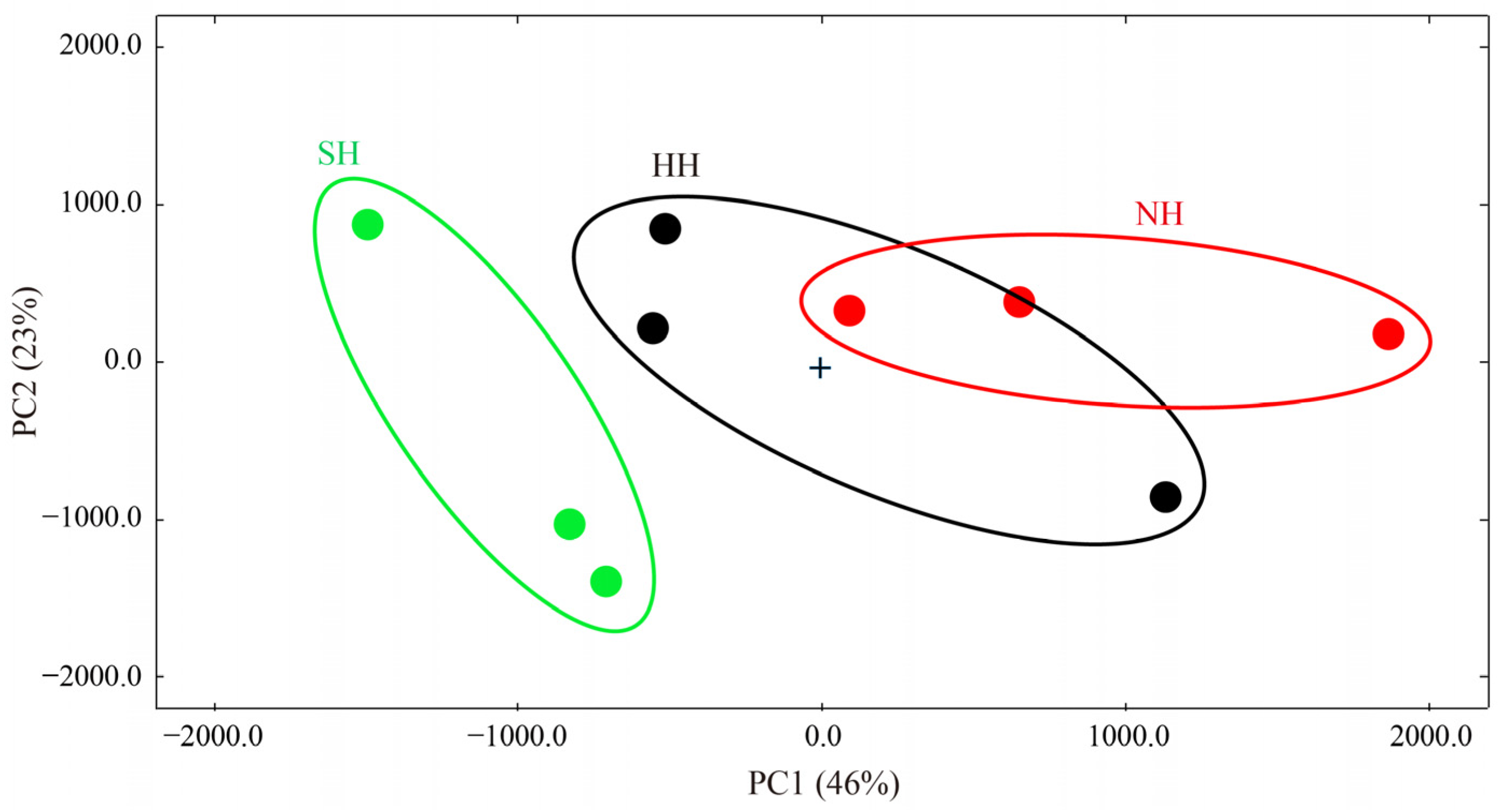
3.4. Identification of Volatile Flavor Compounds in the F1 Crossbred Lambs and Construction of Fingerprints
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cabrera, M.C.; Saadoun, A. An overview of the nutritional value of beef and lamb meat from South America. Meat Sci. 2014, 98, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.M.; Morris, S.; Hopkins, D.L. Nutritional composition of lamb retail cuts from the carcases of extensively finished lambs. Meat Sci. 2019, 154, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Luo, J.; Chai, J.; Ren, L.; Zhou, Y.; Huang, F.; Liu, X.; Chen, Y.; Zhang, C.; Tao, M.; et al. Genomic incompatibilities in the diploid and tetraploid offspring of the goldfish × common carp cross. Proc. Natl. Acad. Sci. USA 2016, 113, 1327–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, R. Drivers of consumer liking for beef, pork, and lamb: A review. Foods 2020, 9, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prache, S.; Schreurs, N.; Guillier, L. Review: Factors affecting sheep carcass and meat quality attributes. Animal 2022, 16, 100330. [Google Scholar] [CrossRef]
- Huo, W.; Weng, K.; Gu, T.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Effect of muscle fiber characteristics on meat quality in fast- and slow-growing ducks. Poult. Sci. 2021, 100, 101264. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kim, G.D.; Jeong, J.Y.; Hur, S.J.; Joo, S.T. The relationship between muscle fiber characteristics and meat quality traits of highly marbled Hanwoo (Korean native cattle) steers. Meat Sci. 2010, 86, 456–461. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M. Muscle fiber properties in cattle and their relationships with meat qualities: An overview. J. Agric. Food Chem. 2020, 68, 6021–6039. [Google Scholar] [CrossRef]
- Antonelo, D.S.; Cônsolo, N.R.B.; Gómez, J.F.M.; Beline, M.; Goulart, R.S.; Corte, R.; Colnago, L.A.; Schilling, M.W.; Gerrard, D.E.; Silva, S.L. Metabolite profile and consumer sensory acceptability of meat from lean Nellore and Angus × Nellore crossbreed cattle fed soybean oil. Food Res. Int. 2020, 132, 109056. [Google Scholar] [CrossRef]
- Chen, G.; Cai, Y.; Su, Y.; Wang, D.; Pan, X.; Zhi, X. Study of meat quality and flavour in different cuts of Duroc-Bamei binary hybrid pigs. Vet. Med. Sci. 2021, 7, 724–734. [Google Scholar] [CrossRef]
- Kim, J.A.; Cho, E.S.; Jeong, Y.D.; Choi, Y.H.; Kim, Y.S.; Choi, J.W.; Kim, J.S.; Jang, A.; Hong, J.K.; Sa, S.J. The effects of breed and gender on meat quality of Duroc, Pietrain, and their crossbred. J. Anim. Sci. Technol. 2020, 62, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Joo, S.T.; Ryu, Y.C. Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Sci. 2010, 86, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, M.; Niemiec, J.; Wnuk, A.; Mroczek-Sosnowska, N. Meat quality and the histological structure of breast and leg muscles in Ayam Cemani chickens, Ayam Cemani × Sussex hybrids and slow-growing Hubbard JA 957 chickens. J. Sci. Food Agric. 2015, 95, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How muscle structure and composition influence meat and flesh quality. Sci. World J. 2016, 2016, 3182746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokoszyński, D.; Piwczyński, D.; Arpášová, H.; Hrnčar, C.; Saleh, M.; Wasilewski, R. A comparative study of carcass characteristics and meat quality in genetic resources Pekin ducks and commercial crossbreds. Asian-Australas. J. Anim. Sci. 2019, 32, 1753–1762. [Google Scholar] [CrossRef]
- Zhao, G.P.; Cui, H.X.; Liu, R.R.; Zheng, M.Q.; Chen, J.L.; Wen, J. Comparison of breast muscle meat quality in 2 broiler breeds. Poult. Sci. 2011, 90, 2355–2359. [Google Scholar] [CrossRef]
- Blasco, M.; Campo, M.M.; Balado, J.; Saudo, C. Effect of Texel crossbreeding on productive traits, carcass and meat quality of Segurea lambs. J. Sci. Food Agric. 2019, 99, 3335–3342. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.K.; Cheng, J.; Huang, Y.Z.; Wang, X.G.; Ma, Y.L.; Peng, S.J.; Chaogetu, B.; Zhuoma, Z.; Chen, H. Growth performance and meat quality evaluations in three-way cross cattle developed for the Tibetan Plateau and their molecular understanding by integrative omics analysis. J. Agric. Food Chem. 2019, 67, 541–550. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, J.; Luo, Z.; He, H.; Chen, L.; Liu, X.; Zhou, Q. Meat and nutritional quality comparison of purebred and crossbred pigs. Anim. Sci. J. 2018, 89, 202–210. [Google Scholar] [CrossRef]
- Purslow, P.P. Contribution of collagen and connective tissue to cooked meat toughness; some paradigms reviewed. Meat Sci. 2018, 144, 127–134. [Google Scholar] [CrossRef]
- Roy, B.C.; Das, C.; Aalhus, J.L.; Bruce, H.L. Relationship between meat quality and intramuscular collagen characteristics of muscles from calf-fed, yearling-fed and mature crossbred beef cattle. Meat Sci. 2021, 173, 108375. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, E.; Martin, J.F.; Bauchart, D.; Abouelkaram, S.; Picard, B. Meat quality and composition of three muscles from French cull cows and young bulls. Anim. Sci. 2003, 76, 387–399. [Google Scholar] [CrossRef]
- Chen, Q.; Peng, Z.; Shen, M.; Wu, H.; Wang, F.; Lu, G. Correlation between beef tenderness and fiber diameter or connective tissue content. Food Sci. 2012, 33, 126–129. [Google Scholar]
- Picard, B.; Cassar-Malek, I. Evidence for expression of IIb myosin heavy chain isoform in some skeletal muscles of Blonde d’Aquitaine bulls. Meat Sci. 2009, 82, 30–36. [Google Scholar] [CrossRef]
- Sun, B.; Hou, Y.; Xu, L.; Wang, C.; Jin, Y.; Zhao, L.; Tu, Y.; Su, L. Effects of different feeding patterns on muscle fiber type composition and mutton quality of Sunit sheep. Sci. Technol. Food Ind. 2022, 43, 96–103. [Google Scholar]
- Zhou, L.; Gao, Z.; Hou, S.; Yang, B.; Wang, Z.; Gui, L. Difference analysis of meat quality and muscle fiber characteristics between newborn and adult black Tibetan sheep. Acta Vet. Zootech. Sinica 2022, 53, 700–710. [Google Scholar]
- Bocian, M.; Wojtysiak, D.; Jankowiak, H.; Cebulska, A.; Kapelański, W.; Migdał, W. Carcass, meat quality and histochemical traits of m. longissimus lumborum from Złotnicka Spotted pigs and commercial pigs. Folia Biol. 2012, 60, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Wojtysiak, D.; Gorska, M.; Wojciechowska, J. Muscle fibre characteristics and physico-chemical parameters of M. semimembranosus from Pulawska, polish large white and Pietrain pigs. Folia Biol. 2016, 64, 197–204. [Google Scholar] [CrossRef]
- Arshad, M.S.; Sohaib, M.; Ahmad, R.S.; Nadeem, M.T.; Imran, A.; Arshad, M.U.; Kwon, J.H.; Amjad, Z. Ruminant meat flavor influenced by different factors with special reference to fatty acids. Lipids Health Dis. 2018, 17, 223. [Google Scholar] [CrossRef] [Green Version]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Pewan, S.B.; Otto, J.R.; Huerlimann, R.; Budd, A.M.; Mwangi, F.W.; Edmunds, R.C.; Holman, B.W.B.; Henry, M.L.E.; Kinobe, R.T.; Adegboye, O.A.; et al. Genetics of omega-3 long-chain polyunsaturated fatty acid metabolism and meat eating quality in tattykeel Australian white lambs. Genes 2020, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Derr, J.; Etherton, T.D.; Kris-Etherton, P.M. Plasma cholesterol-predictive equations demonstrate that stearic acid is neutral and monounsaturated fatty acids are hypocholesterolemic. Am. J. Clin. Nutr. 1995, 61, 1129–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayakawa, K.; Sakamoto, T.; Ishii, A.; Yamaji, K.; Uemoto, Y.; Sasago, N.; Kobayashi, E.; Kobayashi, N.; Matsuhashi, T.; Maruyama, S.; et al. The g.841G>C SNP of FASN gene is associated with fatty acid composition in beef cattle. Anim. Sci. J. 2015, 86, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Lunt, D.K.; Smith, D.R.; Walzem, R.L. Producing high-oleic acid beef and the impact of ground beef consumption on risk factors for cardiovascular disease: A review. Meat Sci. 2020, 163, 108076. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Butler, K.L.; McDonagh, M.B.; Jacobs, J.L.; Hopkins, D.L. Relationship between muscle antioxidant status, forms of iron, polyunsaturated fatty acids and functionality (retail colour) of meat in lambs. Meat Sci. 2012, 90, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yan, P.; Zhang, S.; Huang, H.; Huang, F.; Sun, T.; Deng, Q.; Huang, Q.; Chen, S.; Ye, K.; et al. Long-term dietary alpha-linolenic acid supplement alleviates cognitive impairment correlate with activating hippocampal CREB signaling in natural aging rats. Mol. Neurobiol. 2016, 53, 4772–4786. [Google Scholar] [CrossRef]
- Mottin, C.; Ornaghi, M.G.; Passetti, R.A.C.; Torrecilhas, J.A.; Ramos, T.R.; Guerrero, A.; Bridi, A.M.; Prado, I.N.D. Lipid composition of raw and grilled beef cattle slaughtered at four body weights. Res. Soc. Dev. 2020, 10, e1109108351. [Google Scholar] [CrossRef]
- Şahin, A.; Aksoy, Y.; Uğurlutepe, E.; Kul, E.; Ulutaş, Z. Fatty acid profilies and some meat quality traits at different slaughter weights of Brown Swiss bulls. Trop. Anim. Health Prod. 2021, 53, 380. [Google Scholar] [CrossRef]
- Chikwanha, O.C.; Vahmani, P.; Muchenje, V.; Dugan, M.E.R.; Mapiye, C. Nutritional enhancement of sheep meat fatty acid profile for human health and wellbeing. Food Res. Int. 2018, 104, 25–38. [Google Scholar] [CrossRef]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Lu, Z.; Li, J.; Yuan, C.; Xi, B.; Yang, B.; Meng, X.; Guo, T.; Yue, Y.; Gao, Y.; Liu, J.; et al. Evaluation of mutton quality characteristics of Dongxiang tribute sheep based on membership function and gas chromatography and ion mobility spectrometry. Front. Nutr. 2022, 9, 852399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, H.; Liu, M.; Zhao, X.; Luo, H. Effect of breed on the volatile compound precursors and odor profile attributes of lamb meat. Foods 2020, 9, 1178. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors—A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Amadou, I.; Zhou, G.Y.; Qian, L.Y.; Zhang, J.L.; Wang, D.L.; Cheng, X.R. Flavor components comparison between the neck meat of donkey, swine, bovine, and sheep. Food Sci. Anim. Resour. 2020, 40, 527–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Wang, Z.; Chen, Y.; Liu, X.; Liu, K.; Zhang, Y.; Luo, H. Carcass traits, meat quality, and volatile compounds of lamb meat from different restricted grazing time and indoor supplementary feeding systems. Foods 2021, 10, 2822. [Google Scholar] [CrossRef] [PubMed]
- Ba, H.V.; Seo, H.W.; Seong, P.N.; Cho, S.H.; Kang, S.M.; Kim, Y.S.; Moon, S.S.; Choi, Y.M.; Kim, J.H. Live weights at slaughter significantly affect the meat quality and flavor components of pork meat. Anim. Sci. J. 2019, 90, 667–679. [Google Scholar] [CrossRef]
- Sohail, A.; Al-Dalali, S.; Wang, J.; Xie, J.; Shakoor, A.; Asimi, S.; Shah, H.; Patil, P. Aroma compounds identified in cooked meat: A review. Food Res. Int. 2022, 157, 111385. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Yang, Y.; Zhu, J.; He, W.; Zhao, Q.; Tang, C.; Qin, Y.; Zhang, J. Comparative characterization of lipids and volatile compounds of Beijing Heiliu and Laiwu Chinese black pork as markers. Food Res. Int. 2021, 146, 110433. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Tang, C.; Yue, S.; Zhao, Q.; Li, F.; Zhang, J. Changes in lipids and aroma compounds in intramuscular fat from Hu sheep. Food Chem. 2022, 383, 132611. [Google Scholar] [CrossRef]
- Legako, J.F.; Cramer, T.; Yardley, K.; Murphy, T.J.; Gardner, T.; Chail, A.; Pitcher, L.R.; MacAdam, J.W. Retail stability of three beef muscles from grass-, legume-, and feedlot-finished cattle. J. Anim. Sci. 2018, 96, 2238–2248. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Yang, J.J.; Zou, Y.Z. Comparative analysis of flavor components in pork from different breeds by SPME with different fiber coatings and GC-MS. Food Sci. 2012, 33, 169–172. [Google Scholar]
- Legako, J.F.; Brooks, J.C.; O’Quinn, T.G.; Hagan, T.D.; Polkinghorne, R.; Farmer, L.J.; Miller, M.F. Consumer palatability scores and volatile beef flavor compounds of five USDA quality grades and four muscles. Meat Sci. 2015, 100, 291–300. [Google Scholar] [CrossRef] [PubMed]


| Types of Fatty Acids | HH Group | NH Group | SH Group | p Value |
|---|---|---|---|---|
| C10:0 | 8.50 ± 0.27 a | 5.20 ± 0.53 b | 5.98 ± 0.45 b | <0.001 |
| C12:0 | 7.55 ± 1.55 a | 4.40 ± 0.82 b | 3.23 ± 0.77 b | 0.008 |
| C14:0 | 4.10 ± 0.69 | 3.29 ± 0.49 | 3.82 ± 0.06 | 0.197 |
| C15:0 | 45.91 ± 1.59 | 42.81 ± 0.59 | 41.15 ± 0.16 | 0.081 |
| C16:0 | 4.94 ± 0.62 | 3.82 ± 0.31 | 4.54 ± 0.48 | 0.074 |
| C17:0 | 2.92 ± 0.57 | 2.37 ± 0.31 | 2.36 ± 0.06 | 0.201 |
| C18:0 | 544.18 ± 51.56 c | 660.22 ± 22.91 b | 730.72 ± 14.36 a | 0.001 |
| C20:0 | 3.45 ± 0.34 a | 1.80 ± 0.48 b | 1.63 ± 0.30 b | 0.002 |
| C21:0 | 3.95 ± 0.21 | 3.48 ± 0.28 | 3.46 ± 0.27 | 0.094 |
| C22:0 | 6.61 ± 0.57 | 6.61 ± 0.69 | 6.73 ± 0.72 | 0.970 |
| SFA | 632.13 ± 47.12 c | 733.99 ± 21.69 b | 803.62 ± 15.30 a | 0.002 |
| C14:1 | 26.91 ± 1.78 a | 13.16 ± 0.90 b | 15.04 ± 0.42 b | <0.001 |
| C15:1 | 4.99 ± 0.87 | 3.90 ± 0.65 | 4.47 ± 0.44 | 0.085 |
| C16:1 | 3.17 ± 0.20 | 2.94 ± 0.27 | 3.20 ± 0.46 | 0.581 |
| C17:1 | 3.63 ± 0.16 | 3.40 ± 0.38 | 2.97 ± 0.23 | 0.074 |
| C18:1n9c | 13.63 ± 0.45 a | 6.20 ± 0.61 c | 8.07 ± 0.55 b | <0.001 |
| C18:1n9t | 2314.24 ± 219.60 a | 1453.49 ± 93.39 b | 1846.77 ± 134.55 b | 0.002 |
| C20:1 | 27.95 ± 0.67 a | 6.45 ± 1.29 b | 8.38 ± 1.02 b | <0.001 |
| C22:1n9 | 107.29 ± 6.55 a | 88.61 ± 4.42 b | 87.95 ± 3.35 b | 0.005 |
| MUFA | 2501.82 ± 219.52 a | 1578.15 ± 90.48 c | 1976.65 ± 136.40 b | 0.001 |
| C18:2n6c | 4.48 ± 0.14 b | 6.11 ± 0.33 a | 6.26 ± 0.03 a | <0.001 |
| C18:2n6t | 10.98 ± 0.17 a | 7.91 ± 0.73 b | 8.72 ± 1.30 b | 0.012 |
| C18:3n3 | 4.07 ± 0.37 c | 6.26 ± 0.55 b | 7.89 ± 0.60 a | <0.001 |
| C20:4n6 | 77.15 ± 2.44 | 77.38 ± 2.37 | 79.58 ± 2.86 | 0.483 |
| C20:2 | 10.88 ± 0.38 a | 8.06 ± 0.73 b | 7.32 ± 0.42 b | <0.001 |
| PUFA | 107.56 ± 2.00 | 105.72 ± 3.20 | 109.77 ± 2.24 | 0.226 |
| PUFA/SFA | 0.17 ± 0.02 a | 0.14 ± 0.01 b | 0.14 ± 0.01 b | 0.009 |
| n-6/n-3 | 22.89 ± 2.55 a | 14.69 ± 1.44 b | 12.02 ± 0.75 b | 0.001 |
| Volatiles | Compounds | RI | Rt (s) | Dt (ms) | Intensity (V) | p Value | ||
|---|---|---|---|---|---|---|---|---|
| HH | NH | SH | ||||||
| Alcohols | 1-Propanol | 557.0 | 103.0 | 1.118 | 422.27 ± 89.71 b | 457.55 ± 108.65 b | 883.67 ± 194.27 a | 0.009 |
| 1-Pentanol (M) | 751.0 | 208.7 | 1.255 | 368.86 ± 106.43 | 586.77 ± 492.17 | 120.78 ± 26.68 | 0.226 | |
| 1-Pentanol (D) | 749.9 | 207.8 | 1.520 | 58.21 ± 25.47 | 463.84 ± 652.28 | 62.45 ± 24.37 | 0.379 | |
| Aldehyde | Octanal | 999.7 | 536.6 | 1.407 | 2401.29 ± 123.86 | 2478.25 ± 185.01 | 2351.89 ± 109.99 | 0.583 |
| Benzaldehyde (M) | 948.3 | 447.6 | 1.150 | 987.45 ± 109.72 b | 1429.16 ± 169.29 a | 1325.33 ± 65.83 a | 0.002 | |
| Benzaldehyde (D) | 947.3 | 445.9 | 1.467 | 896.17 ± 163.05 b | 1255.74 ± 153.61 a | 1241.86 ± 177.03 a | 0.011 | |
| Methional | 897.1 | 360.2 | 1.091 | 992.68 ± 144.87 a | 573.80 ± 116.22 b | 488.91 ± 98.45 b | 0.026 | |
| Heptanal | 890.8 | 350.3 | 1.334 | 1902.59 ± 192.42 a | 1919.41 ± 177.39 a | 1663.95 ± 109.15 b | 0.027 | |
| Hexanal (M) | 784.0 | 234.9 | 1.562 | 1943.18 ± 193.30 | 1921.06 ± 178.00 | 1877.59 ± 168.18 | 0.621 | |
| Hexanal (D) | 784.0 | 234.9 | 1.265 | 1538.80 ± 172.17 a | 1483.36 ± 175.62 a | 884.48 ± 108.56 b | 0.008 | |
| Pentanal (M) | 688.8 | 159.3 | 1.191 | 2227.79 ± 182.03 | 2283.46 ± 210.81 | 1827.18 ± 229.91 | 0.083 | |
| Pentanal (D) | 688.0 | 158.9 | 1.422 | 1978.37 ± 153.23 a | 1299.80 ± 115.09 b | 118.66 ± 32.06 c | <0.001 | |
| 3-Methylbutyraldehyde (M) | 642.5 | 139.5 | 1.402 | 3173.46 ± 219.09 a | 2415.42 ± 109.40 b | 2354.49 ± 146.77 b | 0.016 | |
| 3-Methylbutyraldehyde (D) | 638.8 | 137.9 | 1.180 | 1563.54 ± 138.59 a | 1168.37 ± 161.49 b | 852.65 ± 120.29 b | 0.024 | |
| Butanal | 612.1 | 126.5 | 1.286 | 196.79 ± 56.85 | 639.46 ± 576.82 | 159.08 ± 67.69 | 0.231 | |
| ketones | 2-Pentanone (M) | 676.8 | 154.1 | 1.368 | 2621.22 ± 188.28 | 2336.56 ± 190.78 | 2640.25 ± 167.31 | 0.261 |
| 2-Pentanone (D) | 676.8 | 154.1 | 1.120 | 2348.94 ± 187.85 | 2059.00 ± 198.72 | 2326.53 ± 178.79 | 0.113 | |
| 2-Butanone (M) | 583.5 | 114.3 | 1.062 | 1594.18 ± 82.19 b | 1851.37 ± 115.61 a | 1875.26 ± 97.72 a | 0.033 | |
| 2-Butanone (D) | 578.4 | 112.1 | 1.245 | 1361.33 ± 88.11 a | 1122.68 ± 32.88 b | 1153.82 ± 55.72 b | 0.012 | |
| 2,3-Butanedione | 568.6 | 107.9 | 1.182 | 559.20 ± 58.12 | 486.18 ± 85.84 | 909.27 ± 312.03 | 0.071 | |
| 3-Hydroxy-2-butanone (M) | 715.2 | 180.3 | 1.062 | 719.99 ± 242.96 | 276.36 ± 322.07 | 969.29 ± 318.31 | 0.392 | |
| 3-Hydroxy-2-butanone (D) | 707.5 | 174.2 | 1.331 | 195.28 ± 193.46 | 173.39 ± 210.59 | 656.73 ± 536.55 | 0.242 | |
| Esters | Methyl 2-methylbutyrate | 766.4 | 220.9 | 1.172 | 226.19 ± 174.65 | 77.52 ± 14.63 | 553.35 ± 656.28 | 0.376 |
| Methyl acetate | 529.6 | 91.3 | 1.184 | 1515.07 ± 198.75 | 1613.22 ± 116.18 | 1461.68 ± 105.67 | 0.388 | |
| Pyrazines | 2-Ethyl-5-methylpyrazine | 1000.1 | 537.4 | 1.672 | 2824.14 ± 278.11 | 2963.25 ± 123.54 | 2949.90 ± 169.36 | 0.504 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Yue, Y.; Shi, H.; Zhang, J.; Liu, T.; Liu, J.; Yang, B. Effects of Sheep Sires on Muscle Fiber Characteristics, Fatty Acid Composition and Volatile Flavor Compounds in F1 Crossbred Lambs. Foods 2022, 11, 4076. https://doi.org/10.3390/foods11244076
Lu Z, Yue Y, Shi H, Zhang J, Liu T, Liu J, Yang B. Effects of Sheep Sires on Muscle Fiber Characteristics, Fatty Acid Composition and Volatile Flavor Compounds in F1 Crossbred Lambs. Foods. 2022; 11(24):4076. https://doi.org/10.3390/foods11244076
Chicago/Turabian StyleLu, Zengkui, Yaojing Yue, Haina Shi, Jinxia Zhang, Tiaoguo Liu, Jianbin Liu, and Bohui Yang. 2022. "Effects of Sheep Sires on Muscle Fiber Characteristics, Fatty Acid Composition and Volatile Flavor Compounds in F1 Crossbred Lambs" Foods 11, no. 24: 4076. https://doi.org/10.3390/foods11244076
APA StyleLu, Z., Yue, Y., Shi, H., Zhang, J., Liu, T., Liu, J., & Yang, B. (2022). Effects of Sheep Sires on Muscle Fiber Characteristics, Fatty Acid Composition and Volatile Flavor Compounds in F1 Crossbred Lambs. Foods, 11(24), 4076. https://doi.org/10.3390/foods11244076






